Abstract
Purpose
Postoperative sleep disturbances are common. Although several studies have examined the effect of melatonin on postoperative sleep disturbances, the results have not reached any definitive conclusion. We sought to conduct a systematic review to compare the effects of melatonin and melatonin agonists on postoperative sleep quality with those of placebo or no treatment in adult patients who underwent surgery under general or regional anesthesia.
Methods
We searched MEDLINE, Cochrane Central Register of Controlled Trials, Embase, Web of Science, ClinicalTrials.gov, and the UMIN Clinical Trials Registry up to 18 April 2022. Randomized clinical trials examining the effects of melatonin or melatonin agonists in patients undergoing general or regional anesthesia with sedation for any surgery were eligible for inclusion. The primary outcome was sleep quality measured using a visual analog scale (VAS). The secondary outcomes were postoperative sleep duration, sleepiness, pain, opioid consumption, quality of recovery, and adverse events. A random-effects model was used to combine the results. We assessed study quality with the Cochrane Risk of Bias Tool version 2. We applied a trial sequential analysis to assess the precision of the combined results.
Results
Eight studies (516 participants) were analyzed for sleep quality. Of those, four studies used only a short duration of melatonin, either on the night before and the day of surgery or only on the day of surgery. A random-effects meta-analysis showed that melatonin did not improve sleep quality measured by VAS compared with placebo (mean difference, -0.75 mm; 95% confidence interval, -4.86 to 3.35), with low heterogeneity (I2, 5%). Trial sequential analysis revealed that the accrued information size (n = 516) reached the estimated required information size (n = 295). We downgraded the certainty of the evidence because of the high risk of bias. The effect on postoperative adverse events was comparable between the melatonin and control groups.
Conclusion
Our results indicate that melatonin supplementation does not improve postoperative sleep quality measured with the VAS compared with placebo in adult patients (GRADE: moderate).
Study registration
PROSPERO (CRD42020180167); registered 27 October 2022.
Résumé
Objectif
Les troubles du sommeil postopératoires sont fréquents. Bien que plusieurs études aient examiné l’effet de la mélatonine sur les troubles du sommeil postopératoires, les résultats n’ont abouti à aucune conclusion définitive. Nous avons tenté de réaliser une revue systématique afin de comparer les effets de la mélatonine et des agonistes de la mélatonine sur la qualité du sommeil postopératoire à ceux d’un placebo ou de l’absence de traitement chez des patients adultes ayant bénéficié d’une intervention chirurgicale sous anesthésie générale ou régionale.
Méthode
Nous avons effectué des recherches dans les bases de données MEDLINE, le registre Cochrane des essais contrôlés, Embase, Web of Science, ClinicalTrials.gov et le registre des essais cliniques UMIN pour en tirer les manuscrits publiés jusqu’au 18 avril 2022. Les études cliniques randomisées examinant les effets de la mélatonine ou des agonistes de la mélatonine chez des patients bénéficiant d’une anesthésie générale ou régionale avec sédation pour toute intervention chirurgicale étaient éligibles pour l’inclusion. Le critère d’évaluation principal était la qualité du sommeil mesurée à l’aide d’une échelle visuelle analogique (EVA). Les critères d’évaluation secondaires étaient la durée du sommeil postopératoire, la somnolence, la douleur, la consommation d’opioïdes, la qualité de la récupération et les événements indésirables. Un modèle à effets aléatoires a été utilisé pour combiner les résultats. Nous avons évalué la qualité des études en utilisant l’outil de risque de biais de Cochrane version 2.0. Nous avons appliqué une analyse séquentielle des études pour évaluer la précision des résultats combinés.
Résultats
Huit études (516 participants) ont été analysées pour déterminer la qualité du sommeil. Parmi celles-ci, quatre études n’ont utilisé la mélatonine que pour une courte durée, c’est-à-dire soit la nuit précédant et le jour de la chirurgie, soit le jour de la chirurgie seulement. Une méta-analyse à effets aléatoires a montré que la mélatonine n’améliorait pas la qualité du sommeil mesurée par une EVA comparativement au placebo (différence moyenne, -0,75 mm; intervalle de confiance à 95 %, -4,86 à 3,35), avec une faible hétérogénéité (I2, 5 %). L’analyse séquentielle des études a révélé que la taille de l’information accumulée (n = 516) avait atteint la taille estimative de l’information requise (n = 295). Nous avons abaissé le niveau de confiance des données probantes en raison du risque élevé de biais. L’effet sur les événements indésirables postopératoires était comparable entre le groupe mélatonine et les groupes témoin.
Conclusion
Nos résultats indiquent que la supplémentation en mélatonine n’améliore pas la qualité du sommeil postopératoire mesurée avec une EVA par rapport au placebo chez les patients adultes (GRADE : modérée).
Enregistrement de l’étude
PROSPERO (CRD42020180167); enregistrée le 27 octobre 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Postoperative sleep disturbances are common. Several studies have reported its incidence as ranging from 19.5% to 70%.1,2,3,4 Although pain is one of the most important contributing factors to postoperative sleep disturbance, patients with low postoperative pain levels also suffer from poor sleep quality.5,6 Postoperative sleep disturbance is also associated with subsequent cognitive dysfunction,7 and there is expert consensus that postoperative sleep quality is one of the patient outcomes to be studied during the postoperative period.8 In response, the importance of its prevention is drawing attention.
Melatonin is a central circadian regulator, primarily produced by the pineal gland, and a normal melatonin rhythm is partly instrumental in the regulation of the sleep–wake cycle.9 The circadian rhythm of melatonin secretion is disturbed after surgery,10,11 and this disturbance is thought to contribute to postoperative sleep disturbance.12 Therefore, melatonin administration may modify the circadian rhythm and improve postoperative sleep disturbance. Furthermore, melatonin is known to have an excellent safety profile13 and has no major adverse effects, such as respiratory depression. Therefore, melatonin has been researched in nonperioperative settings such as insomnia,14 jet lag,15 and shift work.16 In the perioperative period, melatonin is reportedly effective in decreasing preoperative anxiety,17 emergence agitation,18 and postoperative delirium.19 Since ramelteon, one of the melatonin agonists, has a six-fold higher affinity for MT1 receptors and a three-fold higher affinity for MT2 receptors than that of melatonin,20,21 ramelteon may be more effective than melatonin in improving sleep quality.
Few studies investigating the preventive effect of melatonin administration on postoperative sleep quality have reached any definitive conclusion.22,23,24 Therefore, a systematic review and meta-analysis of these small studies could add value to our current knowledge of postoperative melatonin administration. Zhang et al. conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) and suggested that melatonin intervention did not significantly influence sleep quality compared with a control group.25 Nevertheless, since they limited the surgery to laparoscopic cholecystectomy and analyzed only two RCTs for sleep quality, their studies may be underpowered.
The primary purpose of this systematic review was to compare the effects of melatonin or melatonin agonists on postoperative sleep quality with those of no treatment or placebo in adult patients who underwent surgery under general or regional anesthesia. Secondarily, we sought to assess the effects on pain, opioid consumption, quality of recovery, and any adverse events.
Methods and analysis
The manuscript was prepared following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.26 The protocol for this systematic review was registered in PROSPERO on 27 October 2020, with registration number CRD42020180167 and published elsewhere.27
Eligibility criteria
We included all RCTs that tested the effect of melatonin or melatonin agonists on postoperative sleep quality in adult patients (≥ 18 yr) undergoing general or regional anesthesia with sedation for any surgery. We excluded patients who received surgical intervention under regional anesthesia without sedation. We excluded data from case reports, observational studies, reviews, and animal studies. Eligibility was not restricted by language, type of surgery, or type of anesthesia.
The intervention of interest was the perioperative (seven days before and after the date of surgery) administration of melatonin or melatonin agonists. There were no restrictions on dosing, frequency, timing, route of administration, or therapy duration. No treatment or placebo was included as the control intervention.
Information sources and search strategy
We conducted a search in MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, and Web of Science. The final search was conducted on 18 April 2022. We also searched the reference lists of relevant articles. Furthermore, we conducted a search of ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform, and the University Hospital Medical Information Network (UMIN) Clinical Trials Registry. We searched the gray literature using Web of Science. The literature search was limited to studies with human participants. The search strategy, combining free text and Medical Subject Headings terms for PubMed, is shown in Electronic Supplementary Material (ESM) eTable.
Study records
Two authors (A. T. and T. T.) independently scanned the titles and abstracts of the reports identified using the search strategies described above. When eligibility was determined based on the title or abstract, the full paper was retrieved. Potentially relevant studies chosen by at least one author were retrieved and evaluated in full-text versions. Articles that met the inclusion criteria were assessed independently by two authors, and any discrepancies were resolved through discussion.
Two authors (A. T. and T. T.) extracted data independently and in duplicate from each eligible study. The reviewers resolved disagreements through discussion. We contacted the authors of the study to resolve any uncertainties.
Outcomes and prioritization
Primary outcome
The primary outcome was sleep quality measured using a visual analog scale (VAS) (0 mm = best conceivable sleep and 100 mm = worst conceivable sleep) during the early postoperative period. We defined the early postoperative period as the period between the first postoperative night and the fourth night after surgery. If the outcomes were measured several times during the early postoperative period, we considered the mean of these values as our primary outcome. Although we defined the early postoperative period as the interval between the day after surgery and three days after surgery (i.e., between the second postoperative night and the fourth night after surgery) in the protocol,27 we changed the definition as described above because almost all included studies assessed sleep quality from the first night after surgery.
Secondary outcomes
The secondary outcomes were as follows:
-
1.
Total sleep time (minutes);
-
2.
Sleepiness during the daytime was measured using VAS, the Karolinska Sleepiness Scale (KSS),28 or the Stanford Sleepiness Scale (SSS);29
-
3.
Quality of recovery assessed by the QoR-4030 or QoR-15 measures;31
-
4.
Opioid consumption (expressed as cumulative dose [mg] of intravenous morphine or morphine equivalent);
-
5.
Pain as assessed using validated assessment tool scores, such as a VAS, numerical rating scale (NRS), and verbal categorical rating scale; and
-
6.
Any adverse events such as dizziness, desaturation event, or delayed recovery.
Risk of bias in individual studies
We assessed the risk of bias using the Cochrane Risk of Bias Tool version 2 (RoB 2) for randomized controlled trials.32 The RoB 2.0 assessment for individually randomized trials (including crossover trials) has five domains and one overall risk of bias domain, as follows:
-
1.
Bias arising from the randomization process;
-
2.
Bias due to deviations from intended interventions;
-
3.
Bias due to missing outcome data;
-
4.
Bias in the measurement of outcomes;
-
5.
Bias for selection of the reported result; and
-
6.
Overall risk of bias
The risk of bias was assessed as “low,” “some concern,” or “high” in each domain.
Data synthesis
We summarized continuous data using mean differences (MDs) or standardized mean differences (SMDs) with a 95% confidence interval (CI), and dichotomous data as risk ratio (RR) with 95% CI. If the 95% CI included a value of 0 or 1 for continuous or dichotomous data, respectively, we considered the difference not statistically significant. We contacted the original authors of the study to obtain the relevant missing data. Heterogeneity was quantified using the I2 and Cochran’s Q statistics. We considered significant heterogeneity to exist when the I2 statistic exceeded 50%. We conducted a subgroup analysis to explore the possible causes of the high heterogeneity. We used a random-effects model (DerSimonian and Laird methods33) considering clinical and methodological heterogeneity to combine the results.
We conducted a subgroup analysis according to the following predefined factors when the I2 statistic exceeded 50%: 1) type of anesthesia (regional anesthesia, inhaled general anesthesia, or total intravenous general anesthesia); 2) type of surgery; 3) timing of surgery (daytime vs nighttime); 4) drug type (melatonin vs melatonin agonists); 5) dose of melatonin/melatonin agonists; 6) age; or 7) type of control (placebo vs no treatment). Subgroup analyses were not performed if there were fewer than three studies. Sensitivity analysis excluding studies with a high risk of bias was performed for the primary outcome.
Trial sequential analysis (TSA) was performed to correct for random error and repetitive testing of accumulating and sparse data34 using TSA viewer version 0.9.5.10 β (www.ctu.dk/tsa).35 The risk of type 1 error was maintained at 5%, with a power of 90%. We considered a 10-mm reduction in MD for the primary outcome clinically meaningful. We used diversity (D2) as an estimator of heterogeneity for the required information size calculation.36 We assumed D2 is 30% or a model variance-based value if it is higher than 30%. If the cumulative z-curve did not cross the TSA monitoring boundaries, we downgraded the certainty of the evidence owing to imprecision in the results.
Statistical analyses were performed using R software, version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria), and the “meta” package was used to perform the meta-analysis.
Reporting bias and publication bias
To determine whether reporting bias was present, we determined whether the RCT protocol was published before patients were recruited for the study. For studies published after July 1, 2005, we screened the Clinical Trial Register at ClinicalTrials.gov (https://clinicaltrials.gov/), the WHO International Clinical Trials Registry Platform (https://www.who.int/clinical-trials-registry-platform), and the UMIN Clinical Trials Registry (https://www.umin.ac.jp/ctr/). We evaluated whether selective reporting of outcomes was present (outcome reporting bias) by comparing the outcomes mentioned in the published study protocol or trial registry with the outcomes reported in the paper. The small study effect was assessed using a funnel plot and Egger’s regression asymmetry test37 and was considered positive if P < 0.1 in the regression asymmetry test.
Summary of evidence
We graded the certainty of the evidence of the main outcomes using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach38,39 with the GRADEpro guideline development tool (https://gradepro.org/). The certainty of the evidence was judged based on the presence or absence of the following variables: limitations in the study design, inconsistency, indirectness, imprecision of the results, and publication bias. The certainty of the evidence for the main outcomes was graded as very low, low, moderate, or high.
Results
Search selection and study characteristics
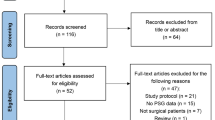
In the initial search of the databases, 2,526 articles were identified. We examined the full texts of 40 articles in detail. Of these, 19 trials were included in this systematic review, and eight trials with 516 patients were included for quantitative synthesis of sleep quality. The PRISMA flow diagram detailing the disposition of the retrieved publications is shown in Fig. 1. The features of the randomized studies included in this meta-analysis are listed in Table 1. Oral or sublingual melatonin or ramelteon was used in 17 trials, intravenous melatonin was used in one trial, and a dermal melatonin patch was used in one trial. The doses of oral melatonin or ramelteon ranged from 5.0 to 12.0 mg, and 13 of the 19 studies used only a short duration of melatonin administration. Of these, six studies investigated melatonin administration on the night before and on the day of surgery, and seven studies assessed melatonin use during the day of the surgery, before surgery only (i.e., lack of nighttime dosing). The type of control was placebo except for one study where there was no control. To date, there have been no studies on regional anesthesia under sedation.
Sleep quality assessed by VAS
Eight trials evaluated postoperative sleep quality using the VAS.23,24,40,41,42,43,44,45 The time point of assessment of sleep quality varied between the included studies. The combined results are shown in Fig. 2. Melatonin did not improve VAS scores compared with placebo (MD, -0.75 mm; 95% CI, -4.86 to 3.35; I2, 5%; Cochran’s Q, 7.38). The CI was corrected to -5.46 to 3.97 by TSA. Trial sequential analysis revealed that the accrued information size (n = 516) reached the estimated required information size (n = 295) (ESM eFig. 1). We considered three trials to have some concerns of risk of bias, whereas five were at a high risk of bias (Fig. 3). Sensitivity analyses that removed studies with a high risk of bias showed consistent results with the primary meta-analyses (MD, -0.37 mm; 95% CI, -8.60 to 7.87, I2, 0%; Cochran’s Q, 0.14; ESM eFig. 2). We did not conduct a subgroup analysis because the I2 statistic did not exceed 50%. We did not conduct an asymmetry test for the funnel plot because only eight trials were included. The overall GRADE certainty of the evidence was rated moderate (Table 2). The evidence was downgraded for the risk of bias.
Sleep time
Three trials41,44,45 evaluated perioperative sleep time on the preoperative night and on the first to third postoperative nights by actigraphy. Postoperative sleep time was defined as the difference from the preoperative sleep time and changed to a mean value of three days. The combined results are shown in ESM eFig. 3. Melatonin and ramelteon did not improve sleep time compared with placebo (MD, 30.2 min; 95% CI, -1.7 to 62.0; I2, 47.5%; Cochran’s Q, 3.81). We considered all trials to be at high risk of bias (ESM eFig. 4). The CI was corrected to -30.81 to 91.10 by TSA. The TSA showed that the estimated required information size was 382; however, the accrued information size was only 125 (32.7%). The z-curve did not cross the TSA monitoring boundary or reach the required information size (ESM eFig. 5). This indicates that sufficient data were not accumulated to conclusively determine whether melatonin improves sleep duration. The overall GRADE certainty of the evidence was rated very low (Table 2). The evidence was downgraded for the risk of bias, inconsistency, and imprecision.
Sleepiness
Five trials evaluated postoperative sleepiness using the VAS,45,46 KSS,40,44 and SSS.41 We did not combine these results because the measurements differed in nature; the VAS score measures only sleepiness whereas the KSS and the SSS contain several conditions (alert/vital/active, relaxed, and sleepy) that are not always considered sequential. Moreover, the absolute scores for the relaxation state considered the best condition differed between the KSS and the SSS. In studies measuring sleepiness with KSS, the MD was 0.00 (95% CI, -2.70 to 2.70)43 and -0.78 (95% CI, -4.51 to 2.95).40 In studies measuring sleepiness with VAS, the MD was 0.51 (95% CI, -1.16 to 2.18)46 and 0.56 (95% CI, -0.24 to 1.36).45 In a study measuring sleepiness with SSS,41 the MD was 0.00 (95% CI, -0.39 to 0.39) (ESM eFig. 6). We considered three studies to be at a high risk of bias and two studies to be at some concerns of risk of bias (ESM eFig. 7).
Pain
Fifteen trials evaluated postoperative pain using a VAS23,24,41,42,43,44,45,46,47,48,49,50,51 and NRS.52,53 Each score until postoperative day 3 was converted to the mean value. We considered an NRS score (measured from 0 to 10) as equivalent to the VAS, and synthesized a ten-fold value of the NRS with the VAS. The combined results are presented in Fig. 4. Melatonin improved pain compared with placebo (MD, -6.89 mm; 95% CI, -11.50 to -2.28; I2, 86%; Cochran’s Q, 103.32). We considered seven, six, and two studies to be at high risk of bias, some concerns of risk of bias, and low risk of bias, respectively (ESM eFig. 8). The CI was corrected to -11.89 -1.88 by TSA. Trial sequential analysis revealed that the accrued information size (n = 962) reached the estimated required information size (n = 562) (ESM eFig. 9). The overall GRADE certainty of the evidence was rated very low (Table 2). In post hoc analysis, we performed subgroup analyses according to the 1) type of anesthesia (inhaled vs total intravenous general anesthesia), 2) type of surgery (major vs minor surgery), and 3) age (> 50 vs < 50 yr), which were preplanned for the subgroup analyses of the primary outcome. The interaction P values were 0.82, 0.10, and 0.43 for the type of anesthesia, type of surgery, and age, respectively.
Opioid consumption
Nine trials23,24,40,41,46,47,49,50,54 evaluated postoperative opioid use. One study40 was excluded from the analysis because it had no opioid use in the control group. We recalculated accumulated morphine consumption. The combined results are shown in ESM eFig. 10. Melatonin and ramelteon reduced opioid consumption compared with placebo (SMD, -3.25 mg; 95% CI, -4.50 to -2.01; I2, 97%; Cochran’s Q, 221.40). We considered 3, 5, and 1 as high, some concern, and low risk of bias, respectively (ESM eFig. 11). Trial sequential analysis showed that the accrued information size (n = 550) reached only 2.1% of the estimated required information size; thus, we could not calculate the TSA-adjusted CI. The z-curve did not cross the TSA monitoring boundary or reach the required information size (ESM eFig. 12). The overall GRADE certainty of the evidence was rated very low (Table 2).
Quality of recovery
Three trials evaluated the postoperative quality of recovery by the QoR-4041,55 or QoR-15 measures.43 The combined results are shown in ESM eFig. 13. Melatonin did not improve postoperative quality of recovery compared with placebo (SMD, 0.26; 95% CI, -0.15 to 0.67; I2, 25%; Cochran’s Q, 2.66). All trials were considered to have a high risk of bias (ESM eFig. 14). The CI was corrected to -0.78 to 1.31 by TSA. The TSA showed that the estimated required information size was 667; however, the accrued information size was only 126 (18.9%). The z-curve did not cross the TSA monitoring boundary or reach the required information size (ESM eFig. 15). The overall GRADE certainty of the evidence was rated very low (Table 2).
Adverse events and other outcomes
Six trials evaluated postoperative nausea and vomiting (PONV) using VAS,46,47,50 NRS,48 or a dichotomous variable.23,41 The results of one study50 were not used for the synthesis because the original data were not available. Visual analog scale scores ranged from 0 (absence of symptoms) to 10 (maximum symptoms). Melatonin did not affect the incidence and severity of PONV (RR, 1.20; 95% CI, 0.63 to 2.28; I2, 45%; Cochran’s Q, 1.82 and MD, -1.91 mm; 95% CI, -4.84 to 1.02; I2, 0%; Cochran’s Q, 0.03) compared with placebo (ESM eFigs 16 and 17).
Three trials23,41,56 evaluated dizziness; the combined results are shown in ESM eFig. 18. Melatonin did not affect the incidence of dizziness compared with placebo (RR, 0.78; 95% CI, 0.60 to 1.02; I2, 0%; Cochran’s Q, 1.91).
Two trials23,56 evaluated headaches; the combined results are shown in ESM eFig. 19. Melatonin did not affect the incidence of headaches compared with placebo (RR, 1.21; 95% CI, 0.78 to 1.88; I2, 0%; Cochran’s Q, 0.16).
Included studies did not report any other secondary outcomes preplanned in the published protocol.27
Discussion
Our systematic review’s results indicate that melatonin supplementation does not improve postoperative sleep quality measured with the VAS compared with placebo in adult patients (GRADE: moderate). Trial sequential analysis indicated sufficient precision for the conclusion, but we downgraded the certainty of evidence because of the high risk of bias. Furthermore, melatonin may not improve sleep time, daytime sleepiness, or quality of recovery (GRADE: very low). Nevertheless, melatonin may reduce postoperative pain scores (GRADE: very low) and opioid consumption (GRADE: very low).
Compared with placebo, moderate evidence indicates that melatonin has no positive effect on postoperative sleep disturbance in adult patients. In the CI of the present results, the minimum and maximum values were less than the clinically minimally important difference (10 mm of VAS), indicating reasonably strong evidence. There are several possible reasons for the finding that melatonin did not improve postoperative sleep disturbance. First, the doses of melatonin intervention ranged from 5 to 10 mg, which may be inadequate for improving postoperative sleep disturbances. In healthy volunteers, the same dose mediates the circadian rhythm and improves sleep disturbances.57,58 In contrast, this dose might be insufficient to shift the circadian rhythm in the perioperative period. The effects of melatonin, such as circadian phase-shifting and hypnotic effects,59 have also been reported to be dose-dependent. Therefore, a higher dose might mediate circadian rhythm and improve sleep disturbances. Second, the duration of melatonin administration was short in four of eight studies, only during the day of the surgery before surgery40,42 or the day before surgery and during the day of surgery before surgery.24,43 The half-life of both oral and intravenous melatonin is 45 min;60 therefore, melatonin administration before surgery only might not affect postoperative sleep disturbances. Although four studies23,41,44,45 administering melatonin in the postoperative period might positively affect sleep disturbance, we could not interpret its effect from the forest plot (Fig. 2). Third, even if the dose or duration is sufficient and melatonin shifts the circadian rhythm, it might not have a clinical impact on postoperative sleep disturbances. A prior systematic review found that melatonin or ramelteon compared with placebo was not efficacious for insomnia disorders.14 Fourth, factors other than circadian rhythm may be related to postoperative sleep disturbances. Natural sleep has been recognized to be controlled by the combined actions of two different but related mechanisms: the sleep homeostat and the circadian rhythm.61 Even if melatonin improves disturbed circadian rhythm, postoperative sleep disturbance may remain because of disturbed sleep homeostasis. Slow-wave sleep during nonrapid eye movement is a classical marker of sleep homeostatic processes. Several factors arising from perioperative periods, such as surgical endocrine62,63 and cytokine response,64,65 anesthesia,66 and opioids,67 disturb rapid eye movement sleep and slow-wave sleep. Moreover, daytime surgery might disturb sleep homeostasis more significantly and lead to sleep disturbance than nighttime surgery might.
A prior systematic review,25 which included RCTs on laparoscopic cholecystectomy, synthesized only two RCTs and found that melatonin had no substantial effects; the present results appear to be largely in line with this small study. Because the present study did not restrict the type of surgery and synthesized more studies, our results can be adapted to larger populations with more robust evidence than the previous systematic review.
Melatonin decreased postoperative pain scores, which was in line with a prior study.68 Previous studies suggested that melatonin may mediate antinociceptive antihyperalgesia through central69 and peripheral70 effects. Melatonin may also affect the opioid and gamma-aminobutyric acid systems.70,71,72 Therefore, the present finding that melatonin decreased postoperative pain scores is biologically plausible. To clarify the reason for high heterogeneity, we conducted post hoc subgroup analyses, but could not determine any possible reasons for the high heterogeneity. Moreover, the difference in VAS scores was associated with very low certainty of evidence. Therefore, our study could not conclude that melatonin was clinically effective at improving postoperative pain.
The results of sleep time were in line with the results of the primary outcome. Although there were no statistically significant differences in sleep time, the TSA result indicates that further studies are needed. Melatonin might be biologically effective in reducing sleep disturbance and improving sleep time (MD, 30 min; 95% CI, -1.7 to 62.0), but this might not be enough to improve the patient's subjective assessment of sleep quality as measured by the VAS. Postoperative sleepiness and quality of recovery are concepts that encompass sleep disturbances. Although we did not combine the results of the studies assessing sleepiness, individual studies seemed to have no positive effect on sleepiness. Melatonin did not improve the quality of recovery, which was similar to the primary outcome. Nevertheless, all secondary outcomes had a very low level of evidence, and it was difficult to obtain plausible imprecation.
The present study has several limitations. First, many studies had a high risk of bias. The reasons for the high risk of bias are as follows: 1) An intention-to-treat analysis was not performed; 2) the number of missing outcomes was significant and it could not be considered that “missing” was completely at random; and 3) there was no protocol, and it was difficult to determine whether the reported results were selected. If patients with low melatonin efficacy were missing or if the authors failed to report unfavorable results, the direction of bias would favor melatonin. Nevertheless, our systematic review concluded that melatonin had no effect. Moreover, we did not detect inconsistency, indirectness, imprecision, or publication bias. Therefore, we downgraded the overall GRADE certainty of the evidence by only one level and rated it as moderate for the primary outcome. Second, melatonin was the only intervention drug among the included studies that assessed sleep quality. Since ramelteon has a six-fold higher affinity for MT1 receptors and a three-fold higher affinity for MT2 receptors than melatonin does,20,21 ramelteon may be more effective than melatonin in improving sleep quality. Therefore, our results cannot be extrapolated to melatonin agonists, and further RCTs are needed to know the effects of these drugs.
In conclusion, the findings from this systematic review indicate that melatonin supplementation does not reduce sleep disturbances after general anesthesia but may reduce postoperative pain scores and opioid consumption. Further RCTs with a low risk of bias are needed to assess the effect of melatonin or melatonin agonists on postoperative sleep disturbances.
References
Hou H, Wu S, Qiu Y, Song F, Deng L. The effects of morning/afternoon surgeries on the early postoperative sleep quality of patients undergoing general anesthesia. BMC Anesthesiol 2022; 22: 286. https://doi.org/10.1186/s12871-022-01828-w
Wang SJ, Shen SY, Lin B, Wang F, Yang HY. Factors affecting postoperative sleep quality of patients undergoing flap transfer for head and neck reconstruction. Oral Oncol 2022; 127: 105804. https://doi.org/10.1016/j.oraloncology.2022.105804
Duan G, Wang K, Peng T, Wu Z, Li H. The effects of intraoperative dexmedetomidine use and its different dose on postoperative sleep disturbance in patients who have undergone non-cardiac major surgery: a real-world cohort study. Nat Sci Sleep 2020; 12: 209–19. https://doi.org/10.2147/nss.s239706
Cai J, Chen Y, Hao X, et al. Effect of intraoperative dexmedetomidine dose on postoperative first night sleep quality in elderly surgery patients: a retrospective study with propensity score-matched analysis. Front Med (Lausanne) 2020; 7: 528. https://doi.org/10.3389/fmed.2020.00528
Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Postoperative sleep disturbance: influences of opioids and pain in humans. Sleep 2001; 24: 39–44. https://doi.org/10.1093/sleep/24.1.39
Kain ZN, Caldwell-Andrews AA. Sleeping characteristics of adults undergoing outpatient elective surgery: a cohort study. J Clin Anesth 2003; 15: 505–9. https://doi.org/10.1016/j.jclinane.2003.02.002
Gögenur I, Middleton B, Burgdorf S, Rasmussen LS, Skene DJ, Rosenberg J. Impact of sleep and circadian disturbances in urinary 6-sulphatoxymelatonin levels, on cognitive function after major surgery. J Pineal Res 2007; 43: 179–84. https://doi.org/10.1111/j.1600-079x.2007.00460.x
Myles PS, Boney O, Botti M, et al. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: patient comfort. Br J Anaesth 2018; 120: 705–11. https://doi.org/10.1016/j.bja.2017.12.037
Shochat T, Haimov I, Lavie P. Melatonin--the key to the gate of sleep. Ann Med 1998; 30: 109–14. https://doi.org/10.3109/07853899808999392
Mihara T, Kikuchi T, Kamiya Y, et al. Day or night administration of ketamine and pentobarbital differentially affect circadian rhythms of pineal melatonin secretion and locomotor activity in rats. Anesth Analg 2012; 115: 805–13. https://doi.org/10.1213/ane.0b013e3182632bcb
Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Melatonin secretion after surgery. Lancet 2000; 356: 1244–5. https://doi.org/10.1016/s0140-6736(00)02795-1
Leardi S, Tavone E, Cianca G, et al. The role of melatonin in the immediate postoperative period in elderly patients [Italian]. Minerva Chir 2000; 55: 745–50.
Yousaf F, Seet E, Venkatraghavan L, Abrishami A, Chung F. Efficacy and safety of melatonin as an anxiolytic and analgesic in the perioperative period: a qualitative systematic review of randomized trials. Anesthesiology 2010; 113: 968–76. https://doi.org/10.1097/aln.0b013e3181e7d626
De Crescenzo F, D'Alò GL, Ostinelli EG, et al. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis. Lancet 2022; 400: 170–84. https://doi.org/10.1016/s0140-6736(22)00878-9
Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev 2002; https://doi.org/10.1002/14651858.cd001520
Liira J, Verbeek JH, Costa G, et al. Pharmacological interventions for sleepiness and sleep disturbances caused by shift work. Cochrane Database Syst Rev 2014; https://doi.org/10.1002/14651858.cd009776.pub2
Madsen BK, Zetner D, Møller AM, Rosenberg J. Melatonin for preoperative and postoperative anxiety in adults. Cochrane Database Syst Rev 2020; 12: CD009861. https://doi.org/10.1002/14651858.cd009861.pub3
Mihara T, Nakamura N, Ka K, Oba MS, Goto T. Effects of melatonin premedication to prevent emergence agitation after general anaesthesia in children: a systematic review and meta-analysis with trial sequential analysis. Eur J Anaesthesiol 2015; 32: 862–71. https://doi.org/10.1097/eja.0000000000000323
Han Y, Wu J, Qin Z, et al. Melatonin and its analogues for the prevention of postoperative delirium: a systematic review and meta-analysis. J Pineal Res 2020; 68: e12644. https://doi.org/10.1111/jpi.12644
Miyamoto M. Pharmacology of ramelteon, a selective MT1/MT2 receptor agonist: a novel therapeutic drug for sleep disorders. CNS Neurosci Ther 2009; 15: 32–51. https://doi.org/10.1111/j.1755-5949.2008.00066.x
Hardeland R. Melatonin in aging and disease - multiple consequences of reduced secretion, options and limits of treatment. Aging Dis 2012; 3: 194–225.
Hansen MV, Madsen MT, Andersen LT, et al. Effect of melatonin on cognitive function and sleep in relation to breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Int J Breast Cancer 2014; 2014: 416531. https://doi.org/10.1155/2014/416531
Gögenur I, Kücükakin B, Bisgaard T, et al. The effect of melatonin on sleep quality after laparoscopic cholecystectomy: a randomized, placebo-controlled trial. Anesth Analg 2009; 108: 1152–6. https://doi.org/10.1213/ane.0b013e31819a6cf0
Borazan H, Tuncer S, Yalcin N, Erol A, Otelcioglu S. Effects of preoperative oral melatonin medication on postoperative analgesia, sleep quality, and sedation in patients undergoing elective prostatectomy: a randomized clinical trial. J Anesth 2010; 24: 155–60. https://doi.org/10.1007/s00540-010-0891-8
Zhang J, Wang Y, Xu H, Yang J. The influence of melatonin on sleep quality after laparoscopic cholecystectomy: a meta-analysis of randomized controlled trials. Surg Laparosc Endosc Percutan Tech 2019; 29: 1–6. https://doi.org/10.1097/sle.0000000000000601
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. https://doi.org/10.1371/journal.pmed.1000097
Tsukinaga A, Mihara T, Takeshima T, Tomita M, Goto T, Yamanaka T. Effect of melatonin and melatonin agonists on postoperative sleep quality in adult patients: a protocol for systematic review and meta-analysis with trial sequential analysis. BMJ Open 2021; 11: e047858. https://doi.org/10.1136/bmjopen-2020-047858
Kaida K, Takahashi M, Akerstedt T, et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol 2006; 117: 1574–81. https://doi.org/10.1016/j.clinph.2006.03.011
Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology 1973; 10: 431–6. https://doi.org/10.1111/j.1469-8986.1973.tb00801.x
Myles PS, Weitkamp B, Jones K, Melick J, Hensen S. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth 2000; 84: 11–5. https://doi.org/10.1093/oxfordjournals.bja.a013366
Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology 2013; 118: 1332–40. https://doi.org/10.1097/aln.0b013e318289b84b
Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: I4898. https://doi.org/10.1136/bmj.l4898
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. https://doi.org/10.1016/0197-2456(86)90046-2
Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA), 2011. Available from URL: https://ctu.dk/wp-content/uploads/2021/03/2017-10-10-TSA-Manual-ENG_ER.pdf (accessed January 2023).
Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008; 61: 64–75. https://doi.org/10.1016/j.jclinepi.2007.03.013
Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 2009; 9: 86. https://doi.org/10.1186/1471-2288-9-86
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. https://doi.org/10.1136/bmj.315.7109.629
Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490. https://doi.org/10.1136/bmj.328.7454.1490
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–6. https://doi.org/10.1136/bmj.39489.470347.ad
Andersen LP, Kücükakin B, Werner MU, Rosenberg J, Gögenur I. Absence of analgesic effect of intravenous melatonin administration during daytime after laparoscopic cholecystectomy: a randomized trial. J Clin Anesth 2014; 26: 545–50. https://doi.org/10.1016/j.jclinane.2014.03.008
Kirksey MA, Yoo D, Danninger T, Stundner O, Ma Y, Memtsoudis SG. Impact of melatonin on sleep and pain after total knee arthroplasty under regional anesthesia with sedation: a double-blind, randomized, placebo-controlled pilot study. J Arthroplasty 2015; 30: 2370–5. https://doi.org/10.1016/j.arth.2015.06.034
Seet E, Liaw CM, Tay S, Su C. Melatonin premedication versus placebo in wisdom teeth extraction: a randomised controlled trial. Singapore Med J 2015; 56: 666–71. https://doi.org/10.11622/smedj.2015186
Ivry M, Goitein D, Welly W, Berkenstadt H. Melatonin premedication improves quality of recovery following bariatric surgery – a double blind placebo controlled prospective study. Surg Obes Relat Dis 2017; 13: 502–6. https://doi.org/10.1016/j.soard.2016.11.001
Madsen MT, Hansen MV, Andersen LT, et al. Effect of melatonin on sleep in the perioperative period after breast cancer surgery: a randomized, double-blind, placebo-controlled trial. J Clin Sleep Med 2016; 12: 225–33. https://doi.org/10.5664/jcsm.5490
Vij V, Dahiya D, Kaman L, Behera A. Efficacy of melatonin on sleep quality after laparoscopic cholecystectomy. Indian J Pharmacol 2018; 50: 236–41. https://doi.org/10.4103/ijp.ijp_250_18
Caumo W, Torres F, Moreira NL Jr, et al. The clinical impact of preoperative melatonin on postoperative outcomes in patients undergoing abdominal hysterectomy. Anesth Analg 2007; 105: 1263–71. https://doi.org/10.1213/01.ane.0000282834.78456.90
Caumo W, Levandovski R, Hidalgo MP. Preoperative anxiolytic effect of melatonin and clonidine on postoperative pain and morphine consumption in patients undergoing abdominal hysterectomy: a double-blind, randomized, placebo-controlled study. J Pain 2009; 10: 100–8. https://doi.org/10.1016/j.jpain.2008.08.007
Hosseini VS, Yekta RA, Marashi S, Marashi SM. The efficacy of melatonin, clonidine and gabapentin in reducing preoperative anxiety and postoperative pain in patients undergoing laparoscopic cholecystectomy: a randomized clinical trial. AACC 2015; 1: 120–5
Esmat IM, Kassim DY. Comparative study between transdermal nicotine and melatonin patches on postoperative pain relief after laparoscopic cholecystectomy, a double-blind, placebo-controlled trial. Egypt J Anaesth 2016; 32: 299–307. https://doi.org/10.1016/j.egja.2016.05.002
Tunay DL, Ilgınel MT, Ünlügenç H, Tunay M, Karacaer F, Biricik E. Comparison of the effects of preoperative melatonin or vitamin C administration on postoperative analgesia. Bosn J Basic Med Sci 2020; 20: 117–24. https://doi.org/10.17305/bjbms.2019.4379
de Carvalho Nogueira EF, de Carvalho Melo V, Catunda IS, Afonso Ferreira JC, de Aguiar Soares Carneiro SC, do Egito Vasconcelos BC. Evaluation of the effects of exogenous melatonin in zygomatic complex fractures. J Maxillofac Oral Surg 2022; 21: 923–8. https://doi.org/10.1007/s12663-021-01568-3
Lotfy M, Ayaad M. Preoperative oral melatonin can reduce preoperative anxiety and postoperative analgesia in a dose-dependent manner. Ain-Shams J Anesthesiol 2021; 13: 32. https://doi.org/10.1186/s42077-021-00146-6
Capuzzo M, Zanardi B, Schiffino E, et al. Melatonin does not reduce anxiety more than placebo in the elderly undergoing surgery. Anesth Analg 2006; 103: 121–3. https://doi.org/10.1213/01.ane.0000222476.62547.ed
Jaiswal SJ, Vyas AD, Heisel AJ, et al. Ramelteon for prevention of postoperative delirium: a randomized controlled trial in patients undergoing elective pulmonary thromboendarterectomy. Crit Care Med 2019; 47: 1751–8. https://doi.org/10.1097/ccm.0000000000004004
Yamaguchi Y, Mihara T, Taguri M, Yamaguchi O, Goto T. Melatonin receptor agonist for the prevention of postoperative delirium in elderly patients: a randomized, double-blind, placebo-controlled trial. Int Care Med 2014; 40: S246.
Barati S, Jahangirifard A, Ahmadi ZH, Tavakoli-Ardakani M, Dastan F. The effects of melatonin on the oxidative stress and duration of atrial fibrillation after coronary artery bypass graft surgery: a randomized controlled trial. Endocr Metab Immune Disord Drug Targets 2021; 21: 1142–9. https://doi.org/10.2174/1871530320666200728152307
Deacon S, Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res 1995; 688: 77–85. https://doi.org/10.1016/0006-8993(95)96872-i
Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol Regul Integr Comp Physiol 2002; 282: R454–63. https://doi.org/10.1152/ajpregu.00135.2001
Naguib M, Hammond DL, Schmid PG 3rd, et al. Pharmacological effects of intravenous melatonin: comparative studies with thiopental and propofol. Br J Anaesth 2003; 90: 504–7. https://doi.org/10.1093/bja/aeg092
Harpsøe NG, Andersen LP, Gögenur I, Rosenberg J. Clinical pharmacokinetics of melatonin: a systematic review. Eur J Clin Pharmacol 2015; 71: 901–9. https://doi.org/10.1007/s00228-015-1873-4
Dijk DJ, Lockley SW. Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol 2002; 92: 852–62. https://doi.org/10.1152/japplphysiol.00924.2001
Friess E, U VB, Wiedemann K, Lauer CJ, Holsboer F. Effects of pulsatile cortisol infusion on sleep-EEG and nocturnal growth hormone release in healthy men. J Sleep Res 1994; 3: 73–9. https://doi.org/10.1111/j.1365-2869.1994.tb00110.x
Bohlhalter S, Murck H, Holsboer F, Steiger A. Cortisol enhances non-REM sleep and growth hormone secretion in elderly subjects. Neurobiol Aging 1997; 18: 423–9. https://doi.org/10.1016/s0197-4580(97)00036-5
Høgevold HE, Lyberg T, Kähler H, Haug E, Reikerås O. Changes in plasma IL-1beta, TNF-alpha and IL-6 after total hip replacement surgery in general or regional anaesthesia. Cytokine 2000; 12: 1156–9. https://doi.org/10.1006/cyto.2000.0675
Späth-Schwalbe E, Hansen K, Schmidt F, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab 1998; 83: 1573–9. https://doi.org/10.1210/jcem.83.5.4795
Lehmkuhl P, Prass D, Pichlmayr I. General anesthesia and postnarcotic sleep disorders. Neuropsychobiology 1987; 18: 37–42. https://doi.org/10.1159/000118390
Kay DC, Eisenstein RB, Jasinski DR. Morphine effects on human REM state, waking state and NREM sleep. Psychopharmacologia 1969; 14: 404–16. https://doi.org/10.1007/bf00403581
Oh SN, Myung SK, Jho HJ. Analgesic efficacy of melatonin: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Clin Med 2020; 9: 1553 https://doi.org/10.3390/jcm9051553
Dubocovich ML. Melatonin receptors in the central nervous system. Adv Exp Med Biol 1991; 294: 255–65. https://doi.org/10.1007/978-1-4684-5952-4_23
Ambriz-Tututi M, Rocha-González HI, Cruz SL, Granados-Soto V. Melatonin: a hormone that modulates pain. Life Sci 2009; 84: 489–98. https://doi.org/10.1016/j.lfs.2009.01.024
Mantovani M, Kaster MP, Pertile R, Calixto JB, Rodrigues AL, Santos AR. Mechanisms involved in the antinociception caused by melatonin in mice. J Pineal Res 2006; 41: 382–9. https://doi.org/10.1111/j.1600-079x.2006.00380.x
Golombek DA, Escolar E, Burin LJ, De Brito Sánchez MG, Cardinali DP. Time-dependent melatonin analgesia in mice: inhibition by opiate or benzodiazepine antagonism. Eur J Pharmacol 1991; 194: 25–30. https://doi.org/10.1016/0014-2999(91)90119-b
Author contributions
Akito Tsukinaga and Takahiro Mihara were involved in the study design, data analysis, data interpretation, and manuscript drafting. Teppei Takeshima was involved in data analysis and data interpretation, critically revised the report, and commented on drafts of the manuscript. Makoto Tomita and Takahisa Goto were involved in study design and data interpretation, critically revised the report, and commented on the drafts of the manuscript.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Disclosures
None.
Funding statement
This work was supported by Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science, Grant Number 20K09201.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsukinaga, A., Mihara, T., Takeshima, T. et al. Effects of melatonin on postoperative sleep quality: a systematic review, meta-analysis, and trial sequential analysis. Can J Anesth/J Can Anesth 70, 901–914 (2023). https://doi.org/10.1007/s12630-023-02442-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02442-1