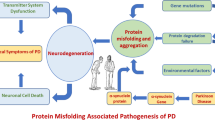
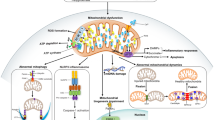
Abstract
Causes of dopaminergic neuronal loss in Parkinson’s disease (PD) are subject of investigation and the common use of models of acute neurodegeneration induced by neurotoxins 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine, and rotenone contributed to advances in the study of PD. However, the use of study models more similar to the pathophysiology of PD is required for advances in early diagnosis and translational pharmacology. Aminochrome (AMI), a compound derived from dopamine oxidation and a precursor of neuromelanin, is able to induce all the mechanisms associated with neurodegeneration. Previously, we showed AMI is cytotoxic in primary culture of mesencephalic cells (PCMC) and induces in vitro and in vivo neuroinflammation. On the other hand, the effect of rutin in central nervous system cells has revealed anti-inflammatory, antioxidative, and neuroprotective potential. However, there have been no data studies on the effect of rutin against aminochrome neurotoxicity. Here, we show that rutin prevents lysosomal dysfunction and aminochrome-induced cell death in SHSY-5Y cells, protects PCMC against aminochrome cytotoxicity, and prevents in vivo loss of dopaminergic neurons in substantia nigra pars compacta (SNPc), as well as microgliosis and astrogliosis. Additionally, we show that rutin decreases levels of interleukin-1β (IL-1β) mRNA and increases levels of glia-derived neurotrophic factor (GDNF) and nerve-derived neurotrophic factor (NGF) mRNA. We evidence for the first time the protective effect of rutin on PD aminochrome-induced models and suggest the potential role of the anti-inflammatory activity and upregulation of NGF and GDNF in the mechanism of rutin action against aminochrome neurotoxicity.









Similar content being viewed by others
Data availability
The datasets used and analyzed in this study are available from the corresponding author upon reasonable request.
References
Arriagada C et al (2004) On the neurotoxicity mechanism of leukoaminochrome o-semiquinone radical derived from dopamine oxidation: mitochondria damage, necrosis, and hydroxyl radical formation. Neurobiol Dis 16(2):468–477
Aydin BS, Bulduk I (2020) A validated HPLC-UV method for determination of dopamine HCl in injectable solutions. Eurasian J Bio Chem Sci 3(2):116–120
Bhidayasiri R, Martinez-Martin P (2017) Clinical assessments in Parkinson’s disease: scales and monitoring. Int Rev Neurobiol 132:129–182
Briceño A et al (2016) Aminochrome toxicity is mediated by inhibition of microtubules polymerization through the formation of adducts with tubulin. Neurotox Res 29(3):381–393
Chaudhuri KR et al (2006) International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease:the NMSQuest study. Movement Disorders: Official Journal of the Movement Disorder Society 21(7):916–923
Costa SL et al (2016) Impact of plant-derived flavonoids on neurodegenerative diseases. Neurotox Res 30(1):41–52
Croisier E et al (2005) Microglial inflammation in the Parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation 2(1):1–8
Cuenca L et al (2018) Parkinson’s disease: a short story of 200 years. Histol Histopathol 34(6):573–591
Cuevas C et al (2015) Glutathione transferase-M2-2 secreted from glioblastoma cell protects SH-SY5Y cells from aminochrome neurotoxicity. Neurotox Res 27(3):217–228
da Silva AB et al (2017) The flavonoid rutin modulates microglial/macrophage activation to a CD150/CD206 M2 phenotype. Chem Biol Interact 274:89–99
da Silva AB et al (2020) The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav Immun 85:170–185
de Araújo FM et al (2018) Aminochrome decreases NGF, GDNF, and induces neuroinflammation in organotypic midbrain slice cultures. Neurotoxicology 66:98–106
de Araújo FM et al (2021) Role of microgliosis and NLRP3 inflammasome in Parkinson’s disease pathogenesis and therapy. Cell Mol Neurobiol p. 1–18
De Araújo FM et al (2022) Aminochrome induces neuroinflammation and dopaminergic neuronal loss: a new preclinical model to find anti-inflammatory and neuroprotective drugs for Parkinson’s disease. Cell Mol Neurobiol p. 1–17
Dıaz-Véliz G et al (2002) Behavioral effects of aminochrome and dopachrome injected in the rat substantia nigra. Pharmacol Biochem Behav 73(4):843–850
Elkouzi A et al (2019) Emerging therapies in Parkinson’s disease—repurposed drugs and new approaches. Nat Rev Neurol 15(4):204–223
Enogieru AB et al (2018) Rutin as a potent antioxidant: implications for neurodegenerative disorders. Oxid Med Cell Longev 2018
Enogieru AB et al (2021) Regulation of AKT/AMPK signaling, autophagy and mitigation of apoptosis in rutin-pretreated SH-SY5Y cells exposed to MPP+. Metab Brain Dis 36(2):315–326
Ferreira RS et al (2021) Rutin improves glutamate uptake and inhibits glutamate excitotoxicity in rat brain slices. Mol Biol Rep 48(2):1475–1483
Gil-Martínez AL et al (2018) Unexpected exacerbation of neuroinflammatory response after a combined therapy in old Parkinsonian mice. Front Cell Neurosci 12:451
Herrera A et al (2016) Aminochrome induces dopaminergic neuronal dysfunction: a new animal model for Parkinson’s disease. Cell Mol Life Sci 73(18):3583–3597
Herrera-Soto A et al (2017) On the role of DT-diaphorase inhibition in aminochrome-induced neurotoxicity in vivo. Neurotox Res 32(1):134–140
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection?. The Lancet Neurology 8(4):382–397
Huenchuguala S et al (2014) Glutathione transferase mu 2 protects glioblastoma cells against aminochrome toxicity by preventing autophagy and lysosome dysfunction. Autophagy 10(4):618–630
Huenchuguala S et al (2016) DT-diaphorase protects astrocytes from aminochrome-induced toxicity. Neurotoxicology 55:10–12
Javed H et al (2012) Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 210:340–352
Karabiyik C, Lee MJ, Rubinsztein DC (2017) Autophagy impairment in Parkinson’s disease. Essays Biochem 61(6):711–720
Khan M et al (2012) Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease. Neurotox Res 22(1):1–15
Lang G-P, Li C, Han Y-Y (2021) Rutin pretreatment promotes microglial M1 to M2 phenotype polarization. Neural Regen Res 16(12):2499
Lecca D et al (2018) Boosting phagocytosis and anti-inflammatory phenotype in microglia mediates neuroprotection by PPARγ agonist MDG548 in Parkinson’s disease models. Br J Pharmacol 175(16):3298–3314
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. San Diego, Calif. https://doi.org/10.1006/meth.2001.1262
Macdonald R et al (2018) Mitochondrial abnormalities in Parkinson’s disease and Alzheimer’s disease: can mitochondria be targeted therapeutically?. Biochem Soc Trans 46(4):891–909
Magalingam KB, Radhakrishnan A, Haleagrahara N (2013) Rutin, a bioflavonoid antioxidant protects rat pheochromocytoma (PC-12) cells against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity. Int J Mol Med 32(1):235–240
Mahapatra KK et al (2021) The lysosome as an imperative regulator of autophagy and cell death. Cell Mol Life Sci 78(23):7435–7449
Martinez-Martin P et al (2017) Measurement of nonmotor symptoms in clinical practice. Int Rev Neurobiol 133:291–345
Meléndez C, Muñoz P, Segura-Aguilar J (2019) DT-diaphorase prevents aminochrome-induced lysosome dysfunction in SH-SY5Y cells. Neurotox Res 35(1):255–259
Menzies FM et al (2017) Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93(5):1015–1034
Moghbelinejad S et al (2014) Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol Lett 224(1):108–113
Moosavi F et al (2016) Modulation of neurotrophic signaling pathways by polyphenols. Drug Des Dev Ther 10:23
Muñoz P et al (2015) DT-diaphorase prevents aminochrome-induced alpha-synuclein oligomer formation and neurotoxicity. Toxicol Sci 145(1):37–47
Obeso J et al (2017) Past, present, and future of Parkinson’s disease: a special essay on the 200th anniversary of the Shaking Palsy. Mov Disord 32(9):1264–1310
Ochs SD, Westfall TC, Macarthur H (2005) The separation and quantification of aminochromes using high-pressure liquid chromatography with electrochemical detection. J Neurosci Methods 142(2):201–208
Ola MS et al (2015) Neuroprotective effects of rutin in streptozotocin-induced diabetic rat retina. J Mol Neurosci 56(2):440–448
Paris I et al (2010) Aminochrome induces disruption of actin, alpha-, and beta-tubulin cytoskeleton networks in substantia-nigra-derived cell line. Neurotox Res 18(1):82–92
Paris I et al (2011) Autophagy protects against aminochrome-induced cell death in substantia nigra-derived cell line. Toxicol Sci 121(2):376–388
Paris Pizarro I et al (2011) Autophagy protects against aminochrome-induced cell death in substantia nigra-derived cell line
Park S-E et al (2014) Rutin from Dendropanax morbifera Leveille protects human dopaminergic cells against rotenone induced cell injury through inhibiting JNK and p38 MAPK signaling. Neurochem Res 39(4):707–718
Patel D et al (2019) Cinnamon and its metabolite protect the nigrostriatum in a mouse model of Parkinson’s disease via astrocytic GDNF. J Neuroimmune Pharmacol 14(3):503–518
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, ed. A. Press
Paxinos G, Watson C (2013) The Rat Brain in Stereotaxic Coordinates. Elsevier(7th Edition), 472
Phatnani H, Maniatis T (2015) Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol 7(6):a020628
Poewe W et al (2017) Parkinson’s disease. Nat Rev Dis Primers 3(1):1–21
Reichmann H (2017) Premotor diagnosis of Parkinson’s disease. Neurosci Bull 33(5):526–534
Remião F et al (2003) Synthesis and analysis of aminochromes by HPLC‐photodiode array. Adrenochrome evaluation in rat blood. Biomed Chromatogr 17(1): p. 6–13
Rodríguez-Chinchilla T et al (2020) [18F]-DPA-714 PET as a specific in vivo marker of early microglial activation in a rat model of progressive dopaminergic degeneration. Eur J Nucl Med Mol Imaging 47(11):2602–2612
Santos CC et al (2017) Aminochrome induces microglia and astrocyte activation. Toxicol in Vitro 42:54–60
Santos CC et al (2020) The flavonoid agathisflavone from Poincianella pyramidalis prevents aminochrome neurotoxicity. Neurotox Res p. 1–6
Santos CC et al (2022) JM-20, a denzodiazepine-dihydropyridine hybrid molecule, inhibits the formation of alpha-synuclein-aggregated species. Neurotox Res p. 1–13
Sarkar S, Raymick J, Imam S (2016) Neuroprotective and therapeutic strategies against Parkinson’s disease: recent perspectives. Int J Mol Sci 17(6):904
Schrag A et al (2015) Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. The Lancet Neurology 14(1):57–64
Segura-Aguilar J (2017) On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease. Neural Regen Res 12(6):897
Segura-Aguilar J et al (2022) Neuroprotection against aminochrome neurotoxicity: glutathione transferase M2–2 and DT-diaphorase. Antioxidants 11(2):296
Segura-Aguilar J, Lind C (1989) On the mechanism of the Mn3+-induced neurotoxicity of dopamine: prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem Biol Interact 72(3):309–324
Segura-Aguilar J, Muñoz P, Paris I (2016) Aminochrome as new preclinical model to find new pharmacological treatment that stop the development of Parkinson’s disease. Curr Med Chem 23(4):346–359
Silva AR et al (2008) The flavonoid rutin induces astrocyte and microglia activation and regulates TNF-alpha and NO release in primary glial cell cultures. Cell Biol Toxicol 24(1):75–86
Silva V, Segura-Aguilar J (2021) State and perspectives on flavonoid neuroprotection against aminochrome-induced neurotoxicity. Neural Regen Res 16(9):1797
Song K et al (2015) Rutin upregulates neurotrophic factors resulting in attenuation of ethanol-induced oxidative stress in HT22 hippocampal neuronal cells. J Sci Food Agric 95(10):2117–2123
Tome D et al (2017) Role of neurotrophic factors in Parkinson’s disease. Curr Pharm Des 23(5):809–838
Uddin MS et al (2021) Natural products for neurodegeneration: regulating neurotrophic signals. Oxid Med Cell Longev 2021
Whone A et al (2019) Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 142(3):512–525
Xiong R, Siegel D, Ross D (2014) Quinone-induced protein handling changes: implications for major protein handling systems in quinone-mediated toxicity. Toxicol Appl Pharmacol 280(2):285–295
Xu SL et al (2013) Flavonoids induce the synthesis and secretion of neurotrophic factors in cultured rat astrocytes: a signaling response mediated by estrogen receptor. Evid-Based Complement Altern Med 2013
Yao S et al (2019) FTY720 inhibits MPP+-induced microglial activation by affecting NLRP3 inflammasome activation. J Neuroimmune Pharmacol 14(3):478–492
Yun SP et al (2018) Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 24(7):931–938
Zafar KS, Siegel D, Ross D (2006) A potential role for cyclized quinones derived from dopamine, DOPA, and 3, 4-dihydroxyphenylacetic acid in proteasomal inhibition. Mol Pharmacol 70(3):1079–1086
Acknowledgements
We are grateful to the Postgraduate Program in Immunology at the Federal University of Bahia. We are thankful to Biorender for the design of the figures. We acknowledge the collaboration of the Master’s degree student Martinho Vaz Martinho in performing the dopamine and aminochrome HPLC analysis.
Funding
F.M.A., C.C.S., M.T.H., and J.S.A. were supported by grants from the Coordenação de Apoio de Pessoal de Nível Superior (PDSE-47/2017; CAPES/PVE– 189576/09–2014; PVB CAPES-PRINT/UFBA 2021); V.D.A.S. and S.L.C. were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ Edital Universal/2018—429127/2018–9, 402051/2022–0, and Research Fellowship). L.C.B. thanks the Spanish Ministry of Science, Innovation and Universities (FPU 18/02549) for its support; M.T.H. thanks the Federación Española de Parkinson (FIS PI13 01293) and the Fundación Séneca (19540/PI/14) for its support; V.D.A.S. and S.L.C. thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ E. Universal/2018—429127/2018–9; and CNPQ—Research Fellowship) for its support.
Author information
Authors and Affiliations
Contributions
V.D.A.S., M.T.H., and J.S.A. made the conception and design of the study; F.M.A, A.F., L.B.J., L.C.B., K.F., C.C.S., E.N.S., J.T.S., F.S., A.C.S.C., A.A.F. P.M., and J.A.M.F. were in charge of the acquisition of data; V.D.A.S., M.T.H., J.S.A., S.L.C., M.F.D.C., and J.A.M.F. made the analysis and the interpretation of the data; V.D.A.S., M.T.H., and J.S.A. made the draft of the article or critical review for important intellectual content.
Corresponding authors
Ethics declarations
Ethical approval
All procedures were approved by the Animal Use Ethics Committee of the Federal University of Bahia (CEUA/UFBA-Protocol n° 127/2017 and 011/2017).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Araújo, F.M., Frota, A.F., de Jesus, L.B. et al. Protective Effects of Flavonoid Rutin Against Aminochrome Neurotoxicity. Neurotox Res 41, 224–241 (2023). https://doi.org/10.1007/s12640-022-00616-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00616-1