Abstract
Purpose
Acetaminophen is the most common drug used to treat acute pain in the pediatric population, given its wide safety margin, low cost, and multiple routes for administration. We sought to determine the most efficacious route of acetaminophen administration for postoperative acute pain relief in the pediatric surgical population.
Methods
We conducted a systematic review of randomized controlled trials (RCTs) that included children aged between 30 days and 17 yr who underwent any type of surgical procedure and that evaluated the analgesic efficacy of different routes of administration of acetaminophen for the treatment of postoperative pain. We searched MEDLINE, CENTRAL, Embase, CINAHL, LILACs, and Google Scholar databases for trials published from inception to 16 April 2023. We assessed the risk of bias in the included studies using the Cochrane Risk of Bias 1.0 tool. We performed a frequentist network meta-analysis using a random-effects model. Our primary outcome was postoperative pain using validated pain scales.
Results
We screened 2,344 studies and included 14 trials with 829 participants in the analysis. We conducted a network meta-analysis for the period from zero to two hours, including six trials with 496 participants. There was no evidence of differences between intravenous vs rectal routes of administration of acetaminophen (difference in means, −0.28; 95% confidence interval [CI], −0.62 to 0.06; very low certainty of the evidence) and intravenous vs oral acetaminophen (difference in means, −0.60; 95% CI, −1.20 to 0.01; low certainty of the evidence). For the comparison of oral vs rectal routes, we found evidence favouring the oral route (difference in means, −0.88; 95% CI, −1.44 to −0.31; low certainty of the evidence). Few trials reported secondary outcomes of interest; when comparing the oral and rectal routes in the incidence of nausea and vomiting, there was no evidence of differences (relative risk, 1.20; 95% CI, 0.81 to 1.78).
Conclusion
The available evidence on the effect of the administration route of acetaminophen on postoperative pain in children is very uncertain. The outcomes of postoperative pain control and postoperative vomiting may differ very little between the oral and rectal route. Better designed and executed RCTs are required to address this important clinical question.
Study registration
PROSPERO (CRD42021286495); first submitted 19 November 2021.
Résumé
Objectif
Compte tenu de sa large marge de sécurité, de son faible coût et de ses multiples voies d’administration, l’acétaminophène est le médicament le plus couramment utilisé pour traiter la douleur aiguë dans la population pédiatrique. Nous avons cherché à déterminer la voie d’administration d’acétaminophène la plus efficace pour le soulagement de la douleur aiguë postopératoire dans la population chirurgicale pédiatrique.
Méthode
Nous avons réalisé une revue systématique d’études randomisées contrôlées (ERC) qui ont inclus des enfants âgé·es de 30 jours à 17 ans ayant bénéficié de n’importe quel type d’intervention chirurgicale et qui ont évalué l’efficacité analgésique de différentes voies d’administration d’acétaminophène pour le traitement de la douleur postopératoire. Nous avons mené des recherches dans les bases de données MEDLINE, CENTRAL, Embase, CINAHL, LILAC et Google Scholar pour en tirer les études publiées depuis leur création jusqu’au 16 avril 2023. Le risque de biais dans les études incluses a été évalué à l’aide de l’outil de Risque de biais 1.0 de Cochrane. Nous avons réalisé une méta-analyse de réseau fréquentiste à l’aide d’un modèle à effets aléatoires. Notre critère d’évaluation principal était la douleur postopératoire mesurée à l’aide d’échelles de douleur validées.
Résultats
Nous avons passé en revue 2344 études et inclus 14 études incluant un total de 829 enfants dans l’analyse. Nous avons mené une méta-analyse en réseau pour une période allant de zéro à deux heures, comprenant six études avec 496 participant·es. Il n’y avait aucune preuve de différences entre les voies d’administraion intraveineuse vs rectale de l’acétaminophène (différence de moyennes, −0,28; intervalle de confiance [IC] à 95 %, −0,62 à 0,06; très faible certitude des données probantes) et entre les voies intraveineuse vs orale (différence de moyennes, −0,60; IC 95 %, −1,20 à 0,01; faible certitude des données probantes). Pour la comparaison des voies orale vs rectale, nous avons trouvé des données probantes en faveur de la voie orale (différence de moyennes, −0,88; IC 95 %, −1,44 à −0,31; faible degré de certitude des données probantes). Peu d’études ont rapporté des résultats secondaires d’intérêt; en comparant les voies orale et rectale dans l’incidence des nausées et des vomissements, il n’y avait aucune preuve de différences (risque relatif, 1,20; IC 95 %, 0,81 à 1,78).
Conclusion
Les données probantes disponibles sur l’effet de la voie d’administration de l’acétaminophène sur la douleur postopératoire chez les enfants sont très incertaines. Les résultats de contrôle de la douleur postopératoire et de vomissements postopératoires peuvent différer très peu entre la voie orale et la voie rectale. Des ERC mieux conçues et mieux exécutées sont nécessaires pour répondre à cette importante question clinique.
Enregistrement de l’étude
PROSPERO (CRD42021286495); première soumission le 19 novembre 2021.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pain is defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.”1 In certain special populations like children, postoperative pain represents a challenging situation. This is mainly because the methods to assess pain are not based on self-reported experience, but on behavioural, expression, and comfort parameters.2,3 These situations may lead to suboptimal management of postoperative pain. In a long-term follow-up study, 52% of children experienced moderate to severe pain three days after adenotonsillectomy, a common surgery performed in this population.4
Acetaminophen is a nonopioid analgesic with multiple theorized mechanisms of action,5 available in oral, rectal, and intravenous formulations. It has emerged as an attractive medication for the treatment of postoperative pain in children and adolescents because of its wide safety margin without modifying platelet function5,6 and because it decreases the use of postoperative opioids,7 thereby reducing the risk of opioid-related adverse effects.
Rectal acetaminophen is an attractive route in patients experiencing postoperative nausea and vomiting (which can influence plasma concentrations of orally administered acetaminophen8) or in patients who refuse to take an oral prescription.3 Despite its potential benefits, absorption of rectal acetaminophen is irregular, and the uptake is dependent on factors like the composition of the suppository (lipophilic or hydrophilic) and the size of the suppository. A pharmacokinetic study reported that a rectal dose of 30 mg·kg−1 achieves serum concentrations of 10–20 μg·mL−1 in approximately 210 min (range, 120–300 min), and these concentrations are associated with antipyretic effects.9 Despite the uncertainty of the plasma concentrations needed for analgesia in children, studies suggest that < 10 μg·mL−1 to 20 μg·mL−1 is sufficient to achieve an analgesic effect8 and can be achieved with rectal doses as low as 20 mg·kg−1.9
By convention, the oral route is ideal for the administration of drugs if it is available. Nevertheless, in the perioperative context, when fasting is recommended or if the patient is not cooperative, the use of other routes should be considered to ensure adequate analgesia. The intravenous route ensures a fast and reliable plasma concentration, although rectal administration of acetaminophen has been proposed for treating postoperative acute pain in children.3,8,9
A systematic review in adults found no evidence of differences between oral and intravenous routes of acetaminophen administration; however, there were serious concerns about the certainty of the evidence from randomized controlled trials (RCTs) graded as “low quality.”10 A recent systematic review by Ulrich et al. concluded that, despite its benefits in the pharmacokinetic profile, the widespread use of intravenous acetaminophen is not supported by solid data proving its superiority over the far less expensive and simpler oral route.11
There is a lack of clinical evidence that supports the use of one route of administration (oral, intravenous, or rectal) over another in the perioperative period. It is important to summarize the available evidence to provide pooled information to improve the decision-making process in anesthesia or to adequately design further comprehensive studies. Therefore, we sought to perform a systematic review of RCTs to evaluate the comparative effectiveness for postoperative pain management of each of these routes in children aged 30 days to 17 yr undergoing any type of surgical procedure. We hypothesized that the three routes of administration would not differ in terms of safety and effectiveness for acute pain relief. In addition, we sought to assess nausea/vomiting, pruritus, sedation, opioid consumption in 24 hr, length of stay in the postanesthesia care unit (PACU), and patient satisfaction.
Methods
This systematic review and network meta-analysis was registered with PROSPERO (CRD42021286495; first submitted 19 November 2021) and it is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Network Meta-Analyses (PRISMA-NMA) statement.12
Eligibility criteria
We included randomized controlled trials that included children aged between 30 days and 17 yr with at least ten participants randomly allocated to each treatment group undergoing any type of surgical procedure, and that evaluated the analgesic efficacy of various routes of acetaminophen administration for the treatment of postoperative pain. We considered studies using single or multiple doses of drugs and studies in which interventions were administered isolated or as a supplement to other analgesics.
Exclusion criteria
We excluded all articles that were review articles, editorials, and case reports. We also excluded studies of experimental pain and studies not conducted in humans.
Information sources and search strategy
A comprehensive literature search was performed with the support of Erasmus MC University Library (Rotterdam, The Netherlands). The search strategy was developed for MEDLINE using controlled vocabulary Medical Subject Headings terms and free-text terms in various permutations combined with Boolean operators (Electronic Supplementary Material [ESM] eTable 1). This strategy was extended to include CENTRAL, Embase, CINAHL, LILACs, and Google Scholar databases from inception to 26 January 2022 and updated on 16 April 2023. There were no language restrictions and filters were applied to exclude experimental studies and studies in animals. We also performed a hand search of the reference lists of included trials or related articles.
Study selection
Screening of abstracts and full texts for the prespecified inclusion and exclusion criteria was independently performed by two review authors (D. M. and D. O.) and differences were resolved by discussion with a third author (J. A. C.). Review authors used the information from the retrieved reports to help identify any duplicate or retracted publications.
Data collection process
From each trial, data were independently extracted by D. M. and D. O. into an electronic database using standardized data extraction forms including authors, year of publication, number and age of participants, doses of the intervention, details about the anesthetic management, other analgesic interventions administered, and type of surgery. Data presented in a graphical format only were extrapolated with plot digitization software (Plot Digitizer, 2.1, Free Software Foundation, Boston, MA, USA). When data were not contained within the original research report, we attempted to contact the corresponding author twice.
Geometry of the network
We presented a conventional network graph including three nodes representing the routes of acetaminophen administration (oral, intravenous, and rectal), edges representing available direct comparisons between pairs of interventions (oral vs intravenous, intravenous vs rectal, and oral vs rectal), line thicknesses indicating the amount of included RCTs, and colour of the lines representing the average risk of bias across studies.12 We presented a qualitative description of the geometry assessment.
Risk-of-bias assessment
The risk of bias in the included studies was assessed using the Cochrane Risk of Bias 1.0 tool, which evaluates seven specific domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias). Two review authors (D. M. and D. O.) independently assessed the risk of bias study and recorded supporting information and justifications for the risk of bias judgements for each domain (low, unclear, or high) and differences were resolved by a third author (J. A. C.).
Summary measures
The primary outcome was pain assessment using validated pain scales (pain intensity and pain relief in the form of a visual analog scale [VAS]; a numeric rating scale; a categorical scale; the Children and Infants Postoperative Pain Scale; the Face, Legs, Activity, Cry, Consolability scale; the Wong–Baker Pain Scale; the COMFORT score; or nursing pain scores). Where a 0–10 numeric rating scale (NRS) was used, we converted scores to a 0–100 scale by multiplying the number by 10. We used four time frames related to the end of surgery to assess pain during the postoperative period: 1) 0–2 hours, 2) 2–6 hours, 3) 6–24 hr, and 4) beyond 24 hr. These time frames were selected post hoc and were based on previous research.10,13
Secondary outcomes included opioid consumption during the first 24 hr. We converted all opioid consumption to intravenous morphine equivalents (mg) using conversion tables.14 We also extracted data from the time to first analgesic request or rescue dosage in minutes, patient satisfaction, and length of stay at the PACU or hospital. The incidence of side effects was collected to include nausea and vomiting, pruritus, and sedation during follow-up of each RCT. Finally, we also assessed the serum/plasma acetaminophen concentrations reported by the authors. Where mean and standard deviation were not presented for any outcomes examined, we used the median, interquartile range, and range to estimate these using standardized conversion equations.15
Planned methods of analysis
Data were entered into Review Manager 5.016 by one author (D. O.) and checked by two authors (D. M. and J. A. C.). Data were analyzed by another author (K. R.) with RStudio17 (Posit Software, PBC, Boston, MA, USA), using the packages, “netmeta” (version 0.9-8) and “meta” (version 4.9-7).
We aimed to conduct a network meta-analysis according to the frequentist method described by Rücker et al.18 In network meta-analysis, interventions (in this case, routes of administration) can be compared directly and indirectly, the latter via mathematical manipulation of the estimates of the direct intervention effect as a common comparator. Importantly, indirect comparisons and network meta-analysis facilitate the estimation of the relative effects of interventions that have not been previously compared directly within an RCT and provide a potential ranking of interventions.
We rated the certainty of evidence for each outcome subjected to network meta-analysis using the grading of recommendations assessment, development, and evaluation system (GRADE)19 by using the Confidence in Network Meta-Analysis methodology (CINeMA, Institute of Social and Preventive Medicine, University of Bern, Switzerland).20 This approach requires assigning a judgement as “no concern,” “some concerns,” or “major concerns” according to the relative contributions of the studies with high or moderate risk of bias included in the within-study bias and indirectness domains. Each domain was classified from high certainty to moderate-, low-, or very low certainty. The classification was undertaken independently by two review authors (D. O. and J. A. C.) and agreement was reached by consensus by including a third author (M. K.). Characteristics that caused a downgrade of the certainty of the evidence included: 1) risk of bias, 2) inconsistency of results, 3) indirectness, 4) imprecision, and 5) publication bias. In addition to the risk of bias assessment, imprecision was assessed by considering the confidence intervals (CIs) and minimal clinically important difference defined as 1.0 unit in pain VAS or 10 units in NRS, as previously described by Powell et al. in children.21 Inconsistency includes disagreement between direct and indirect estimates for the same comparison. Included outcomes in this evaluation were presented in a “summary of findings” table.22
We expressed treatment effects as risk ratios or mean differences with corresponding 95% CIs. To assess heterogeneity in the network analysis, we used the I2 statistic. We assessed inconsistency in the model by looking at differences between estimates from direct and indirect comparisons. We ranked the treatments by P-scores18,23 and presented the outcomes of the analysis in a league table sorted by the P-scores.
Corresponding authors were contacted for any data missing from the original publication, irrespective of publication date.8,24,25 In certain cases, we extracted data from published graphs.24
For secondary outcomes, we conducted a conventional pairwise meta-analysis using random-effects modelling and expected high clinical heterogeneity between studies. For dichotomous outcomes, we calculated the risk ratio (95% CI) using random-effects modelling. We assessed statistical heterogeneity with the use of the I2 statistic. As an indicator of statistical significance, we used a P value of less than 0.05.
Results
Study selection
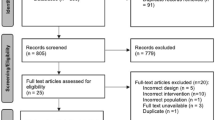
Initially, 4,596 article citations were identified, with 2,252 duplicates removed. Following screening, 2,316 records were excluded based on title and abstract, leaving 29 references. One reference could not be retrieved,25 and after a full-text assessment of 28 reports, 17 were excluded primarily because of incorrect comparisons. Three additional RCTs were found through a Google Scholar search and included in the review. Ultimately, 14 RCTs involving 829 patients met the inclusion criteria.8,24,25,26,27,28,29,30,31,32,33,34,35,36,37 Additional details about the selection process are outlined in the PRISMA flowchart (Fig. 1).
Characteristics of the included studies
Of the 14 RCTs included, eight administered the intervention in the intraoperative period,8,26,28,29,30,31,35,36 two in the preoperative period,34,37 one in the postoperative period,33 and three in a combination of the preoperative and intraoperative period because of the need for intravenous access.24,27,32 Three RCTs compared intravenous and oral routes.24,32,37 Six studies compared intravenous and rectal routes,28,29,30,31,33,35 and five studies compared oral and rectal routes.8,26,27,34,36 In addition, one trial, by Aslani et al.,24 was available only as a poster meeting from which we extracted the relevant data. Only two trials, by Khalili et al. and Nour et al., included placebo as a control group.31,32 Finally, Kumar et al.35 reported pain scores with unexplained low variability.
Included RCTs recruited children between three months and 15 yr of age undergoing general anesthesia with orotracheal intubation as the main anesthetic technique. One study included patients undergoing primary cleft repair,32 one included patients for surgical repair of a unilateral inguinal hernia,31 two included patients undergoing surgical correction of major craniofacial malformations,8,33 and ten included patients undergoing tonsillectomy with or without adenoidectomy.24,26,27,28,29,30,34,35,36,37 Four RCTs were conducted in the USA,24,32,34,37 two in Iran,26,31 two in the Netherlands,8,32 two in India,28,29 and one each in Australia, Italy, Pakistan, and Denmark.27,30,35,36 Trials testing the rectal route used acetaminophen doses ranging from 15 mg·kg−1 to 90 mg·kg−1, those testing the oral route used doses ranging from 15 mg·kg−1 to 60 mg·kg−1, and those investigating the intravenous route used doses ranging from 10 mg·kg−1 to 40 mg·kg−1. Characteristics of the included trials are detailed in Table 1 and ESM eTables 2 and 3, the risk of bias assessment is shown in Fig. 2, and the summary of the risk of bias is presented in ESM eFig. 1. Of the 14 RCTs included, eight did not declare their source of funding,8,24,28,31,33,34,35,36 five declared funding by universities or no external sources,26,29,30,32,37 and one declared funding by a pharmaceutical company27 (for the analyses of plasma concentrations) (ESM eTable 4).
Risk of bias within studies
Among the 14 RCTs included in our analysis, seven provided sufficient detail regarding the randomization process,8,26,27,30,32,35,37 and seven provided insufficient information.24,28,29,31,33,34,36 Seven RCTs described the methods for allocation concealment,8,26,27,30,32,33,37 one RCT allowed patients to choose their intervention route,36 and six RCTs provided insufficient information about concealment of the intervention.24,28,29,31,34,35 In three RCTs, the anesthesiologist was not blinded to the intervention;28,35,36 in two RCTs, insufficient information was provided about blinding of the investigators;24,34 and in the remaining nine RCTs, patients and investigators were blinded to the interventions.8,26,27,29,30,31,32,33,37 In two RCTs, the data collectors were unblinded to the intervention;34,36 in four RCTs, insufficient information was provided about blinding of the outcome;8,24,29,35 and in the remaining eight RCTs, data collectors were blinded to the intervention.26,27,28,30,31,32,33,37 Only one RCT had an available online protocol32 and no significant deviations were identified. In two RCTs,35,36 the data were collected by untrained personnel and we judged them as high risk of bias in the domain “other sources of bias” (Fig. 2, ESM eFig. 1).
Primary outcome
Pain assessment using validated scales was reported for the 0–2-hour period in seven trials including 525 participants,24,26,27,28,29,30,31,32 2–6 hours in five trials including 435 participants,26,28,29,31,35 6–24 hr in three trials including 230 participants,28,29,31 and beyond 24 hr in three trials including 92 participants.30,33,36
Because of the limited availability of data, we were able to conduct a network meta-analysis that included six trials and 496 participants for the 0–2-hour period only.24,26,27,28,29,31 The resulting network is presented in Fig. 3. We did not include data from Nour et al.,32 because they reported the primary outcome using medians and 95% CIs without crude data for this period. For the primary outcome comparing intravenous and rectal routes at 0–2 hours, the mean difference in the pain score was 0.28 lower (95% CI, −0.62 to 0.06). The mean difference in the pain score in the intravenous and oral route was 0.60 lower (95% CI, −1.20 to 0.01), and the mean pain score in the oral route was 0.88 lower than that in the rectal route (difference in means, −0.88; 95% CI, −1.44 to −0.31) (I2 = 60.7%; 95% CI, 0.0 to 85.3) (Table 2).
Network plot for the primary outcome of pain assessment at 0–2 hours. Connecting lines between the circles indicate the direct comparisons of interventions, their width proportional to the number of trials evaluating the comparison, and their colour representing the average risk of bias across trials (intravenous vs rectal routes in three trials; rectal vs oral routes in two trials; intravenous vs oral routes in one trial).
Green = low risk; yellow = unclear risk; red = high risk
Table 2 presents the league table ranking of the routes of administration of acetaminophen to treat postoperative pain in children at 0–2 hours. In this network meta-analysis, the certainty of the evidence was rated as very low to low according to GRADE. In the comparison of intravenous vs rectal, evidence certainty was downgraded to three levels (up to very low certainty) because of the risk of bias, imprecision of the estimates, and heterogeneity of the results (Table 3). A funnel plot for the primary outcome for the 0–2-hour period is presented in ESM eFig. 3.
As a secondary analysis, we expanded the definition of our primary outcome to the period of 0–6 hours, resulting in the inclusion of data from one additional RCT.35 For the comparison of intravenous and rectal routes, the mean pain score in the intravenous group was zero on average (difference in means, 0; 95% CI, −0.48 to 0.48). For the comparison of oral and intravenous routes, the pain mean score for the intravenous route was on average 0.54 lower than that in the oral route (95% CI, −1.34 to −0.27) and for the comparison of oral and rectal routes, the mean pain score in the oral route was 0.53 lower than that in the rectal route (95% CI, −1.26 to 0.19) (I2 = 98.4%; 95% CI, 97.9 to 98.8) (ESM eTable 5).
Secondary outcomes
Very few RCTs reported opioid consumption after surgery,24,33,35,37 time to first analgesic request,24,28,29,30,34 and plasma acetaminophen concentrations.27,32,37 When available, outcomes were reported by different summary measures and time frames that limited pooled analysis. Patient satisfaction was reported only by one study,24 showing no differences between the oral and intravenous routes. Length of PACU stay and postoperative hospital stay were reported only by two studies.31,32 Data were reported as medians and mean differences, which limited the ability to compare these estimates. Individual studies showed no evidence of differences between the compared routes (intravenous vs oral in 24-hr opioid consumption or plasma concentration, intravenous vs oral in PACU length of stay and hospital stay, and intravenous vs rectal in PACU length of stay). Detailed information and data extraction are available in ESM eTable 3.
We performed a conventional meta-analysis for postoperative vomiting comparing oral vs rectal route that included three trials and 267 participants.8,26,27 There was no evidence of differences between these two routes (relative risk, 1.20; 95% CI, 0.81 to 1.78; I2 = 0%) (ESM eFig. 2). Finally, no studies reported pruritus. Lack of data prevented us from conducting additional analyses of secondary outcomes.
Discussion
This systematic review and network meta-analysis aimed to compare the efficacy of three routes of acetaminophen administration on the outcomes of pain and adverse effects in a postoperative pediatric population. The available evidence is very uncertain about the effect of the route of administration for acetaminophen on postoperative pain in children. Included RCTs presented significant heterogeneity mainly due to differences in participants’ ages, dosages and cointerventions, regimes for the drug, and outcome measurement strategies.
As stated in our protocol, we intended to include RCTs with participants between two and 17 yr old. Having performed a literature search, we found that the age range in a relevant number of the studies was broader and because of a lack of a sound scientific rationale behind the previous age range, we adapted our inclusion criteria to include participants between 30 days and 17 yr of age. Nevertheless, we found only 14 RCTs to answer this question, and individual trials included ages ranging from months to years. For oral administration, the overall plasma elimination of acetaminophen has been described to be slow in neonates but comparable to that of adults in both children and adolescents, as judged by half-life determinations.38 In children from two to 15 yr of age, a similar serum concentration of the drug was found when given a standard dose of propacetamol of 30 mg·kg−1 every six hours, but clearance was reduced in children less than one year of age39 consistent with immaturity of some of the metabolic pathways responsible for acetaminophen metabolism.40 Nevertheless, these insights are already translated to age- and weight-specific dosing protocols with decades of experience. Several of our included RCTs considered patients below one year of age and pharmacologic heterogeneity was cautiously considered during interpretation of our findings.
The included pediatric RCTs used a wide range of acetaminophen doses, ranging from 15–60 mg·kg−1 (oral), 10–40 mg·kg−1 (intravenous), and 15–90 mg·kg−1 (rectal). This wide range of dosages does not necessarily ensure equipotent plasma concentrations of acetaminophen within the therapeutic range for adequate analgesia. Pharmacokinetic studies suggest that there is a wide variability in bioavailability between the routes of acetaminophen reported between 11% and 91% in the oral administration and between 24% and 98% in rectal administration, with a delayed absorption relative to the oral route and peak plasma concentrations achieved from two to four hours.41 In addition, in contrast to the recommendations of the Association of Pediatric Anaesthetists of Great Britain and Ireland,42 among the included RCTs using the oral route, only Lammers et al. provided a loading dose of 30 mg·kg−1.37 None of the included RCTs using the rectal route administered a recommended loading dose (e.g., 40 mg·kg−1), and one of the included RCTs26 used a maintenance dose below the recommended one for the rectal route (20 mg·kg−1). These strong variations in dose regimens limit comparisons regarding analgesic efficacy according to the administration route.
Cointerventions are another source of heterogeneity. Five RCTs26,29,30,32,37 used dexamethasone in the perioperative period, which has an antiemetic effect; nevertheless, it has also been proposed as a component of a multimodal analgesia regimen in clinical practice guidelines,43,44 potentially modifying postoperative pain scores and the primary outcome, as stated by Afman et al.45 Two RCTs evaluating major craniofacial surgery8,33 and one in adenotonsillectomy37 used infiltration of surgical wounds with 0.25% bupivacaine as an analgesic technique. The only study evaluating cleft lip and palate surgery32 used infiltration of the surgical wound with lidocaine. Romej et al.34 used a dose of morphine at the time of anesthetic induction, and Bhandari et al.29 used pentazocine at anesthetic induction in addition to hourly administration of diclofenac in all study participants. Khalili et al.31 administered a dose of morphine to all included patients in addition to the induction dose of fentanyl used for anesthetic maintenance. Without considering the differences in postoperative analgesic management, the use of these intraoperative cointerventions likely affected pain scores, especially in the immediate postoperative period.
Finally, several instruments were used to measure the primary outcome of postoperative pain. Each corresponded to a validated scale, but they are not necessarily interchangeable, limiting our capability to pool the data and compare outcomes between RCTs. Even in RCTs considering similar ages, instruments to assess postoperative pain differ.28,29,35,36,37 The lack of standardization in the measurement of outcomes is a problem recognized and analyzed in the fields of anesthesiology and perioperative medicine. The Core Outcome Measures for Perioperative and Anaesthetic Care initiative46 stands out, as it seeks to standardize outcomes measured in clinical trials. Nevertheless, there is no clear recommendation for measurement instruments, which is one of the major sources of heterogeneity found in our study and clearly limits the ability to pool data and improve the precision of the estimates. Likewise, this strategy also highlights the importance of focusing on measuring patient-reported outcomes including hospital discharge times, incidence of nausea and vomiting, and patient satisfaction with the surgical process.
Primary outcome
With very low and low certainty, there was neither evidence of superiority nor evidence of equivalence between intravenous vs rectal routes of administration of acetaminophen, and intravenous vs oral acetaminophen, respectively. With low certainty, we found evidence favouring the oral route without clinical significance but considering the amount of clinical and statistical heterogeneity, this finding must be interpreted with caution. In addition, the inclusion of RCTs that differ in administered dosages may threaten the validity of our NMA because it may violate the assumption of transitivity.47
Powell et al.21 described that the minimum clinically significant difference in VAS for children is 10 mm. When comparing rectal vs oral routes, and across the three routes of administration, differences were small and CIs included clinical irrelevant findings. When rating confidence in the comparison of oral vs rectal routes using the GRADE approach, we downgraded the certainty of the evidence based on concerns about heterogeneity in the pooled data. We also downgraded the certainty of the evidence to very low in the comparison of intravenous vs rectal owing to concerns of bias, imprecision, and heterogeneity within the included RCTs. These findings are useful to inform the development of further RCTs.
Based on the available knowledge and the low certainty of evidence, for practical purposes, the decision to choose one route over another should be based on the clinical setting. In practice, the choice for the route of administration of acetaminophen seems much more frequently based on local preferences (including costs) than on clinical factors, scientific aspects, environmental sustainability, and the carbon footprint of anesthetic practice.48 While there are situations where the choice is easy because there is no real choice (e.g., when the enteral route is not feasible), there is a lack of decision support when more than one option is possible. Then, it is worth mentioning that Mallama et al.,10 in a systematic review conducted in adults, found that the perioperative route of acetaminophen administration (intravenous vs oral) did not affect pain or any other postoperative outcome—with insufficient evidence to exclude important clinical effects and a poor quality of the evidence overall. Nevertheless, they also reported that intravenous acetaminophen was approximately ten times more expensive than an equivalent oral dose (GBP 1.95 [approximately CAD 3.35] vs GBP 0.19 [approximately CAD 0.33]) and the selection of the route of administration should always consider this aspect, especially in this “absence of evidence” scenario. These results agree with our findings in the pediatric population when clinical indications should be strongly considered until more robust evidence is available. Finally, the intravenous route achieves more rapid analgesic plasma concentrations and could be administered as a rescue medication in the setting of breakthrough pain in the postoperative period or in the setting of tonsillectomy or appendicectomy in which the oral route might be limited or contraindicated.49
Secondary outcomes
Very few of the included RCTs reported relevant secondary outcomes and the heterogeneity between estimates made it difficult to pool the data. We were able to pool data from three RCTs8,26,27 for the outcome of postoperative vomiting. We found no evidence of differences in the incidence of vomiting between the oral and rectal administration routes. Contraindications for the use of the rectal route include immunosuppression, pre-existing rectal lesions, and recent colorectal surgery; thus, in the absence of contraindications, the rectal route could be considered. It is also important to mention that rectal administration is less expensive than intravenous acetaminophen.50
Patient-reported outcome measures (PROMs) allow for the assessment of the impact of an intervention from a patient or caregiver’s perspective, and they are often the most important outcomes for patients and families.51 Their relevance in clinical research is often underestimated and they are not frequently reported in anesthesiology. In our protocol, we aimed to evaluate patient satisfaction as a PROM; however, only one study reported parents’ satisfaction with pain control, with no evidence of a difference between the oral and intravenous routes.24 Patient-reported outcome measures can be difficult to assess, particularly in a postoperative setting (in children), where no tool is universally accepted to measure this domain. There is an urgent need for specific guidelines for using PROMs in clinical perioperative studies and for standardizing tools for collecting valid, reliable, and reproducible data in all age groups.
Limitations and differences between protocol and review
We had three major differences between our protocol and review process. First, we aimed to include children aged between two and 17 yr old in our protocol and finally we included children from three months of age; this resulted in the inclusion of four additional trials that considered children below two years old; intravenous vs oral one trial,32 intravenous vs rectal two trials,31,33 and oral vs rectal one trial.8 As stated previously, there is a paucity of evidence in this area, and we decided to expand our inclusion criteria to be conscientious about the increase in heterogeneity of the included populations and measurement methods. While we are far from providing definitive results, we decided to show a wide scope of available evidence to solve this important clinical question in a wide range of pediatric ages.
Second, we originally aimed to study and establish the time-point for administration of acetaminophen to prevent postoperative pain. Unfortunately, there was a wide heterogeneity in included trials regarding the timing of administration and overall postoperative regimen, which precluded any additional analysis. Eight trials administered the study drug during the intraoperative period,8,26,28,29,30,31,35,36 two preoperatively,34,37 one postoperatively,33 and three preoperatively and intraoperatively.24,27,32 Ultimately, our visual examination of the funnel plot for the primary outcome did not reveal evidence of publication bias. Nevertheless, because of insufficient data, we were unable to conduct additional subgroup analyses.
Our review has several limitations that should be carefully considered when interpreting our results. The heterogeneity of the included trials reduced the certainty of our conclusions from three main sources: 1) the inclusion of patients with a wide range of ages; 2) the inclusion of a wide variety of administered doses for all three routes of administration; and 3) the use of different instruments to assess pain that are not necessarily interchangeable. These findings not only prevented us from providing more robust conclusions but also reflect the limited standardization and wide variability in practice during the administration of analgesics in the pediatric population worldwide. The broad range of doses used reflects the low consensus and potentially low use of clinical practice guidelines in this area and population. It remains unclear whether equipotent doses were compared among the three different routes of administration. Finally, incomplete and poor reporting, including inappropriate summary of results in some RCTs, remains a major limitation of the current literature.
Conclusion
The available evidence is very uncertain about the effect of the route of acetaminophen administration on postoperative pain in children. The oral route may result in little to no difference when compared with the rectal route in the outcomes of postoperative pain control and postoperative vomiting. Included RCTs presented significant heterogeneity mainly due to differences in participants ages, dosages, and regimens for the drug and outcome measurement strategies. Additionally, the included RCTs showed concerns about selection and reporting bias. To answer this important clinical question, there is a need for methodologically better designed and executed RCTs that include children of different age categories, test equipotent doses of acetaminophen using validated pain assessment instruments, and explore relevant outcomes for patients and caregivers.
References
IASP Taxonomy Working Group. Pain terms: a current list with definitions and notes on usage; 1994. Available from URL: https://iaspfiles.s3.amazonaws.com/production/public/2021/Part_III-PainTerms.pdf (accessed February 2023)
Zieliński J, Morawska-Kochman M, Zatoński T. Pain assessment and management in children in the postoperative period: a review of the most commonly used postoperative pain assessment tools, new diagnostic methods and the latest guidelines for postoperative pain therapy in children. Adv Clin Exp Med 2020; 29: 365–74. https://doi.org/10.17219/acem/112600
Brasher C, Gafsous B, Dugue S, et al. Postoperative pain management in children and infants: an update. Paediatr Drugs 2014; 16: 129–40. https://doi.org/10.1007/s40272-013-0062-0
Stanko D, Bergesio R, Davies K, Hegarty M, von Ungern-Sternberg BS. Postoperative pain, nausea and vomiting following adeno-tonsillectomy—a long-term follow-up. Paediatr Anaesth 2013; 23: 690–6. https://doi.org/10.1111/pan.12170
Shastri N. Intravenous acetaminophen use in pediatrics. Pediatr Emerg Care 2015; 31: 444–8. https://doi.org/10.1097/pec.0000000000000463
Kokki H. Ketoprofen pharmacokinetics, efficacy, and tolerability in pediatric patients. Paediatr Drugs 2010; 12: 313–29. https://doi.org/10.2165/11534910-000000000-00000
Zhu C, Zhang S, Gu Z, Tong Y, Wei R. Caudal and intravenous dexamethasone as an adjuvant to pediatric caudal block: a systematic review and meta-analysis. Paediatr Anaesth 2018; 28: 195–203. https://doi.org/10.1111/pan.13338
Van Der Marel CD, Van Lingen RA, Pluim MA, et al. Analgesic efficacy of rectal versus oral acetaminophen in children after major craniofacial surgery. Clin Pharmacol Ther 2001; 70: 82–90. https://doi.org/10.1067/mcp.2001.116794
Birmingham PK, Tobin MJ, Henthorn TK, et al. Twenty-four-hour pharmacokinetics of rectal acetaminophen in children: an old drug with new recommendations. Anesthesiology 1997; 87: 244–52. https://doi.org/10.1097/00000542-199708000-00010
Mallama M, Valencia A, Rijs K, Rietdijk WJ, Klimek M, Calvache JA. A systematic review and trial sequential analysis of intravenous vs. oral peri-operative paracetamol. Anaesthesia 2021; 76: 270–6. https://doi.org/10.1111/anae.15163
Ulrich M, Chamberland M, Bertoldi C, Garcia-Bournissen F, Kleiber N. Newly approved IV acetaminophen in Canada: switching from oral to IV acetaminophen. Is IV worth the price difference? A systematic review. Paediatr Child Health 2021; 26: 337–43. https://doi.org/10.1093/pch/pxaa137
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–84. https://doi.org/10.7326/m14-2385
Pushpanathan E, Setty T, Carvalho B, Sultan P. A systematic review of postoperative pain outcome measurements utilised in regional anesthesia randomized controlled trials. Anesthesiol Res Pract 2018; 2018: 9050239. https://doi.org/10.1155/2018/9050239
Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep 2022; 71: 1–95. https://doi.org/10.15585/mmwr.rr7103a1
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. https://doi.org/10.1186/1471-2288-5-13
The Cochrane Collaboration. Review Manager (RevMan). Available from URL: https://training.cochrane.org/online-learning/core-software/revman (accessed February 2024).
Posit. Homepage. Available from URL: https://posit.co (accessed February 2024).
Rücker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods 2012; 3: 312–24. https://doi.org/10.1002/jrsm.1058
Salanti G, Giovane CD, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014; 9: e99682. https://doi.org/10.1371/journal.pone.0099682
Nikolakopoulou A, Higgins JP, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med 2020; 17: e1003082. https://doi.org/10.1371/journal.pmed.1003082
Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med 2001; 37: 28–31. https://doi.org/10.1067/mem.2001.111517
Papakonstantinou T, Nikolakopoulou, A, Higgins JP, Egger M, Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev 2020; 16: e1080. https://doi.org/10.1002/cl2.1080
Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 2015; 15: 58. https://doi.org/10.1186/s12874-015-0060-8
Aslani K, Hozella J, Han RE, Sternberg DM, Collier PJ, Soto RG. Oral versus intravenous acetaminophen for post-tonsillectomy pain in children. Reg Anesth Pain Med 2017; 42; 802–18. https://doi.org/10.1097/aap.0000000000000680
Seyedhejazie M, Moghaddam YJ, Sheikhzadeh D, Sohrabpour M, Taghizadieh N. Comparison of analgesic effects of intravenous or rectal acetaminophen for controlling post-tonsillectomy pain. Adv Biosci Clin Med 2018; 6: 5. https://doi.org/10.7575/aiac.abcmed.v.6n.2p.5
Etemadi Aleagha A, Behzadi M, Jafarieh A, Yousefi A, Hajimohamadi F. Effects of prophylactic rectal versus oral acetaminophen on postoperative conditions in pediatric adenotonsillectomy patients: a randomized clinical trial. Arch Anesthesiol Crit Care 2015; 1: 39–41.
Anderson B, Kanagasundarum S, Woollard G. Analgesic efficacy of paracetamol in children using tonsillectomy as a pain model. Anaesth Intensive Care 1996; 24: 669–73. https://doi.org/10.1177/0310057x9602400606
Elango P, Balasubramaniaguhan V, Sivakumar G, Anitha K, Srinivasan A. Comparison of intravenous and rectal paracetamol for pain relief in children undergoing tonsillectomy surgery. J Evol Med Dent Sci 2017; 6: 2845. https://doi.org/10.14260/jemds/2017/611
Bhandari G, Mitra S, Shahi KS, Rani A, Chauhan R, Satrunjay. Analgesic efficacy of intravenous versus rectal acetaminophen after adeno tonsillectomy in children. J Evol Med Dent Sci 2015; 4: 3933–9.
Capici F, Ingelmo PM, Davidson A, et al. Randomized controlled trial of duration of analgesia following intravenous or rectal acetaminophen after adenotonsillectomy in children. Br J Anaesth 2008; 100: 251–5. https://doi.org/10.1093/bja/aem377
Khalili GR, Shafa A, Yousefi R. Comparison of the effects of preemptive intravenous and rectal acetaminophen on pain management after inguinal herniorrhaphy in children: a placebo-controlled study. Middle East J Anaesthesiol 2016; 23: 543–8.
Nour C, Ratsiu J, Singh N, et al. Analgesic effectiveness of acetaminophen for primary cleft palate repair in young children: a randomized placebo controlled trial. Paediatr Anaesth 2014; 24: 574–81. https://doi.org/10.1111/pan.12393
Prins SA, Van Dijk M, Van Leeuwen P, et al. Pharmacokinetics and analgesic effects of intravenous propacetamol vs rectal paracetamol in children after major craniofacial surgery. Paediatr Anaesth 2008; 18: 582–92. https://doi.org/10.1111/j.1460-9592.2008.02619.x
Romej M, Voepel-Lewis T, Merkel SI, Reynolds PI, Quinn P. Effect of preemptive acetaminophen on postoperative pain scores and oral fluid intake in pediatric tonsillectomy patients. AANA J 1996; 64: 535–40.
Kumar U, Kainat, Kumar R, Mahenda, Khan B, Hiranand. Comparison of pain scores between intravenous versus rectal acetaminophen in children undergoing tonsillectomy. Pak J Med Health Sci 2022; 16: 324–6. https://doi.org/10.53350/pjmhs22167324
Romsing J, Moiniche S, Dahl JB. Rectal and parenteral paracetamol, and paracetamol in combination with NSAIDs, for postoperative analgesia. Br J Anaesth 2002; 88: 215–26. https://doi.org/10.1093/bja/88.2.215
Lammers CR, Schwinghammer AJ, Hall B, et al. Comparison of oral loading dose to intravenous acetaminophen in children for analgesia after tonsillectomy and adenoidectomy: a randomized clinical trial. Anesth Analg 2021; 133: 1568–76. https://doi.org/10.1213/ane.0000000000005678
Peterson RG, Rumack BH. Pharmacokinetics of acetaminophen in children. Pediatrics 1978; 62: 877–9
Anderson BJ, Pons G, Autret-Leca E, Allegaert K, Boccard E. Pediatric intravenous paracetamol (propacetamol) pharmacokinetics: a population analysis. Paediatr Anaesth 2005; 15: 282–92. https://doi.org/10.1111/j.1460-9592.2005.01455.x
Anderson BJ, Woollard GA, Holford NH. A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br J Clin Pharmacol 2000; 50: 125–34. https://doi.org/10.1046/j.1365-2125.2000.00231.x
Ward B, Alexander-Williams JM. Paracetamol revisited: a review of the pharmacokinetics and pharmacodynamics. Acute Pain 1999; 2: 139–49. https://doi.org/10.1016/S1366-0071(99)80006-0
Association of Paediatric Anaesthetists of Great Britain and Ireland. Good practice in postoperative and procedural pain management, 2nd Edition. Paediatr Anaesth 2012; 22: 1–79. https://doi.org/10.1111/j.1460-9592.2012.03838.x
Aldamluji N, Burgess A, Pogatzki-Zahn E, Raeder J, Beloeil, PROSPECT Working Group Collaborators. PROSPECT guideline for tonsillectomy: systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2021; 76: 947–61. https://doi.org/10.1111/anae.15299
Mitchell RB, Archer SM, Ishman SL, et al. Clinical practice guideline: tonsillectomy in children (update). Otolaryngol Head Neck Surg 2019; 160: S1–42. https://doi.org/10.1177/0194599818801757
Afman CE, Welge JA, Steward DL. Steroids for post-tonsillectomy pain reduction: meta-analysis of randomized controlled trials. Otolaryngol Head Neck Surg 2006; 134: 181–6. https://doi.org/10.1016/j.otohns.2005.11.010
Boney O, Moonesinghe SR, Myles PS, Grocott MP; StEP-COMPAC Group. Core outcome measures for perioperative and anaesthetic care (COMPAC): a modified Delphi process to develop a core outcome set for trials in perioperative care and anaesthesia. Br J Anaesth 2022; 128: 174–85. https://doi.org/10.1016/j.bja.2021.09.027
Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Chapter 11: undertaking network meta-analyses. In: Higgins JP, Thomas J, Chandler J, et al. (Eds.). Cochrane Handbook for Systematic Reviews of Interventions version 6.4. Available from URL: https://training.cochrane.org/handbook/current/chapter-11 (accessed February 2024).
Myo J, Pooley S, Brennan F. Oral, in place of intravenous, paracetamol as the new normal for elective cases. Anaesthesia 2021; 76: 1143–4. https://doi.org/10.1111/anae.15482
Vittinghoff M, Lönnqvist PA, Mossetti V, et al. Postoperative pain management in children: guidance from the pain committee of the European Society for Paediatric Anaesthesiology (ESPA Pain Management Ladder Initiative). Paediatr Anaesth 2018; 28: 493–506. https://doi.org/10.1111/pan.13373
Puntillo F, Giglio M, Varrassi G. the routes of administration for acute postoperative pain medication. Pain Ther 2021; 10: 909–25. https://doi.org/10.1007/s40122-021-00286-5
Johnston BC, Patrick DL, Devji T, et al. Chapter 18: patient-reported outcomes. In: Higgins JP, Thomas J, Chandler J, et al. (Eds.). Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Available from URL: https://training.cochrane.org/handbook/current/chapter-18 (accessed February 2024).
Authors’ contributions
Danilo Osorio and Diana Maldonado contributed to investigation and writing the original draft. Koen Rijs contributed to the methodology and formal analysis. Caroline van der Marel and Markus Klimek contributed to writing supervision and reviewing and editing the manuscript. Jose A. Calvache contributed to study conceptualization and methodology, writing the original draft, and supervision.
Acknowledgments
The authors wish to thank Elise Krabbendam and her team from the Erasmus MC Medical Library for developing and updating the search strategies.
Disclosures
None.
Funding statement
No external funding was received for this study. Their respective employers covered the time of the authors.
Prior conference presentations
This paper was presented at the XXXV Colombian Congress of Anesthesiology (13–15 July 2023, Cartagena, Colombia).
Editorial responsibility
This submission was handled by Dr. Vishal Uppal, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Osorio, D., Maldonado, D., Rijs, K. et al. Efficacy of different routes of acetaminophen administration for postoperative pain in children: a systematic review and network meta-analysis. Can J Anesth/J Can Anesth 71, 1103–1116 (2024). https://doi.org/10.1007/s12630-024-02760-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-024-02760-y