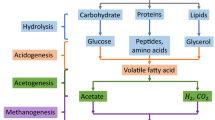
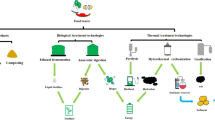
Abstract
The conventional approaches for handling food waste has been incineration, composting, landfilling, and anaerobic digestion for producing biogas. In light of organic waste bans and newly discovered presence of per- and polyfluorinated substances (PFAS) in food, food packaging materials, and compost, this review provides a critical summary of what has been investigated and reported and what needs to be considered when choosing suitable pathways for food waste. In addition to the fundamental principles inherent to anaerobic digestion and hydrothermal liquefaction, challenges for each process are identified followed by discussion of potential solutions to resolve the bottlenecks.
Graphic Abstract


Similar content being viewed by others
References
Kibler, K.M., Reinhart, D., Hawkins, C., Motlagh, A.M., Wright, J.: Food waste and the food-energy-water nexus: a review of food waste management alternatives. Waste Manage. 74, 52–62 (2018). https://doi.org/10.1016/j.wasman.2018.01.014
Karthikeyan, O.P., Trably, E., Mehariya, S., Bernet, N., Wong, J.W., Carrere, H.: Pretreatment of food waste for methane and hydrogen recovery: a review. Biores. Technol. 249, 1025–1039 (2018)
Ren, Y., Yu, M., Wu, C., Wang, Q., Gao, M., Huang, Q., et al.: A comprehensive review on food waste anaerobic digestion: research updates and tendencies. Biores. Technol. 247, 1069–1076 (2018). https://doi.org/10.1016/j.biortech.2017.09.109
Uçkun Kiran, E., Trzcinski, A.P., Ng, W.J., Liu, Y.: Bioconversion of food waste to energy: a review. Fuel 134, 389–399 (2014). https://doi.org/10.1016/j.fuel.2014.05.074
Xiong, X., Yu, I.K.M., Tsang, D.C.W., Bolan, N.S., Sik Ok, Y., Igalavithana, A.D., et al.: Value-added chemicals from food supply chain wastes: state-of-the-art review and future prospects. Chem. Eng. J. 375, 121983 (2019). https://doi.org/10.1016/j.cej.2019.121983
Strazzera, G., Battista, F., Garcia, N.H., Frison, N., Bolzonella, D.: Volatile fatty acids production from food wastes for biorefinery platforms: a review. J. Environ. Manage. 226, 278–288 (2018). https://doi.org/10.1016/j.jenvman.2018.08.039
Zhou, M., Yan, B., Wong, J.W., Zhang, Y.: Enhanced volatile fatty acids production from anaerobic fermentation of food waste: a mini-review focusing on acidogenic metabolic pathways. Biores. Technol. 248, 68–78 (2018)
Dahiya, S., Kumar, A.N., Shanthi Sravan, J., Chatterjee, S., Sarkar, O., Mohan, S.V.: Food waste biorefinery: sustainable strategy for circular bioeconomy. Biores. Technol. 248, 2–12 (2018). https://doi.org/10.1016/j.biortech.2017.07.176
Ma, Y., Liu, Y.: Turning food waste to energy and resources towards a great environmental and economic sustainability: an innovative integrated biological approach. Biotechnol. Adv. 37(7), 107414 (2019). https://doi.org/10.1016/j.biotechadv.2019.06.013
Nielsen, C., Rahman, A., Rehman, A.U., Walsh, M.K., Miller, C.D.: Food waste conversion to microbial polyhydroxyalkanoates. Microb. Biotechnol. 10(6), 1338–1352 (2017)
Ong, K.L., Kaur, G., Pensupa, N., Uisan, K., Lin, C.S.K.: Trends in food waste valorization for the production of chemicals, materials and fuels: case study South and Southeast Asia. Biores. Technol. 248, 100–112 (2018). https://doi.org/10.1016/j.biortech.2017.06.076
Jayathilakan, K., Sultana, K., Radhakrishna, K., Bawa, A.: Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. J. Food Sci. Technol. 49(3), 278–293 (2012)
Wang, W., Xu, Y., Wang, X., Zhang, B., Tian, W., Zhang, J.: Hydrothermal liquefaction of microalgae over transition metal supported TiO2 catalyst. Biores. Technol. 250, 474–480 (2018). https://doi.org/10.1016/j.biortech.2017.11.051
Upadhyay, A., Lama, J.P., Tawata, S.: Utilization of pineapple waste: a review. J. Food Sci. Technol. Nepal 6, 10–18 (2010)
Du, C., Abdullah, J.J., Greetham, D., Fu, D., Yu, M., Ren, L., et al.: Valorization of food waste into biofertiliser and its field application. J. Clean. Prod. 187, 273–284 (2018). https://doi.org/10.1016/j.jclepro.2018.03.211
Wainaina, S., Horváth, I.S., Taherzadeh, M.J.: Biochemicals from food waste and recalcitrant biomass via syngas fermentation: a review. Biores. Technol. 248, 113–121 (2018). https://doi.org/10.1016/j.biortech.2017.06.075
Office BT. Biofuels and bioproducts from wet and gaseous waste streams: challenges and opportunities. DOE, EERE. Jan 2017
Thyberg, K.L., Tonjes, D.J., Gurevitch, J.: Quantification of food waste disposal in the United States: a meta-analysis. Environ. Sci. Technol. 49(24), 13946–13953 (2015). https://doi.org/10.1021/acs.est.5b03880
EPA. Advancing sustainable materials management: 2017 fact sheet. Nov 2019
EPA. United States 2030 food loss and waste reduction goal. US Environ Prot Agency (2017) www.epa.gov/sustainable-management-food/united-states-2030-food-loss-and-waste-reduction-goal#goal
EPA. Food recovery hierarchy. US Environ Prot Agency (2017) www.epa.gov/sustainable-management-food/food-recovery-hierarchy
EPA. Wasted food programs and resources across the United States. US Environ Prot Agency (2017) www.epa.gov/sustainable-management-food/wasted-food-programs-and-resources-across-united-states#1
Badgett, A., Milbrandt, A.: A summary of standards and practices for wet waste streams used in waste-to-energy technologies in the United States. Renew. Sustain. Energy Rev. 117, 109425 (2020)
NYC. Waste characterization https://dsny.cityofnewyork.us/wp-content/uploads/2018/04/2017-Waste-Characterization-Study.pdf (2017)
Lee, J.P., Lee, J.S., Park, S.C.: Two-phase methanization of food wastes in pilot scale. In: Davison, B.H., Finkelstein, M. (eds.) Twentieth symposium on biotechnology for fuels and chemicals, pp. 585–93. Humana Press, Totowa (1999)
Thyberg, K.L., Tonjes, D.J.: The environmental impacts of alternative food waste treatment technologies in the US. J. Clean. Prod. 158, 101–8 (2017). https://doi.org/10.1016/j.jclepro.2017.04.169
USDA. Biogas opportunities roadmap: voluntary actions to reduce methane emissions and increase energy independent (2014)
Chen, Y., Cheng, J.J., Creamer, K.S.: Inhibition of anaerobic digestion process: a review. Biores. Technol. 99(10), 4044–4064 (2008). https://doi.org/10.1016/j.biortech.2007.01.057
Council, Ab.: Current and potential biogas production.https://www.americanbiogascouncilorg/pdf/biogas101pdf (2015)
Pilli, S., Bhunia, P., Yan, S., LeBlanc, R., Tyagi, R., Surampalli, R.: Ultrasonic pretreatment of sludge: a review. Ultrason. Sonochem. 18(1), 1–18 (2011)
Yoshida, H., Mønster, J., Scheutz, C.: Plant-integrated measurement of greenhouse gas emissions from a municipal wastewater treatment plant. Water Res. 61, 108–118 (2014). https://doi.org/10.1016/j.watres.2014.05.014
Smith, A.L., Stadler, L.B., Cao, L., Love, N.G., Raskin, L., Skerlos, S.J.: Navigating wastewater energy recovery strategies: a life cycle comparison of anaerobic membrane bioreactor and conventional treatment systems with anaerobic digestion. Environ. Sci. Technol. 48(10), 5972–5981 (2014)
Smith, A.L., Skerlos, S.J., Raskin, L.: Psychrophilic anaerobic membrane bioreactor treatment of domestic wastewater. Water Res. 47(4), 1655–1665 (2013). https://doi.org/10.1016/j.watres.2012.12.028
Hartley, K., Lant, P.: Eliminating non-renewable CO2 emissions from sewage treatment: an anaerobic migrating bed reactor pilot plant study. Biotechnol. Bioeng. 95(3), 384–398 (2006)
Singh, K.S., Harada, H., Viraraghavan, T.: Low-strength wastewater treatment by a UASB reactor. Biores. Technol. 55(3), 187–194 (1996)
Pauss, A., Andre, G., Perrier, M., Guiot, S.R.: Liquid-to-gas mass transfer in anaerobic processes: inevitable transfer limitations of methane and hydrogen in the biomethanation process. Appl. Environ. Microbiol. 56(6), 1636–1644 (1990)
Bandara, W.M., Satoh, H., Sasakawa, M., Nakahara, Y., Takahashi, M., Okabe, S.: Removal of residual dissolved methane gas in an upflow anaerobic sludge blanket reactor treating low-strength wastewater at low temperature with degassing membrane. Water Res. 45(11), 3533–3540 (2011)
Bandara, W.M., Kindaichi, T., Satoh, H., Sasakawa, M., Nakahara, Y., Takahashi, M., et al.: Anaerobic treatment of municipal wastewater at ambient temperature: analysis of archaeal community structure and recovery of dissolved methane. Water Res. 46(17), 5756–5764 (2012)
Giménez, J., Martí, N., Ferrer, J., Seco, A.: Methane recovery efficiency in a submerged anaerobic membrane bioreactor (SAnMBR) treating sulphate-rich urban wastewater: evaluation of methane losses with the effluent. Biores. Technol. 118, 67–72 (2012)
McCarty, P.L., Bae, J., Kim, J.: Domestic wastewater treatment as a net energy producer–can this be achieved? ACS Publications, Washington (2011)
Haroon, M.F., Hu, S., Shi, Y., Imelfort, M., Keller, J., Hugenholtz, P., et al.: Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500(7464), 567 (2013)
Stein, L.Y., Roy, R., Dunfield, P.F.: Aerobic methanotrophy and nitrification processes and connections. eLS. Wiley, Chichester (2012)
Zhu, J., Wang, Q., Yuan, M., Tan, G.-Y.A., Sun, F., Wang, C., et al.: Microbiology and potential applications of aerobic methane oxidation coupled to denitrification (AME-D) process: a review. Water Res. 90, 203–15 (2016). https://doi.org/10.1016/j.watres.2015.12.020
Caldwell, S.L., Laidler, J.R., Brewer, E.A., Eberly, J.O., Sandborgh, S.C., Colwell, F.S.: Anaerobic oxidation of methane: mechanisms, bioenergetics, and the ecology of associated microorganisms. Environ. Sci. Technol. 42(18), 6791–6799 (2008). https://doi.org/10.1021/es800120b
Knittel, K., Boetius, A.: Anaerobic oxidation of methane: progress with an unknown process. Annu. Rev. Microbiol. 63(1), 311–334 (2009). https://doi.org/10.1146/annurev.micro.61.080706.093130
Borrel, G., Jézéquel, D., Biderre-Petit, C., Morel-Desrosiers, N., Morel, J.-P., Peyret, P., et al.: Production and consumption of methane in freshwater lake ecosystems. Res. Microbiol. 162(9), 832–847 (2011)
Raghoebarsing, A.A., Pol, A., Van de Pas-Schoonen, K.T., Smolders, A.J., Ettwig, K.F., Rijpstra, W.I.C., et al.: A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440(7086), 918 (2006)
Deutzmann, J.S., Schink, B.: Anaerobic oxidation of methane in sediments of lake constance, an oligotrophic freshwater lake. Appl. Environ. Microbiol. 77(13), 4429–4436 (2011)
Kojima, H., Tsutsumi, M., Ishikawa, K., Iwata, T., Mußmann, M., Fukui, M.: Distribution of putative denitrifying methane oxidizing bacteria in sediment of a freshwater lake Lake Biwa. Syst. Appl. Microbiol. 35(4), 233–238 (2012)
Shen, L.-d, Liu, S., Zhu, Q., Li, X.-y, Cai, C., Cheng, D.-q, et al.: Distribution and diversity of nitrite-dependent anaerobic methane-oxidising bacteria in the sediments of the Qiantang River. Microbial Ecology. 67(2), 341–9 (2014)
Hu, B.-l, Shen, L.-d, Lian, X., Zhu, Q., Liu, S., Huang, Q., et al.: Evidence for nitrite-dependent anaerobic methane oxidation as a previously overlooked microbial methane sink in wetlands. Proc. Nat. Acad. Sci. USA 111(12), 4495–500 (2014)
Shen, L.-d, Huang, Q., He, Z.-f, Lian, X., Liu, S., He, Y.-f, et al.: Vertical distribution of nitrite-dependent anaerobic methane-oxidising bacteria in natural freshwater wetland soils. Appl. Microbiol. Biotechnol. 99(1), 349–57 (2015)
Shen, L.-d, Liu, S., He, Z.-f, Lian, X., Huang, Q., He, Y.-f, et al.: Depth-specific distribution and importance of nitrite-dependent anaerobic ammonium and methane-oxidising bacteria in an urban wetland. Soil Biol. Biochem. 83, 43–51 (2015). https://doi.org/10.1016/j.soilbio.2015.01.010
Shen, L.-d, Liu, S., Huang, Q., Lian, X., He, Z.-f, Geng, S., et al.: Evidence for the cooccurrence of nitrite-dependent anaerobic ammonium and methane oxidation processes in a flooded paddy field. Appl. Environ. Microbiol. 80(24), 7611–9 (2014)
Wang, Y., Zhu, G., Harhangi, H.R., Zhu, B., Jetten, M.S., Yin, C., et al.: Co-occurrence and distribution of nitrite-dependent anaerobic ammonium and methane-oxidizing bacteria in a paddy soil. FEMS Microbiol. Lett. 336(2), 79–88 (2012)
Zhu, G., Zhou, L., Wang, Y., Wang, S., Guo, J., Long, X.E., et al.: Biogeographical distribution of denitrifying anaerobic methane oxidizing bacteria in Chinese wetland ecosystems. Environ. Microbiol. Rep. 7(1), 128–138 (2015)
Li-dong, S., Qun, Z., Shuai, L., Ping, D., Jiang-ning, Z., Dong-qing, C., et al.: Molecular evidence for nitrite-dependent anaerobic methane-oxidising bacteria in the Jiaojiang Estuary of the East Sea (China). Appl. Microbiol. Biotechnol. 98(11), 5029–5038 (2014)
Chen, J., Zhou, Z.-C., Gu, J.-D.: Occurrence and diversity of nitrite-dependent anaerobic methane oxidation bacteria in the sediments of the South China Sea revealed by amplification of both 16S rRNA and pmoA genes. Appl. Microbiol. Biotechnol. 98(12), 5685–5696 (2014)
Ettwig, K.F., Butler, M.K., Le Paslier, D., Pelletier, E., Mangenot, S., Kuypers, M.M., et al.: Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464(7288), 543–548 (2010)
Luesken, F.A., Zhu, B., van Alen, T.A., Butler, M.K., Diaz, M.R., Song, B., et al.: pmoA primers for detection of anaerobic methanotrophs. Appl. Environ. Microbiol. (2011). https://doi.org/10.1128/AEM.02960-10
Valentine, D.L., Reeburgh, W.S.: New perspectives on anaerobic methane oxidation. Environ. Microbiol. 2(5), 477–484 (2000)
Zehnder, A.J., Brock, T.D.: Anaerobic methane oxidation: occurrence and ecology. Appl. Environ. Microbiol. 39(1), 194–204 (1980)
Hoehler, T.M., Alperin, M.J., Albert, D.B., Martens, C.S.: Field and laboratory studies of methane oxidation in an anoxic marine sediment: evidence for a methanogen-sulfate reducer consortium. Global Biogeochem. Cycles 8(4), 451–463 (1994)
Thauer, R.K.: Biochemistry of methanogenesis: a tribute to Marjory Stephenson: 1998 Marjory Stephenson prize lecture. Microbiology 144(9), 2377–2406 (1998)
Schreiber, L., Holler, T., Knittel, K., Meyerdierks, A., Amann, R.: Identification of the dominant sulfate-reducing bacterial partner of anaerobic methanotrophs of the ANME-2 clade. Environ. Microbiol. 12(8), 2327–2340 (2010)
Beal, E.J., House, C.H., Orphan, V.J.: Manganese-and iron-dependent marine methane oxidation. Science 325(5937), 184–187 (2009)
Knittel, K., Boetius, A., Lemke, A., Eilers, H., Lochte, K., Pfannkuche, O., et al.: Activity, distribution, and diversity of sulfate reducers and other bacteria in sediments above gas hydrate (Cascadia Margin, Oregon). Geomicrobiol. J. 20(4), 269–294 (2003)
Orphan, V.J., House, C.H., Hinrichs, K.-U., McKeegan, K.D., DeLong, E.F.: Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science 293(5529), 484–487 (2001)
Orphan, V.J., House, C.H., Hinrichs, K.-U., McKeegan, K.D., DeLong, E.F.: Multiple archaeal groups mediate methane oxidation in anoxic cold seep sediments. Proc. Natl. Acad. Sci. USA 99(11), 7663–7668 (2002)
Treude, T., Krüger, K., Boetius, A., Jorgensen, B.: Environmental control on anaerobic oxidation of methane in gassy sediments of Eckernförde Bay (German Baltic). Limnol. Oceanogr. 50(6), 1771–1786 (2005)
Lösekann, T., Knittel, K., Nadalig, T., Fuchs, B., Niemann, H., Boetius, A., et al.: Diversity and abundance of aerobic and anaerobic methane oxidizers at the Haakon Mosby mud volcano Barents sea. Appl. Environ. Microbiol. 73(10), 3348–3362 (2007)
Milucka J, Ferdelman TG, Polerecky L, Franzke D, Wegener G, Schmid M, et al: Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature. 491(7425), 541–546 (2012) http://www.nature.com/nature/journal/v491/n7425/abs/nature11656.html#supplementary-information
Modin, O., Fukushi, K., Yamamoto, K.: Denitrification with methane as external carbon source. Water Res. 41(12), 2726–2738 (2007)
Islas-Lima, S., Thalasso, F., Gomez-Hernandez, J.: Evidence of anoxic methane oxidation coupled to denitrification. Water Res. 38(1), 13–16 (2004)
Zhu, B., Sánchez, J., van Alen, T.A., Sanabria, J., Jetten, M.S., Ettwig, K.F., et al.: Combined anaerobic ammonium and methane oxidation for nitrogen and methane removal. Portland Press Limited, London (2011)
Rice, E.W., Baird, R.B., Eaton, A.D. (eds.): Standard methods for the examination of water and wastewater. Standard methods for the examination of water and wastewater. American Public Health Association, Washington (2017)
Luesken, F.A., van Alen, T.A., van der Biezen, E., Frijters, C., Toonen, G., Kampman, C., et al.: Diversity and enrichment of nitrite-dependent anaerobic methane oxidizing bacteria from wastewater sludge. Appl. Microbiol. Biotechnol. 92(4), 845 (2011)
Wegener, G., Krukenberg, V., Ruff, S.E., Kellermann, M.Y., Knittel, K.: Metabolic capabilities of microorganisms involved in and associated with the anaerobic oxidation of methane. Front. Microbiol. (2016). https://doi.org/10.3389/fmicb.2016.00046
Lee, H.-S., Tang, Y., Rittmann, B.E., Zhao, H.-P.: Anaerobic oxidation of methane coupled to denitrification: fundamentals, challenges, and potential. Crit. Rev. Environ. Sci. Technol. 48(19–21), 1067–1093 (2018)
Wang, D., Wang, Y., Liu, Y., Ngo, H.H., Lian, Y., Zhao, J., et al.: Is denitrifying anaerobic methane oxidation-centered technologies a solution for the sustainable operation of wastewater treatment plants? Biores. Technol. 234, 456–465 (2017). https://doi.org/10.1016/j.biortech.2017.02.059
Liu, T., Hu, S., Guo, J.: Enhancing mainstream nitrogen removal by employing nitrate/nitrite-dependent anaerobic methane oxidation processes. Crit. Rev. Biotechnol. 39(5), 732–745 (2019)
Welte, C.U., Rasigraf, O., Vaksmaa, A., Versantvoort, W., Arshad, A., Op den Camp, H.J., et al.: Nitrate-and nitrite-dependent anaerobic oxidation of methane. Environ. Microbiol. Rep. 8(6), 941–55 (2016)
Costa, C., Dijkema, C., Friedrich, M., Garcia-Encina, P., Fernandez-Polanco, F., Stams, A.: Denitrification with methane as electron donor in oxygen-limited bioreactors. Appl. Microbiol. Biotechnol. 53(6), 754–762 (2000)
Jena, U., Das, K.C.: Comparative evaluation of thermochemical liquefaction and pyrolysis for bio-oil production from microalgae. Energy Fuels 25(11), 5472–82 (2011)
Akhtar, J., Amin, N.A.S.: A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 15(3), 1615–24 (2011)
Jena, U., Das, K.C., Kastner, J.R.: Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis. Biores. Technol. 102(10), 6221–6229 (2011)
Yin, S., Dolan, R., Harris, M., Tan, Z.: Subcritical hydrothermal liquefaction of cattle manure to bio-oil: effects of conversion parameters on bio-oil yield and characterization of bio-oil. Biores. Technol. 101(10), 3657–64 (2010)
Biller, P., Ross, A.B.: Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Biores. Technol. 102(1), 215–25 (2011)
Liang, Y., Yesuf, J., Schmitt, S., Bender, K., Bozzola, J.: Study of cellulases from a newly-isolated thermophilic and cellulolytic Brevibacillus sp. strain JXL. J. Ind. Microbiol. Biotechnol. 36, 961–70 (2009)
Liang, Y., Feng, Z., Yesuf, J., Blackburn, J.W.: Optimization of growth medium and enzyme assay conditions for crude cellulases produced by a novel thermophilic and cellulolytic bacterium, Anoxybacillus sp. 527. Appl. Biochem. Biotechnol. 160, 1841–52 (2010)
Bi, Z., Zhang, J., Peterson, E., Zhu, Z., Xia, C., Liang, Y., et al.: Biocrude from pretreated sorghum bagasse through catalytic hydrothermal liquefaction. Fuel 188, 112–120 (2017). https://doi.org/10.1016/j.fuel.2016.10.039
Zhu, Y., Lee, Y., Elander, R.T.: Dilute-acid pretreatment of corn stover using a high-solids percolation reactor. Appl. Biochem. Biotechnol. 117(2), 103–114 (2004)
Wyman, C.E., Decker, S.R., Himmel, M.E., Brady, J.W., Skopec, C.E., Viikari, L.: Hydrolysis of cellulose and hemicellulose. Polysaccharides: structural diversity and functional versatility. Sci Res 1, 1023–62 (2005)
Brebu, M., Vasile, C.: Thermal degradation of lignin—a review. Cellul. Chem. Technol. 44(9), 353 (2010)
He, B.J., Zhang, Y., Yin, Y., Funk, T.L., Riskowski, G.L.: Operating temperature and retention time effects on the thermochemical conversion process of swine manure. Trans. ASAE Am. Soc. Agric. Eng. 43(6), 1821–1826 (2000)
White, D.H., Wolf, D.: Direct biomass liquefaction by an extruder-feeder system. Chem. Eng. Commun. 135(1), 1–19 (1995). https://doi.org/10.1080/00986449508936335
Goudnaan, F., van de Beld, B., Boerefijn, F.R., Bos, G.M., Naber, J.E., van der Wal, S., et al.: Thermal efficiency of the HTU® process for biomass liquefaction. In: Bridgwater, A.V. (ed.) Progress in thermochemical biomass conversion, pp. 1312–25. Blackwell Science Ltd, Oxford (2008)
Minowa, T., Murakami, M., Dote, Y., Ogi, T., Yokoyama, S.-y: Oil production from garbage by thermochemical liquefaction. Biomass Bioenergy 8(2), 117–20 (1995)
Minowa, T., Yokoyama, S.-y, Kishimoto, M., Okakura, T.: Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel 74(12), 1735–8 (1995). https://doi.org/10.1016/0016-2361(95)80001-X
Shuping, Z., Yulong, W., Mingde, Y., Kaleem, I., Chun, L., Tong, J.: Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy 35(12), 5406–11 (2010)
Peterson, A.A., Vogel, F., Lachance, R.P., Froling, M., Antal, M.J., Jr., Tester, J.W.: Thermochemical biofuel production in hydrothermal media: a review of sub-and supercritical water technologies. Energy Environ. Sci. 1(1), 32–65 (2008)
Abu El-Rub, Z., Bramer, E.A., Brem, G.: Review of catalysts for tar elimination in biomass gasification processes. Ind. Eng. Chem. Res. 43(22), 6911–6919 (2004)
Wainaina, S., Parchami, M., Mahboubi, A., Horváth, I.S., Taherzadeh, M.J.: Food waste-derived volatile fatty acids platform using an immersed membrane bioreactor. Biores. Technol. 274, 329–334 (2019). https://doi.org/10.1016/j.biortech.2018.11.104
Zhang, B., von Keitz, M., Valentas, K.: Thermochemical liquefaction of high-diversity grassland perennials. J. Anal. Appl. Pyrol. 84(1), 18–24 (2009)
Skaggs, R.L., Coleman, A.M., Seiple, T.E., Milbrandt, A.R.: Waste-to-energy biofuel production potential for selected feedstocks in the conterminous United States. Renew. Sustain. Energy Rev. 82, 2640–2651 (2018)
Kostyukevich, Y., Vlaskin, M., Borisova, L., Zherebker, A., Perminova, I., Kononikhin, A., et al.: Investigation of bio-oil produced by hydrothermal liquefaction of food waste using ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometry. Eur. J. Mass Spectrom. 24(1), 116–123 (2018)
Maddi, B., Panisko, E., Wietsma, T., Lemmon, T., Swita, M., Albrecht, K., et al.: Quantitative characterization of aqueous byproducts from hydrothermal liquefaction of municipal wastes, food industry wastes, and biomass grown on waste. ACS Sustain. Chem. Eng. 5(3), 2205–2214 (2017)
Cantero-Tubilla, B., Cantero, D.A., Martinez, C.M., Tester, J.W., Walker, L.P., Posmanik, R.: Characterization of the solid products from hydrothermal liquefaction of waste feedstocks from food and agricultural industries. J. Supercrit. Fluids 133, 665–673 (2018). https://doi.org/10.1016/j.supflu.2017.07.009
Gollakota, A., Savage, P.E.: Hydrothermal liquefaction of model food waste biomolecules and ternary mixtures under isothermal and fast conditions. ACS Sustain. Chem. Eng. 6(7), 9018–9027 (2018)
Posmanik, R., Labatut, R.A., Kim, A.H., Usack, J.G., Tester, J.W., Angenent, L.T.: Coupling hydrothermal liquefaction and anaerobic digestion for energy valorization from model biomass feedstocks. Biores. Technol. 233, 134–143 (2017). https://doi.org/10.1016/j.biortech.2017.02.095
Posmanik, R., Martinez, C.M., Cantero-Tubilla, B., Cantero, D.A., Sills, D., Cocero, M.J., et al.: Acid and alkali catalyzed hydrothermal liquefaction of dairy manure digestate and food waste. ACS Sustain. Chem. Eng. 6(2), 2724–2732 (2018)
Maag, A.R., Paulsen, A.D., Amundsen, T.J., Yelvington, P.E., Tompsett, G.A., Timko, M.T.: Catalytic hydrothermal liquefaction of food waste using CeZrOx. Energies 11(3), 564 (2018)
Déniel, M., Haarlemmer, G., Roubaud, A., Weiss-Hortala, E., Fages, J.: Modelling and predictive study of hydrothermal liquefaction: application to food processing residues. Waste Biomass Valor. 8(6), 2087–2107 (2017)
Aierzhati, A., Stablein, M.J., Wu, N.E., Kuo, C.-T., Si, B., Kang, X., et al.: Experimental and model enhancement of food waste hydrothermal liquefaction with combined effects of biochemical composition and reaction conditions. Biores. Technol. 284, 139–147 (2019). https://doi.org/10.1016/j.biortech.2019.03.076
Xiu, S., Shahbazi, A.: Bio-oil production and upgrading research: a review. Renew. Sustain. Energy Rev. 16(7), 4406–4414 (2012). https://doi.org/10.1016/j.rser.2012.04.028
Toor, S.S., Rosendahl, L., Rudolf, A.: Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy 36(5), 2328–2342 (2011)
Dimitriadis, A., Bezergianni, S.: Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: a state of the art review. Renew. Sustain. Energy Rev. 68, 113–125 (2017). https://doi.org/10.1016/j.rser.2016.09.120
Barreiro, D.L., Prins, W., Ronsse, F., Brilman, W.: Hydrothermal liquefaction (HTL) of microalgae for biofuel production: state of the art review and future prospects. Biomass Bioenerg. 53, 113–127 (2013)
Tian, C., Li, B., Liu, Z., Zhang, Y., Lu, H.: Hydrothermal liquefaction for algal biorefinery: a critical review. Renew. Sustain. Energy Rev. 38, 933–950 (2014)
Guo, Y., Yeh, T., Song, W., Xu, D., Wang, S.: A review of bio-oil production from hydrothermal liquefaction of algae. Renew. Sustain. Energy Rev. 48, 776–790 (2015)
Qian, L., Wang, S., Savage, P.E.: Hydrothermal liquefaction of sewage sludge under isothermal and fast conditions. Biores. Technol. 232, 27–34 (2017)
Snowden-Swan, L.J., Zhu, Y., Jones, S.B., Elliott, D.C., Schmidt, A.J., Hallen, R.T., et al.: Hydrothermal liquefaction and upgrading of municipal wastewater treatment plant sludge: a preliminary techno-economic analysis, rev. 1. Pacific Northwest National Lab, Richland (2016)
Jarvis, J.M., Albrecht, K.O., Billing, J.M., Schmidt, A.J., Hallen, R.T., Schaub, T.M.: Assessment of hydrotreatment for hydrothermal liquefaction biocrudes from sewage sludge, microalgae, and pine feedstocks. Energy Fuels 32(8), 8483–8493 (2018)
Liu, R., Tian, W., Kong, S., Meng, Y., Wang, H., Zhang, J.: Effects of inorganic and organic acid pretreatments on the hydrothermal liquefaction of municipal secondary sludge. Energy Convers. Manage. 174, 661–667 (2018)
Kapusta, K.: Effect of ultrasound pretreatment of municipal sewage sludge on characteristics of bio-oil from hydrothermal liquefaction process. Waste Manage. 78, 183–190 (2018). https://doi.org/10.1016/j.wasman.2018.05.043
Domingo, J.L., Nadal, M.: Per-and polyfluoroalkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. J. Agric. Food Chem. 65(3), 533–543 (2017)
Herzke, D., Huber, S., Bervoets, L., D’Hollander, W., Hajslova, J., Pulkrabova, J., et al.: Perfluorinated alkylated substances in vegetables collected in four European countries; occurrence and human exposure estimations. Environ. Sci. Pollut. Res. 20(11), 7930–7939 (2013)
Hlouskova, V., Hradkova, P., Poustka, J., Brambilla, G., De Filipps, S.P., D’Hollander, W., et al.: Occurrence of perfluoroalkyl substances (PFASs) in various food items of animal origin collected in four European countries. Food Addit. Contam. A. 30(11), 1918–1932 (2013)
D’Hollander, W., Herzke, D., Huber, S., Hajslova, J., Pulkrabova, J., Brambilla, G., et al.: Occurrence of perfluorinated alkylated substances in cereals, salt, sweets and fruit items collected in four European countries. Chemosphere 129, 179–185 (2015)
Klenow, S., Heinemeyer, G., Brambilla, G., Dellatte, E., Herzke, D., de Voogt, P.: Dietary exposure to selected perfluoroalkyl acids (PFAAs) in four European regions. Food Addit. Contam. A. 30(12), 2141–2151 (2013)
Heo, J.-J., Lee, J.-W., Kim, S.-K., Oh, J.-E.: Foodstuff analyses show that seafood and water are major perfluoroalkyl acids (PFAAs) sources to humans in Korea. J. Hazard. Mater. 279, 402–409 (2014)
Pérez, F., Llorca, M., Köck-Schulmeyer, M., Škrbić, B., Oliveira, L.S., da Boit, M.K., et al.: Assessment of perfluoroalkyl substances in food items at global scale. Environ. Res. 135, 181–189 (2014)
Authority EFS: Perfluoroalkylated substances in food: occurrence and dietary exposure. EFSA J. 10(6), 2743 (2012)
Clarke, D., Bailey, V., Routledge, A., Lloyd, A., Hird, S., Mortimer, D., et al.: Dietary intake estimate for perfluorooctanesulphonic acid (PFOS) and other perfluorocompounds (PFCs) in UK retail foods following determination using standard addition LC–MS/MS. Food Addit. Contam. 27(4), 530–545 (2010)
Tittlemier, S.A., Pepper, K., Seymour, C., Moisey, J., Bronson, R., Cao, X.-L., et al.: Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J. Agric. Food Chem. 55(8), 3203–3210 (2007)
Bhavsar, S.P., Zhang, X., Guo, R., Braekevelt, E., Petro, S., Gandhi, N., et al.: Cooking fish is not effective in reducing exposure to perfluoroalkyl and polyfluoroalkyl substances. Environ. Int. 66, 107–114 (2014). https://doi.org/10.1016/j.envint.2014.01.024
Lechner, M., Knapp, H.: Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plant and distribution to the different plant compartments studied in cultures of carrots (Daucus carota ssp. Sativus), potatoes (Solanum tuberosum), and cucumbers (Cucumis sativus). J. Agric. Food Chem. 59(20), 11011–8 (2011)
Blaine, A.C., Rich, C.D., Hundal, L.S., Lau, C., Mills, M.A., Harris, K.M., et al.: Uptake of perfluoroalkyl acids into edible crops via land applied biosolids: field and greenhouse studies. Environ. Sci. Technol. 47(24), 14062–14069 (2013)
Blaine, A.C., Rich, C.D., Sedlacko, E.M., Hundal, L.S., Kumar, K., Lau, C., et al.: Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids-amended soils. Environ. Sci. Technol. 48(14), 7858–7865 (2014)
Scher, D.P., Kelly, J.E., Huset, C.A., Barry, K.M., Hoffbeck, R.W., Yingling, V.L., et al.: Occurrence of perfluoroalkyl substances (PFAS) in garden produce at homes with a history of PFAS-contaminated drinking water. Chemosphere 196, 548–555 (2018)
Xiao, F.: Emerging poly- and perfluoroalkyl substances in the aquatic environment: a review of current literature. Water Res. 124, 482–495 (2017). https://doi.org/10.1016/j.watres.2017.07.024
Schaider, L.A., Balan, S.A., Blum, A., Andrews, D.Q., Strynar, M.J., Dickinson, M.E., et al.: Fluorinated compounds in US fast food packaging. Environ. Sci. Technol. Lett. 4(3), 105–111 (2017)
Schultes, L., Peaslee, G.F., Brockman, J.D., Majumdar, A., McGuinness, S.R., Wilkinson, J.T., et al.: Total fluorine measurements in food packaging: how do current methods perform? Environ. Sci. Technol. Lett. 6(2), 73–78 (2019)
Yeung, L.W., Robinson, S.J., Koschorreck, J., Mabury, S.A.: Part II. A temporal study of PFOS and its precursors in human plasma from two German cities in 1982–2009. Environ. Sci. Technol. 47(8), 3875–82 (2013)
Trier, X., Granby, K., Christensen, J.H.: Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ. Sci. Pollut. Res. 18(7), 1108–1120 (2011)
Trier, X., Nielsen, N.J., Christensen, J.H.: Structural isomers of polyfluorinated di-and tri-alkylated phosphate ester surfactants present in industrial blends and in microwave popcorn bags. Environ. Sci. Pollut. Res. 18(8), 1422–1432 (2011)
Begley, T., White, K., Honigfort, P., Twaroski, M., Neches, R., Walker, R.: Perfluorochemicals: potential sources of and migration from food packaging. Food Addit. Contam. 22(10), 1023–1031 (2005)
Begley, T., Hsu, W., Noonan, G., Diachenko, G.: Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit. Contam. 25(3), 384–390 (2008)
Choi, Y.J., Kim Lazcano, R., Yousefi, P., Trim, H., Lee, L.S.: Perfluoroalkyl acid characterization in US municipal organic solid waste composts. Environ. Sci. Technol. Lett. 6(6), 372–377 (2019)
Maine Department of Environmental Protection. https://www.maine.gov/dep/spills/topics/pfas/Maine-PFAS-Screening-Levels-Rev-6.28.21.pdf
Ross, I., McDonough, J., Miles, J., Storch, P., Thelakkat Kochunarayanan, P., Kalve, E., et al.: A review of emerging technologies for remediation of PFASs. Remediat. J. 28(2), 101–126 (2018)
Kucharzyk, K.H., Darlington, R., Benotti, M., Deeb, R., Hawley, E.: Novel treatment technologies for PFAS compounds: a critical review. J. Environ. Manage. 204, 757–764 (2017). https://doi.org/10.1016/j.jenvman.2017.08.016
McCleaf, P., Englund, S., Östlund, A., Lindegren, K., Wiberg, K., Ahrens, L.: Removal efficiency of multiple poly- and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Res. 120, 77–87 (2017). https://doi.org/10.1016/j.watres.2017.04.057
ITRC.: Remediation technologies and methods for per and polyfluoroalkyl substances (PFAS).https://www.pfas-1itrcweborg/wp-content/uploads/2018/03/pfas_fact_sheet_remediation_3_15_18pdf (2018)
Zhang, D., Zhang, W., Liang, Y.: Adsorption of perfluoroalkyl and polyfluoroalkyl substances (PFASs) from aqueous solution—a review. Sci. Total Environ. 694, 133606 (2019)
Zhang, D., He, Q., Wang, M., Zhang, W., Liang, Y.: Sorption of perfluoroalkylated substances (PFASs) onto granular activated carbon and biochar. Environ. Technol. (2019). https://doi.org/10.1080/09593330.2019.1680744
Bao, Y., Niu, J., Xu, Z., Gao, D., Shi, J., Sun, X., et al.: Removal of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) from water by coagulation: mechanisms and influencing factors. J. Colloid Interface Sci. 434, 59–64 (2014)
Birk, G., Alden, D., Stuart, R.: Ex situ treatments of aqueous film-forming foam impacted water. American Chemical Society, Washington (2016)
Yang, B., Han, Y., Deng, Y., Li, Y., Zhuo, Q., Wu, J.: Highly efficient removal of perfluorooctanoic acid from aqueous solution by H2O2-enhanced electrocoagulation-electroflotation technique. Emerging Contaminants. 2(1), 49–55 (2016)
Lin, H., Wang, Y., Niu, J., Yue, Z., Huang, Q.: Efficient sorption and removal of perfluoroalkyl acids (PFAAs) from aqueous solution by metal hydroxides generated in situ by electrocoagulation. Environ. Sci. Technol. 49(17), 10562–10569 (2015)
Tang, C.Y., Fu, Q.S., Robertson, A., Criddle, C.S., Leckie, J.O.: Use of reverse osmosis membranes to remove perfluorooctane sulfonate (PFOS) from semiconductor wastewater. Environ. Sci. Technol. 40(23), 7343–7349 (2006)
Flores, C., Ventura, F., Martin-Alonso, J., Caixach, J.: Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in N.E. Spanish surface waters and their removal in a drinking water treatment plant that combines conventional and advanced treatments in parallel lines. Sci. Total Environ. 461–462, 618–26 (2013). https://doi.org/10.1016/j.scitotenv.2013.05.026
Tang, C.Y., Fu, Q.S., Criddle, C.S., Leckie, J.O.: Effect of flux (transmembrane pressure) and membrane properties on fouling and rejection of reverse osmosis and nanofiltration membranes treating perfluorooctane sulfonate containing wastewater. Environ. Sci. Technol. 41(6), 2008–2014 (2007)
Steinle-Darling, E., Reinhard, M.: Nanofiltration for trace organic contaminant removal: structure, solution, and membrane fouling effects on the rejection of perfluorochemicals. Environ. Sci. Technol. 42(14), 5292–5297 (2008)
Loi-Brügger, A., Panglisch, S., Hoffmann, G., Buchta, P., Gimbel, R., Nacke, C.-J.: Removal of trace organic substances from river bank filtrate–performance study of RO and NF membranes. Water Science and Technology: Water Supply. 8(1), 85–92 (2008)
Tsai, Y.-T., Yu-Chen Lin, A., Weng, Y.-H., Li, K.-C.: Treatment of perfluorinated chemicals by electro-microfiltration. Environ. Sci. Technol. 44(20), 7914–7920 (2010)
Eberle, D., Ball, R., Boving, T.B.: Impact of ISCO treatment on PFAA co-contaminants at a former fire training area. Environ. Sci. Technol. 51(9), 5127–5136 (2017)
Huang, J., Wang, X., Pan, Z., Li, X., Ling, Y., Li, L.: Efficient degradation of perfluorooctanoic acid (PFOA) by photocatalytic ozonation. Chem. Eng. J. 296, 329–334 (2016)
Vecitis, C.D., Park, H., Cheng, J., Mader, B.T., Hoffmann, M.R.: Kinetics and mechanism of the sonolytic conversion of the aqueous perfluorinated surfactants, perfluorooctanoate (PFOA), and perfluorooctane sulfonate (PFOS) into inorganic products. J. Phys. Chem. A 112(18), 4261–4270 (2008)
Lee, Y.-C., Chen, M.-J., Huang, C.-P., Kuo, J., Lo, S.-L.: Efficient sonochemical degradation of perfluorooctanoic acid using periodate. Ultrason. Sonochem. 31, 499–505 (2016)
Hao, F., Guo, W., Wang, A., Leng, Y., Li, H.: Intensification of sonochemical degradation of ammonium perfluorooctanoate by persulfate oxidant. Ultrason. Sonochem. 21(2), 554–558 (2014)
Lin, J.-C., Hu, C.-Y., Lo, S.-L.: Effect of surfactants on the degradation of perfluorooctanoic acid (PFOA) by ultrasonic (US) treatment. Ultrason. Sonochem. 28, 130–135 (2016)
Zhuo, Q., Li, X., Yan, F., Yang, B., Deng, S., Huang, J., et al.: Electrochemical oxidation of 1H, 1H, 2H, 2H-perfluorooctane sulfonic acid (6:2 FTS) on DSA electrode: operating parameters and mechanism. J. Environ. Sci. 26(8), 1733–1739 (2014)
Gomez-Ruiz, B., Gómez-Lavín, S., Diban, N., Boiteux, V., Colin, A., Dauchy, X., et al.: Efficient electrochemical degradation of poly-and perfluoroalkyl substances (PFASs) from the effluents of an industrial wastewater treatment plant. Chem. Eng. J. 322, 196–204 (2017)
Schaefer, C.E., Andaya, C., Burant, A., Condee, C.W., Urtiaga, A., Strathmann, T.J., et al.: Electrochemical treatment of perfluorooctanoic acid and perfluorooctane sulfonate: insights into mechanisms and application to groundwater treatment. Chem. Eng. J. 317, 424–432 (2017)
Schaefer, C.E., Andaya, C., Urtiaga, A., McKenzie, E.R., Higgins, C.P.: Electrochemical treatment of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in groundwater impacted by aqueous film forming foams (AFFFs). J. Hazard. Mater. 295, 170–175 (2015)
Trautmann, A., Schell, H., Schmidt, K., Mangold, K.-M., Tiehm, A.: Electrochemical degradation of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in groundwater. Water Sci. Technol. 71(10), 1569–1575 (2015)
Chen, J., Zhang, P., Zhang, L.: Photocatalytic decomposition of environmentally persistent perfluorooctanoic acid. Chem. Lett. 35(2), 230–231 (2006)
Zhang, C., Qu, Y., Zhao, X., Zhou, Q.: Photoinduced reductive decomposition of perflurooctanoic acid in water: effect of temperature and ionic strength. CLEAN Soil Air Water 43(2), 223–8 (2015)
Liu, J., Mejia, A.S.: Microbial degradation of polyfluoroalkyl chemicals in the environment: a review. Environ. Int. 61, 98–114 (2013). https://doi.org/10.1016/j.envint.2013.08.022
Wang, N., Buck, R.C., Szostek, B., Sulecki, L.M., Wolstenholme, B.W.: 5:3 Polyfluorinated acid aerobic biotransformation in activated sludge via novel “one-carbon removal pathways.” Chemosphere 87(5), 527–534 (2012). https://doi.org/10.1016/j.chemosphere.2011.12.056
Wang, N., Liu, J., Buck, R.C., Korzeniowski, S.H., Wolstenholme, B.W., Folsom, P.W., et al.: 6:2 Fluorotelomer sulfonate aerobic biotransformation in activated sludge of waste water treatment plants. Chemosphere 82(6), 853–858 (2011)
Wang, N., Szostek, B., Buck, R.C., Folsom, P.W., Sulecki, L.M., Capka, V., et al.: Fluorotelomer alcohol biodegradation direct evidence that perfluorinated carbon chains breakdown. Environ. Sci. Technol. 39(19), 7516–7528 (2005)
Wang, N., Szostek, B., Buck, R.C., Folsom, P.W., Sulecki, L.M., Gannon, J.T.: 8–2 Fluorotelomer alcohol aerobic soil biodegradation: pathways, metabolites, and metabolite yields. Chemosphere 75(8), 1089–1096 (2009). https://doi.org/10.1016/j.chemosphere.2009.01.033
Wang, N., Szostek, B., Folsom, P.W., Sulecki, L.M., Capka, V., Buck, R.C., et al.: Aerobic biotransformation of 14C-labeled 8–2 telomer B alcohol by activated sludge from a domestic sewage treatment plant. Environ. Sci. Technol. 39(2), 531–538 (2005)
Zhao, L., Folsom, P.W., Wolstenholme, B.W., Sun, H., Wang, N., Buck, R.C.: 6:2 Fluorotelomer alcohol biotransformation in an aerobic river sediment system. Chemosphere 90(2), 203–209 (2013). https://doi.org/10.1016/j.chemosphere.2012.06.035
Harding-Marjanovic, K.C., Houtz, E.F., Yi, S., Field, J.A., Sedlak, D.L., Alvarez-Cohen, L.: Aerobic biotransformation of fluorotelomer thioether amido sulfonate (Lodyne) in AFFF-amended microcosms. Environ. Sci. Technol. 49(13), 7666–7674 (2015)
Liou, J.S.C., Szostek, B., DeRito, C.M., Madsen, E.L.: Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 80(2), 176–183 (2010). https://doi.org/10.1016/j.chemosphere.2010.03.009
Yi, L., Chai, L., Xie, Y., Peng, Q., Peng, Q.: Isolation, identification, and degradation performance of a PFOA-degrading strain. Genet. Mol. Res. (2016). https://doi.org/10.4238/gmr.15028043
Yi, L., Peng, Q., Liu, D., Zhou, L., Tang, C., Zhou, Y., et al.: Enhanced degradation of perfluorooctanoic acid by a genome shuffling-modified Pseudomonas parafulva YAB-1. Environ. Technol. (2018). https://doi.org/10.1080/09593330.2018.1466918
Kwon, B.G.: Reply to comment on “biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant.” Chemosphere 138, 1039–1044 (2015). https://doi.org/10.1016/j.chemosphere.2015.03.021
Kwon, B.G., Lim, H.-J., Na, S.-H., Choi, B.-I., Shin, D.-S., Chung, S.-Y.: Biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant. Chemosphere 109, 221–225 (2014). https://doi.org/10.1016/j.chemosphere.2014.01.072
Avendaño, S.M., Zhong, G., Liu, J.: Comment on “biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant.” Chemosphere 138, 1037–1038 (2015)
Huang, S., Jaffé, P.R.: Defluorination of perfluorooctanoic Acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. strain A6. Environ. Sci. Technol. 53(19), 11410–9 (2019)
Hori, H., Nagaoka, Y., Yamamoto, A., Sano, T., Yamashita, N., Taniyasu, S., et al.: Efficient decomposition of environmentally persistent perfluorooctanesulfonate and related fluorochemicals using zerovalent iron in subcritical water. Environ. Sci. Technol. 40(3), 1049–1054 (2006)
Hori, H., Nagaoka, Y., Sano, T., Kutsuna, S.: Iron-induced decomposition of perfluorohexanesulfonate in sub-and supercritical water. Chemosphere 70(5), 800–806 (2008)
Wu, B., Hao, S., Choi, Y., Higgins, C.P., Deeb, R., Strathmann, T.J.: Rapid destruction and defluorination of perfluorooctanesulfonate by alkaline hydrothermal reaction. Environ. Sci. Technol. Lett. 6(10), 630–636 (2019)
Acknowledgements
This material is based upon work supported by the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE) under the Bioenergy Technology Office Award Number DE-EE0008932.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, Y. A Critical Review of Challenges Faced by Converting Food Waste to Bioenergy Through Anaerobic Digestion and Hydrothermal Liquefaction. Waste Biomass Valor 13, 781–796 (2022). https://doi.org/10.1007/s12649-021-01540-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01540-9