Abstract
Biochemical methane potential (BMP) tests are the most reliable method for the direct evaluation of the methane yield from a specific feedstock in anaerobic digestion. However, these tests are time-consuming (about 1 month) and quite expensive (need of no less than two or three replicates). This study evaluates the accuracy of the “first-order kinetic”, “logistic” and “Gompertz” models in predicting the BMP values, calibrating the models’ parameters with the data collected in shorter BMP tests (i.e., 5, 7, 10, 14 and 21 days) than usually (28 days or more). Moreover, the influence of the number of replicates (i.e., two or three) on the model prediction accuracy was also evaluated. A database from 32 BMP tests, previously carried out on different substrates, was adopted for these evaluations. The test duration significantly influences the prediction accuracy for two models (Gompertz and first-order kinetic), while the number of replicates is less influencing. The ultimate methane production is not accurate if the models use parameters from short (less than 10 days) BMP tests. The increase in test duration to 21 days gives BMP predictions with errors below 10% for Gompertz and logistic models.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Prediction models are useful tools to estimate the ultimate biochemical methane potential (BMP) of a feedstock in anaerobic digestion (AD) under optimal conditions. However, in order to calibrate their parameters, these models require preliminary BMP tests, which are expensive (since require replications) and time-consuming (up to 3 months, but usually about 1 month is sufficient). To reduce the test time and save money, it is important to evaluate whether it is possible to reduce the test duration and the number of replicates. This study proposes a novel “hybrid” approach, where the “first order kinetic”, “logistic” and “Gompertz” models are applied in predicting the BMP values, using the data collected in short BMP tests (between 5 and 21 days). Moreover, the model accuracy is also evaluated under the hypothesis of reducing the number of replicates (commonly three) to only two. To the authors’ best knowledge, no relevant evaluations are available in the literature. This is an important approach, since a fast and reliable method for BMP prediction is strictly linked to the management of full-scale AD plants.
Introduction
Anaerobic digestion (AD) is a biochemical process that degrades organic substances thanks to anaerobic microorganisms. The final product of AD is the biogas, a mixture of methane (60–70%), carbon dioxide (30–40%) and traces of other gases (such as nitrogen, hydrogen, ammonia and hydrogen sulphide). The methane produced by the AD (the so-called “biomethane”) is a valid alternative to fossil fuels, as renewable resource. In addition and, more importantly, the biomethane can be produced from biodegradable waste, and this enhances the sustainable management of the organic fraction of municipal and agro-industrial waste and wastewater [1]. As well-known, the methane produced from a specific substrate by AD under optimised environmental conditions is the biochemical methane potential (BMP), generally expressed as mL or L of CH4 per gram of volatile solids (VS) of biodegradable substrate. The BMP depends on the physico-chemical characteristics of the substrate as well as on the environmental conditions of the AD process (such as the temperature, hydraulic retention time, inoculum to substrate ratio, possible presence of inhibitory compounds). The BMP evaluation is an essential step to optimise the AD [2], as it measures, under optimal conditions, the potential biodegradation of the substrate and therefore its methane yield. In other words, the BMP determination measures the substrate biodegradability, considering that the methane production increases with the anaerobic biodegradation. Usually, before feeding full-scale AD plants with a specific substrate, BMP values are previously determined, in order to optimise the environmental conditions (such as temperature, organic loading rate, size of digester, etc.) and thus the methane yield of the converted organic matter. When upgraded to full-scale plant, the energy from a substrate is evaluated using its calorific value. The latter parameter can be compared to the related efficiency of the biological process [3], although few studies played attention on this issue.
The methane yield of a substrate or a mixed feedstock can be estimated using several methods. In addition to the BMP tests, other experimental and theoretical methods have been proposed [4, 5]. Spectrometry [6] and, in particular, near infrared (NIR) spectroscopy predict the enzymatically-digestible organic matter (the organic matter that can be digested under anaerobic conditions) and, therefore, the methane yield of a biomass through prediction models [5, 7, 8]. NIR spectrometry applied to several biomasses (such as municipal green waste, energy crops [9], municipal solid waste [10], household waste [11], plant biomasses [5] and straw and manure [12]) gave accurate BMP predictions. However, NIR spectral information may be influenced by the biomass composition, especially the water content and particle size, and this influence reduces the reliability of the NIR prediction models [5].
The analysis of the chemical composition of the digested substrates is an alternative method to predict the final methane production, since it is influenced by the biomass chemical characteristics [5]. Buswell [13, 14] and Tchobanoglous equations [15], which are based on the elemental composition (C, H, O, and N) of the substrate, are among the best predictors of the final methane production. These stoichiometric equations are extremely accurate in applications to easily-biodegradable substrates (e.g., cellulose) [4]. However, these equations are less or not reliable to predict the BMP of slowly-degradable compounds (such as the lignocellulosic biomasses) [16]. Therefore, these equations are mainly used to evaluate the biodegradability of a substrate, by comparing the methane yield of an experimental BMP batch test and the theoretical stoichiometric value derived by the formulas.
Other theoretical models, based on the complete characterisation of the substrate, have been developed to evaluate the variable level of biodegradability of a given substrate. For instance, the BMP of a lignocellulosic substrate can be predicted by measuring its lipid, protein and carbohydrate contents [17] (e.g., the model proposed by Raposo et al. [18]). Catenacci et al. [19] developed BMP prediction models to measure the sludge bio-methanisation based on its chemical composition. These authors found that the soluble organic nitrogen is an influential parameter, in addition to the organic fraction composition. In contrast, the chemical oxygen demand (COD) does not give information on the content of the biodegradable organic matter, since the chemical oxidation does not separate the effects of degradable and non-degradable organic matter [2]. Angelidaki and Sanders [20] used the COD to VS ratio as an indicator of the anaerobic bio-degradability of a substrate. Biological oxygen demand (BOD) is generally well correlated to the BMP [2], but this parameter is not appropriate for lignocellulosic substrates [21]. Models based on physico-chemical properties of the substrate could provide reliable predictions of BMP, but much effort must be paid to the accurate characterisation of the biomass, because each organic fraction shows different microbial accessibility and degradability [19]. In addition, the theoretical models hardly simulate the problems of the AD process, due to the substrate characteristics and environmental conditions.
A BMP laboratory test is still considered as the most accurate method to evaluate the methane yield of a given substrate [22]. This test is simpler compared to full-scale applications, and somehow allows the simulation of anaerobic digesters on commercial scale [4]. However, the BMP tests are expensive (requiring replications to statistically process the test results) and time-consuming (up to 3 months, with a minimum duration of 1 month) [22, 23].
Much effort has been paid and many studies have been conducted so far, in order to develop standard procedures for BMP tests [24,25,26,27,28,29,30,31,32,33,34,35]. In contrast, to the authors’ best knowledge, little research is available on how to reduce the test duration as well as the number of replicates. Only Strömberg et al. [36] and Ponsá et al. [37] defined mathematical methods for the prediction of the BMP at an early stage of the AD test. Ponsá et al. [37] found a good correlation between the methane produced in the early stage of AD and the final methane yield of the organic fraction of municipal solid waste. In contrast, these authors reported the need of longer time (2 weeks or more) to fit well the final production of mixed (more heterogeneous) municipal waste. Again Strömberg et al. [36], analysing data from more than 100 BMP tests on several substrates, reported accurate predictions of the final BMP after a 1-week AD test using optimised algorithms. From this short analysis of the-state-of-the-art, it is evident that the research question is still open, and therefore more investigations are needed to setup quick and accurate methods for BMP estimations.
To fill these gaps, this study evaluates the accuracy of the “first-order kinetic”, “logistic” and “Gompertz” models in predicting the BMP values. The model parameters are calibrated using the data of BMP tests that are shorter (i.e., the first 5, 7, 10, 14 and 21 days) than usually done (28–30 days or more). Moreover, the influence of the number of replicates (i.e., two or three) on the model prediction accuracy is also evaluated. A database from 32 BMP tests, previously carried out on different substrates throughout six years (2015–2021) in the same laboratory and under very similar experimental conditions, was adopted for the BMP tests of the current study. This database has been prepared using AD substrates of different origin (such as orange peel waste, bioplastics, market waste, anchovy residues, prickly pear cactus), which were tested under a variety of conditions (e.g., as mesophilic or thermophilic temperature, co-digestion) and or pre-treatments (such as ensiling or chemical conditioning). For most of the tests (about 90%), the inoculum was derived from the same full-scale mesophilic digested fed with manure and agro-waste.
Materials and Methods
Description of Substrates and Inocula
The substrates of the experimental dataset (Fig. 1), whose characterisation is reported in Table 1, are by-products or waste of the agro-industrial sector as well as municipal waste:
-
Orange peel waste (OPW), the residue of juice production;
-
anchovy sludge (AS), the by-product of fish after oil extraction;
-
Opuntia Ficus-indica (OFI), a cactus commonly known as “prickly pear” [17];
-
Mater-Bi, compostable bags [38].
In more detail, the OPW is the most abundant residue from orange industry, and consists of seeds (0–9%), peels (60–75%) and membrane residues (23–33%) [39, 40]. OPW has a noticeable water content (> 80%) and a very low pH (3–5), and contains essential oil, mainly composed of D-limonene, in variable concentrations [41, 42]; the latter is toxic for the AD biomass [43]. However, OPW use as a substrate for AD is one of the most promising valorisation patterns [39, 44]. In these BMP tests, OPW was digested as a raw (fresh) substrate or ensiled under three conditions [45]: (i) natural ensiling, (ii) wet ensiling (water, 20% w/w, added to OPW); (iii) dry ensiling (OPW placed over a drainage system made of quartz gravel). In one test, after ensiling, OPW was chemically treated by ethanol addition and then centrifuged, or simply centrifuged without previous ethanol addition [45]. The duration of the ensiling process was also analysed: different samples of OPW were ensiled for increasingly longer periods (0, 7, 14, 21 and 37 days) and then used as AD substrates [46]. To verify the bacteria adaptation, BMP tests on ensiled OPW were carried out by modifying the substrate to inoculum ratio (0.3 and 1, respectively) [47].
Anchovy sludge derived from fish oil extraction by novel process [48] was digested alone or with a co-substrate mimicking fruit and vegetable market waste (MW) [49], composed of 49.0% (w/w) of potatoes, 44.4% of apples, and 6.6% of carrots [48].
OFI is a biomass that is potentially worth to be used as substrate in AD, since it is an excellent source of lignocellulosic substrate, with a yield of 10–50 Mg of dry mass per year and ha [17]. OFI was co-digested alone or with poultry manure and lapillus (unpublished data). Lapillus is an unconsolidated volcanic fragment, which consists of fresh magma, solid magma from prior eruption or basement rocks passed by the eruption.
Compostable bags made of Mater Bi® are designed to deliver organic residues to household waste collection systems. The bags used in the BMP tests were made up of starch and its derivatives (over 60%), and a synthetic resin that is hydrophilic and biodegradable (for the residual 40%) [38, 50].
The inoculum for BMP tests under mesophilic conditions was a liquid digestate collected before each test, from the second stage of a full-scale, two stages, mesophilic (35 °C) anaerobic digester fed with cattle and chicken manure, and several agro-industry residues (e.g., orange peel waste, greenhouse horticulture residues).
Generally, after collection, the inoculum was sieved and stored for about a week at 35 °C to reduce the non-specific biogas production. During the thermophilic tests, the inoculum from the same full-scale plant was subjected to the same pre-treatment, but it was progressively adapted to thermophilic conditions by increasing temperature from 35 to 55 °C [38].
BMP Tests
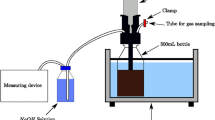
The same method, as described below, was followed for the BMP tests on the substrates of the experimental dataset. The tests were carried out in triplicate in 1-L glass reactors by mixing a proper amount of inoculum and substrate. The nutrient solutions were routinely added following the procedures set by the Italian norm on BMP tests (UNI 1601755—Method for the assessment of potential production of methane from anaerobic digestion in wet conditions [52]) during the tests carried out since the release of the same norm (Table 1). The norm requires the use of three different nutrient solutions defined as Solution A, B and C respectively. Solution A contains specified quantities of KH2PO4, Na2HPO4‧12H2O, NH4Cl, distilled water while the amount to be used is 5% of the final volume of the mixture subjected to BMP test. Solution B contains CaCl2‧2H2O, MgCl2‧6H2O, FeCl2‧4H2O, distilled water and the amount to be used is 5% of the final volume. Solution C contains MnCl2‧4H2O, H3BO3, ZnCl2, CuCl2, Na2MoO4‧2H2O, CoCl2‧6H2O, NiCl2‧6H2O, Na2SeO3, distilled water and the amount to be used is 1% of the final volume of the blend. Blanks (reactors containing only inoculum) were used to evaluate the endogenous methane production (that is, the production due to the digestion of the inoculum). The biogas was periodically measured (on average three times a week) and its methane content was estimated by the fluid displacement method [49, 53, 54]. For this estimation, a three-neck bottle with an alkaline solution (3 M NaOH) was used, and this allows the precipitation of the carbon dioxide in the biogas. From one neck of the bottle, the biogas was transferred to the solution of sodium hydroxide; the pressure in the bottle a corresponding volume of the alkaline solution displaced through the second neck into a graduated cylinder. Hypothesising that the biogas is only composed of methane and carbon dioxide, the methane volume of biogas can be considered equal to the volume of the alkaline solution displaced in the cylinder (as the CO2 was trapped in the solution). The test ended when the methane production throughout three consecutive days was less than 1% of the cumulated volume. The endogenous methane production was subtracted to the production of the blend of inoculum and substrate (which estimates the “net” methane production), and then normalised to standard pressure and temperature conditions (1 bar and 0 °C, respectively). Finally, the cumulative methane production was referred to the weight of substrate added (in terms of VS), to obtain the specific production (hereafter simply indicated as “BMP” and measured in L or its submultiple under standard temperature and pressure, STP).
Detailed BMP tests settings (such as temperature or food to microorganisms’ ratio) as well as possible pre-treatment types and conditions, are explained in the related reference (Table 1).
Modelling
Three well known models [55] (e.g., the first-order kinetics, the modified Gompertz equation and the logistic model) have been used for the purposes of this research. Hereafter, the first-order kinetics model will be simply indicated as “first order” model. The three models are commonly used to estimate the kinetic constants of the AD process and predict the specific cumulative methane production from BMP tests. Their analytical expression is reported in Eqs. (1), (2), and (3), respectively:
where:
-
B0 (or P), B (mLSTP gVS−1) = asymptotic and daily cumulative methane yields, respectively
-
k (d−1) = kinetic constant
-
Rm (mLSTP·gVS−1·d−1) = maximum methane production rate
-
λ (d) = duration of the lag phase
-
b = numeric constant of the model (3)
-
t (d) = hydraulic retention time.
P, Rm, λ in Eq. (2), k and B0 in Eq. (1) and B0, b and k in Eq. (3) were determined through the Excel tool “Solver” by minimizing the sum of square errors between the model and the experimental mean values. In our study, B is assumed as the model prediction of the observed BMP.
In order to estimate the influence of test duration on the model’s prediction accuracy, the BMP was estimated, using the data related to the first 5, 10, 14 and 21 days of the BMP tests, respectively; this value will be indicated hereafter as “BMP at i-th day”. The final BMP value calculated using a given model and all the available data (that is, for the total duration) was used as reference value (“control”), in order to evaluate the model performance.
To compare the model’s accuracy using two or three replicates, four combinations of replicates for the test with three original replicates (indicated as R1, R2 and R3) were prepared (R1-R2, R2-R3 and R1-R3) and the BMP was estimated by each model, using the data related to each couple of replicates or three replicates (R1-R2-R3) as reference.
Statistical Analysis
Preliminarily, all the final BMP values estimated by modelling that were higher than the stoichiometric methane production for lipids (equal to 1014 mLSTP gVS−1) were removed from the experimental dataset. In fact, this yield is the maximum value that is theoretically possible for a given substrate [20]. Then, a one-way ANOVA was used to identify significant differences (p < 0.05) among the three models (Gompertz, first order and logistic) and between the mean BMP predicted by each model and the corresponding reference BMP (control, calculated by the same model but using all the data available).
Finally, two-way ANOVA was applied to evaluate the statistical significance (at p < 0.05) of the differences in the BMP (considered as dependent variables) among the number of replicates and test duration (independent factors) as well as their interaction.
For both tests, which were applied to each prediction model, the data were subjected to normality test or square root-transformed whenever necessary, in order to satisfy the assumptions of the statistical tests (equality of variance and normal distribution).
All the statistical tests were carried out by the XLSTAT software (release 2019).
Results and Discussions
Comparing the performance of the models with reference to the number of replicates, the Gompertz model gave the largest difference (34.3 ± 46.0%) between the mean predicted BMP compared to the control using two replicates, and this difference decreased to 31.4 ± 40.2% in the tests with three replicates. The lowest difference between the predicted BMP and the control were found for the logistic model (31.5 ± 85.0%) in the tests with two replicates, while, surprisingly, the same model gave the highest error (42.7 ± 118.3%) in the tests with three replicates (Fig. 2a and Table 2).
It is worth mentioning that, for the first order and logistic models, the variability is significantly larger than the standard deviation of the control.
Figures 3 report examples of comparisons between the predicted BMP (using 2 or 3 replicates, and different test duration) and the control values for a selection of five substrates. As previously reported, outliers obtained by applying the modelled tests at different time were excluded from the study. A high number of outliers (over 74% of the available data) were observed applying the first order model to the substrate poultry manure in the first 2 weeks of the experiment (Fig. 3), indicating a low reliability of the predictive model at the first stage of the test for some substrate.
Comparison of mean modelled BMP values of selected substrates (naturally, wet and dried ensiled orange peel waste; Opuntia ficus indica; poultry manure) among the number of replicates and the test duration for the three models. The numbers on the bars are the data processed for each BMP test modelling
The two-way ANOVA shows that the test duration significantly influences the estimations of the BMP values predicted by the Gompertz and first order models, while this factor is not significant for the logistic model (Fig. 2b and Table 3).
More specifically, the accuracy of the BMP estimations on average increases with the test duration for the Gompertz and first order models. This is shown by the monotonic decrease in the differences among the durations (from 49.85 ± 21.7% and 40.9 ± 60.7% with test duration of 5 days down to 9.6 ± 19.6% for BMP at the 21-th day, respectively). Moreover, for the first order and logistic models, the BMP estimations are more reliable with the increase in the test duration, although the trend is not always monotonic. The tests with durations of seven and 10 days are two exceptions for the logistic model, since the errors in the estimation are higher compared to a shorter test (Fig. 2b and Table 2).
It is worth to notice that tests with duration of 14 days give estimations of the mean BMP values with mean errors close to 25% and never higher than 30% (28.2 ± 61.5% for the Gompertz model, 24.7 ± 71.6% for the first order, and 22.9 ± 48.3% for the logistic), and this error decreases to only 9.6 ± 19.6% and 9.4 ± 23.4% for the Gompertz and logistic models, respectively. In contrast, the error in the BMP at the 21-st day using the first order model is 20.1 ± 48.1% (Fig. 2b and Table 2). Moreover, the standard deviation of the BMP values at the 14-th and 21-st day for the first order model is noticeably higher compared to the other models. However, these BMP values are statistically similar as the control.
The scatterplots (Figs. 1SM, 2SM, 3SM and 4SM) clearly demonstrate how the accuracy of the Gompertz model based on a test duration of 21 days is very close to the control.
Table 4 clearly shows that the number of outliers (i.e., predicted values higher than 1.014 LSTP gVS−1) is minimum for the Gompertz model; for this model only 10–20% of the estimated values must be discarded.
The fact that, for the logistic model based on a test duration of 5 days, about 75% of the predictions was not reliable reduces its apparent accuracy for the lowest duration. The number of outliers produced by the Gompertz and logistic models based on durations of 14 and 21 days is very similar and even higher compared to the first order model.
Overall, the evaluation of the accuracy and potential usage of the BMP predictions using tests with shorter duration and/or a minimum number of replicates must consider the intrinsic difficulty of BMP tests and their uncertainty, linked to the complexity of AD biochemistry. This complexity is fully acknowledged by the regulations on the BMP tests in view of their practical applications. For example, the Italian Norm (UNI 1601755—Method for the assessment of potential production of methane from anaerobic digestion in wet conditions) tolerates variations of ± 25% in the experimental value of the BMP for microcrystalline cellulose; the latter is a very simple and biodegradable substrate, which is used as a feasible control for the whole procedure of a BMP test. This means that BMP estimates with errors close to this tolerance can be generally accepted.
Conclusions
The accuracy of three models (“first order kinetic”, “logistic” and “Gompertz”) in predicting the BMP values has been verified, calibrating the model parameters with the data collected in BMP tests (i.e., the first 5, 7, 10, 14 and 21 days) that are shorter than usually done (28 days or more). Moreover, the influence of the number of replicates (i.e., two or three) on the model prediction accuracy was also evaluated.
The test duration significantly influences the prediction accuracy of Gompertz and first order models. In contrast, the number of replicates is only significant for the Gompertz model. Performing BMP in triplicates is however highly advisable especially when the failure of BMP tests is possible (e.g., acidification or presence of inhibiting agents).
The BMP model predictions based on tests with the shorter durations (less than 10 days) are not accurate, since the errors are generally higher than 30%. A dataset of 14 days reduces these errors to 20–25% for the first order and logistic models, and below 30% for Gompertz model.
Overall, this study has demonstrated that two of the evaluated models (i.e., first order and logistic models) provide BMP estimations with differences lower than 25% and a limited numbers of outliers compared to full-length tests, using as model’s input parameters the data collected in tests of limited duration (i.e., more than 14 days) compared to the commonly adopted time (28 days or even more). The increase of this test duration to 21 days gives errors in BMP values below 10% (for the Gompertz and logistic models) and a further reduction of outliers.
Data Availability
The datasets analysed during the current study are not publicly available due to internal procedures but are available from the corresponding author on reasonable request.
References
Speece, R.E.: Anaerobic biotechnology for industrial wastewater treatment. Environ. Sci. Technol. 17, 416A-427A (1983). https://doi.org/10.1021/es00115a725
Schievano, A., Scaglia, B., D’Imporzano, G., Malagutti, L., Gozzi, A., Adani, F.: Prediction of biogas potentials using quick laboratory analyses: upgrading previous models for application to heterogeneous organic matrices. Bioresour. Technol. 100, 5777–5782 (2009). https://doi.org/10.1016/j.biortech.2009.05.075
Hülsemann, B., Mächtig, T., Pohl, M., Liebetrau, J., Müller, J., Hartung, E., Oechsner, H.: Comparison of biological efficiency assessment methods and their application to full-scale biogas plants. Energies 14, 2381 (2021). https://doi.org/10.3390/EN14092381
Jingura, R.M., Kamusoko, R.: Methods for determination of biomethane potential of feedstocks: a review. Biofuel Res. J. 14, 573–586 (2017). https://doi.org/10.18331/BRJ2017.4.2.3
Godin, B., Mayer, F., Agneessens, R., Gerin, P., Dardenne, P., Delfosse, P., Delcarte, J.: Biochemical methane potential prediction of plant biomasses: comparing chemical composition versus near infrared methods and linear versus non-linear models. Bioresour. Technol. 175, 382–390 (2015). https://doi.org/10.1016/J.BIORTECH.2014.10.115
Spanjers, H., van Lier, J.B.: Instrumentation in anaerobic treatment—research and practice. Water Sci. Technol. 53, 63–76 (2006). https://doi.org/10.2166/wst.2006.111
De Boever, J.L., Cottyn, B.G., Buysse, F.X., Wainman, F.W., Vanacker, F.W.: The use of an enzymatic technique to predict digestibility, metabolizable and net energy of compound feedstuffs for ruminants. Anim. Feed Sci. Technol. 14, 203–214 (1986)
Rodrigues, R.P., Rodrigues, D.P., Klepacz-Smolka, A., Martins, R.C., Quina, M.J.: Comparative analysis of methods and models for predicting biochemical methane potential of various organic substrates. Sci. Total Environ. 649, 1599–1608 (2019). https://doi.org/10.1016/J.SCITOTENV.2018.08.270
Triolo, J.M., Ward, A.J., Pedersen, L., Løkke, M.M., Qu, H., Sommer, S.G.: Near infrared reflectance spectroscopy (NIRS) for rapid determination of biochemical methane potential of plant biomass. Appl. Energy 116, 52–57 (2014). https://doi.org/10.1016/J.APENERGY.2013.11.006
Doublet, J., Boulanger, A., Ponthieux, A., Laroche, C., Poitrenaud, M., Cacho Rivero, J.A.: Predicting the biochemical methane potential of wide range of organic substrates by near infrared spectroscopy. Bioresour. Technol. 128, 252–258 (2013). https://doi.org/10.1016/J.BIORTECH.2012.10.044
Fitamo, T., Triolo, J.M., Boldrin, A., Scheutz, C.: Rapid biochemical methane potential prediction of urban organic waste with near-infrared reflectance spectroscopy. Water Res. 119, 242–251 (2017). https://doi.org/10.1016/J.WATRES.2017.04.051
Liu, J., Zeng, C., Wang, N., Shi, J., Zhang, B., Liu, C., Sun, Y.: Rapid biochemical methane potential evaluation of anaerobic co-digestion feedstocks based on near infrared spectroscopy and chemometrics. Energies 14, 1460 (2021). https://doi.org/10.3390/EN14051460
Buswell, A.M., Mueller, H.F.: Mechanism of methane fermentation. Ind. Eng. Chem. 44, 550–552 (1952). https://doi.org/10.1021/ie50507a033
Symons, G.E., Buswell, A.M.: The methane fermentation of carbohydrates. J. Am. Chem. Soc. 55, 2028–2036 (1933). https://doi.org/10.1021/ja01332a039
Tchobanoglous, G., Theisen, H., Vigil, S.A.: Integrated solid waste management: engineering principles and management issues. McGraw-Hill Education, New York, US (1993)
Thomsen, S.T., Spliid, H., Østergård, H.: Statistical prediction of biomethane potentials based on the composition of lignocellulosic biomass. Bioresour. Technol. 154, 80–86 (2014). https://doi.org/10.1016/j.biortech.2013.12.029
Calabrò, P.S., Catalán, E., Folino, A., Sánchez, A., Komilis, D.: Effect of three pretreatment techniques on the chemical composition and on the methane yields of Opuntia ficus-indica prickly pear. Waste Manag. Res. (2018). https://doi.org/10.1177/0734242X17741193
Raposo, F., De La Rubia, M.A., Fernández-Cegrí, V., Borja, R.: Anaerobic digestion of solid organic substrates in batch mode: an overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 16, 861–877 (2012). https://doi.org/10.1016/J.RSER.2011.09.008
Catenacci, A., Azzellino, A., Malpei, F.: Development of statistical predictive models for estimating the methane yield of Italian municipal sludges from chemical composition: a preliminary study. Water Sci. Technol. 79, 435–447 (2019). https://doi.org/10.2166/WST.2019.063
Angelidaki, I., Sanders, W.: Assessment of the anaerobic biodegradability of macropollutants. Rev Environ Sci Biotechnol (2004). https://doi.org/10.1007/s11157-004-2502-3
Argiz, L., Reyes, C., Belmonte, M., Franchi, O., Campo, R., Fra-Vázquez, A., Val del Río, A., Mosquera-Corral, A., Campos, J.L.: Assessment of a fast method to predict the biochemical methane potential based on biodegradable COD obtained by fractionation respirometric tests. J. Environ. Manag. 269, 110695 (2020). https://doi.org/10.1016/J.JENVMAN.2020.110695
Da Silva, C., Astals, S., Peces, M., Campos, J.L., Guerrero, L.: Biochemical methane potential (BMP) tests: reducing test time by early parameter estimation. Waste Manag. 71, 19–24 (2017). https://doi.org/10.1016/j.wasman.2017.10.009
Raposo, F., Fernández-Cegrí, V., De la Rubia, M.A., Borja, R., Béline, F., Cavinato, C., Demirer, G., Fernández, B., Fernández-Polanco, M., Frigon, J.C., Ganesh, R., Kaparaju, P., Koubova, J., Méndez, R., Menin, G., Peene, A., Scherer, P., Torrijos, M., Uellendahl, H., Wierinck, I., de Wilde, V.: Biochemical methane potential (BMP) of solid organic substrates: evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 86, 1088–1098 (2011). https://doi.org/10.1002/jctb.2622
Angelidaki, I., Alves, M., Bolzonella, D., Borzacconi, L., Campos, J.L., Guwy, A.J., Kalyuzhnyi, S., Jenicek, P., Van Lier, J.B.: Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Sci. Technol. 59, 927–934 (2009). https://doi.org/10.2166/wst.2009.040
Holliger, C., Astals, S., de Laclos, H.F., Hafner, S.D., Koch, K., Weinrich, S.: Towards a standardization of biomethane potential tests: a commentary. Water Sci. Technol. 83, 247–250 (2021). https://doi.org/10.2166/WST.2020.569
Holliger, C., Alves, M., Andrade, D., Angelidaki, I., Astals, S., Baier, U., Bougrier, C., Buffière, P., Carballa, M., De Wilde, V., Ebertseder, F., Fernández, B., Ficara, E., Ghasimi, S.M., Hack, G., Hartel, M., Heerenklage, J., Horvath, I.S., Jenicek, P., Koch, K., Krautwald, J., Lizasoain, J., Liu, J., Mosberger, L., Nistor, M., Oechsner, H., Oliveira, J.V., Paterson, M., Pauss, A., Pommier, S., Porqueddu, I., Raposo, F., Ribeiro, T., Pfund, F.R., Strömberg, S., Torrijos, M., Van Eekert, M., Van Lier, J., Wedwitschka, H., Wierinck, I., Fotidis, I.: Towards a standardization of biomethane potential tests. Water Sci. Technol. 74, 2515–2522 (2016). https://doi.org/10.2166/wst.2016.336
Hafner, S.D., de Laclos, H.F., Koch, K., Holliger, C.: Improving inter-laboratory reproducibility in measurement of biochemical methane potential (BMP). Water 12, 1752 (2020). https://doi.org/10.3390/W12061752
Koch, K., Hafner, S.D., Weinrich, S., Astals, S.: Identification of critical problems in biochemical methane potential (BMP) tests from methane production curves. Front. Environ. Sci. 7, 1–8 (2019). https://doi.org/10.3389/fenvs.2019.00178
Hafner, S.D., Astals, S.: Systematic error in manometric measurement of biochemical methane potential: sources and solutions. Waste Manag. 91, 147–155 (2019). https://doi.org/10.1016/j.wasman.2019.05.001
Hafner, S.D., Rennuit, C., Triolo, J.M., Richards, B.K.: Validation of a simple gravimetric method for measuring biogas production in laboratory experiments. Biomass Bioenerg. 83, 297–301 (2015). https://doi.org/10.1016/j.biombioe.2015.10.003
Amodeo, C., Hafner, S.D., Franco, R.T., Benbelkacem, H., Moretti, P., Bayard, R., Buffière, P.: How different are manometric, gravimetric, and automated volumetric bmp results? Water 12, 1839 (2020). https://doi.org/10.3390/W12061839
Justesen, C.G., Astals, S., Mortensen, J.R., Thorsen, R., Koch, K., Weinrich, S., Triolo, J.M., Hafner, S.D.: Development and validation of a low-cost gas density method for measuring biochemical methane potential (BMP). Water 11, 2431 (2019). https://doi.org/10.3390/W11122431
Hafner, S.D., Koch, K., Carrere, H., Astals, S., Weinrich, S., Rennuit, C.: Software for biogas research: tools for measurement and prediction of methane production. SoftwareX 7, 205–210 (2018). https://doi.org/10.1016/j.softx.2018.06.005
Filer, J., Ding, H.H., Chang, S.: Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water 11, 921 (2019). https://doi.org/10.3390/W11050921
Koch, K., Hafner, S.D., Weinrich, S., Astals, S., Holliger, C.: Power and limitations of biochemical methane potential (BMP) tests. Front. Energy Res. 8, 1–4 (2020). https://doi.org/10.3389/fenrg.2020.00063
Strömberg, S., Nistor, M., Liu, J.: Early prediction of biochemical methane potential through statistical and kinetic modelling of initial gas production. Bioresour. Technol. 176, 233–241 (2015). https://doi.org/10.1016/j.biortech.2014.11.033
Ponsá, S., Gea, T., Sánchez, A.: Short-time estimation of biogas and methane potentials from municipal solid wastes. J. Chem. Technol. Biotechnol. 86, 1121–1124 (2011). https://doi.org/10.1002/JCTB.2615
Calabro’, P.S., Folino, A., Fazzino, F., Komilis, D.: Preliminary evaluation of the anaerobic biodegradability of three biobased materials used for the production of disposable plastics. J. Hazard. Mater. 390, 1653 (2020). https://doi.org/10.1016/j.jhazmat.2019.121653
Zema, D.A., Calabrò, P.S., Folino, A., Tamburino, V., Zappia, G., Zimbone, S.M.: Valorisation of citrus processing waste: a review. Waste Manag. 80, 252–273 (2018). https://doi.org/10.1016/j.wasman.2018.09.024
Pascual, J.M., Carmona, J.F.: Composition of citrus pulp. Anim. Feed Sci. Technol. 5, 1–10 (1980). https://doi.org/10.1016/0377-8401(80)90005-X
Calsamiglia, S., Ferret, A., Bach, A.: Tablas FEDNA de valor nutritivo de Forrajes y Subproductos fibrosos húmedos, Madrid (2004)
Zema, D.A., Fòlino, A., Zappia, G., Calabrò, P.S., Tamburino, V., Zimbone, S.M.: Anaerobic digestion of orange peel in a semi-continuous pilot plant: an environmentally sound way of citrus waste management in agro-ecosystems. Sci. Total Environ. 630, 401–408 (2018). https://doi.org/10.1016/j.scitotenv.2018.02.168
Ruiz, B., Flotats, X.: Citrus essential oils and their influence on the anaerobic digestion process: an overview. Waste Manag. 34, 2063–2079 (2014). https://doi.org/10.1016/j.wasman.2014.06.026
Calabrò, P.S., Fazzino, F., Folino, A., Paone, E., Komilis, D.: Semi-continuous anaerobic digestion of orange peel waste: effect of activated carbon addition and alkaline pretreatment on the process. Sustainability 11, 3386 (2019). https://doi.org/10.3390/su11123386
Calabrò, P.S., Fazzino, F., Sidari, R., Zema, D.A.: Optimization of orange peel waste ensiling for sustainable anaerobic digestion. Renew. Energy 154, 849–862 (2020). https://doi.org/10.1016/j.renene.2020.03.047
Calabrò, P.S., Panzera, M.F.: Biomethane production tests on ensiled orange peel waste. Int. J. Heat Technol. 35, 130–136 (2017). https://doi.org/10.18280/ijht.35Sp0118
Calabrò, P.S., Panzera, M.F.: Anaerobic digestion of ensiled orange peel waste: Preliminary batch results. Therm. Sci. Eng. Prog. 6, 355–360 (2018). https://doi.org/10.1016/j.tsep.2017.12.011
Fazzino, F., Paone, E., Pedullà, A., Mauriello, F., Calabrò, P.S.: A new biorefinery approach for the full valorisation of anchovy residues: use of the sludge generated during the extraction of fish oil as a nitrogen supplement in anaerobic digestion. Appl. Sci. 11, 10163 (2021). https://doi.org/10.3390/APP112110163
Fazzino, F., Folino, A., Mauriello, F., Pedullà, A., Calabrò, P.S.: Biofuel production from fruit and vegetable market waste and mature landfill leachate by an active filter-anaerobic digestion integrated system. Energy Convers. Manag. X. 12, 100130 (2021). https://doi.org/10.1016/J.ECMX.2021.100130
Folino, A., Karageorgiou, A., Calabrò, P.S., Komilis, D.: Biodegradation of wasted bioplastics in natural and industrial environments: a review. Sustainability 12, 6030 (2020). https://doi.org/10.3390/su12156030
Paone, E., Fazzino, F., Pizzone, D.M., Scurria, A., Pagliaro, M., Ciriminna, R., Calabrò, P.S.: Towards the anchovy biorefinery: biogas production from anchovy processing waste after fish oil extraction with biobased limonene. Sustainability 13, 1–12 (2021). https://doi.org/10.3390/su13052428
UNI/TS 11703:2018 Method for the Assessment of Potential Production of Methane from Anaerobic Digestion in Wet Conditions - Matrix into Foodstuffs (2018)
Calabrò, P.S., Fazzino, F., Folino, A., Komilis, D.: Semi-continuous anaerobic digestion of orange peel waste: preliminary results. In: Naddeo, V., Balakrishnan, M., Choo, K. (eds.) Advances in science, technology & innovation (IEREK interdisciplinary series for sustainable development), pp. 309–311. Springer, Cham, Switzerland (2020)
Folino, A., Calabrò, P.S., Zema, D.A.: Effects of ammonia stripping and other physico-chemical pretreatments on anaerobic digestion of swine wastewater. Energies (2020). https://doi.org/10.3390/en13133413
Donoso-Bravo, A., Pérez-Elvira, S.I., Fdz-Polanco, F.: Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem. Eng. J. 160, 607–614 (2010). https://doi.org/10.1016/j.cej.2010.03.082
Acknowledgements
This paper is dedicated in memoriam of Emeritus Professor Rosario Pietropaolo that unexpectedly passed away on July 1st 2022. Former Rector of the Mediterranea University of Reggio Calabria, first Dean of the Faculty of Engineering as well as a visionary of science, master of ethics, integrity and dedication and inspiration for many of us.
Funding
Open access funding provided by Università degli Studi Mediterranea di Reggio Calabria within the CRUI-CARE Agreement. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, to data collection and analysis. The first draft of the manuscript was written by AF, DP and MM and all authors commented on previous versions of the manuscript. The manuscript was then revised by PSC and DAZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Calabrò, P.S., Folino, A., Maesano, M. et al. Exploring the Possibility to Shorten the Duration and Reduce the Number of Replicates in Biomethane Potential Tests (BMP). Waste Biomass Valor 14, 2481–2493 (2023). https://doi.org/10.1007/s12649-022-01893-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01893-9