Abstract
Purpose
There is lack of consensus regarding the minimum arterial pulse pressure required for confirming permanent cessation of circulation for death determination by circulatory criteria in organ donors. We assessed direct and indirect evidence supporting whether one should use an arterial pulse pressure of 0 mm Hg vs more than 0 (5, 10, 20, 40) mm Hg to confirm permanent cessation of circulation.
Source
We conducted this systematic review as part of a larger project to develop a clinical practice guideline for death determination by circulatory or neurologic criteria. We systematically searched Ovid MEDLINE, Ovid Embase, Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Library, and Web of Science for articles published from inception until August 2021. We included all types of peer-reviewed original research publications related to arterial pulse pressure as monitored by an indwelling arterial pressure transducer around circulatory arrest or determination of death with either direct context-specific (organ donation) or indirect (outside of organ donation context) data.
Principal findings
A total of 3,289 abstracts were identified and screened for eligibility. Fourteen studies were included; three from personal libraries. Five studies were of sufficient quality for inclusion in the evidence profile for the clinical practice guideline. One study measured cessation of cortical scalp electroencephalogram (EEG) activity after withdrawal of life-sustaining measures and showed that EEG activity fell below 2 μV when the pulse pressure reached 8 mm Hg. This indirect evidence suggests there is a possibility of persistent cerebral activity at arterial pulse pressures > 5 mm Hg.
Conclusion
Indirect evidence suggests that clinicians may incorrectly diagnose death by circulatory criteria if they apply any arterial pulse pressure threshold of greater than 5 mm Hg. Moreover, there is insufficient evidence to determine that any pulse pressure threshold greater than 0 and less than 5 can safely determine circulatory death.
Study registration
PROSPERO (CRD42021275763); first submitted 28 August 2021.
Résumé
Objectif
Il n’y a pas de consensus concernant la pression artérielle minimale requise pour confirmer l’arrêt permanent de la circulation pour la détermination du décès selon des critères circulatoires chez les donneurs d’organes. Nous avons évalué les données probantes directes et indirectes soutenant l’utilisation d’une pression pulsée artérielle de 0 mmHg vs plus de 0 (5, 10, 20, 40) mm Hg pour confirmer l’arrêt définitif de la circulation.
Sources
Nous avons réalisé cette revue systématique dans le cadre d’un projet plus vaste visant à élaborer des lignes directrices de pratique clinique pour la détermination du décès selon des critères circulatoires ou neurologiques. Nous avons mené des recherches systématiques dans Ovid MEDLINE, Ovid Embase, le registre Cochrane des études contrôlées (CENTRAL) via la Cochrane Library et Web of Science pour trouver des articles publiés depuis leur création jusqu’en août 2021. Nous avons inclus tous les types de publications de recherches originales évaluées par des pairs liées à la pression pulsée artérielle telle que surveillée par un transducteur de pression artérielle à demeure entourant un arrêt circulatoire ou de une détermination de décès avec des données directes spécifiques au contexte (don d’organes) ou indirectes (en dehors d’un contexte du don d’organes).
Constatations principales
Au total, 3289 résumés ont été identifiés et examinés pour déterminer leur admissibilité. Quatorze études ont été incluses, trois provenant de bibliothèques personnelles. Cinq études étaient de qualité suffisante pour être incluses dans le profil de données probantes des Lignes directrices de pratique clinique. Une étude a mesuré l’arrêt de l’activité de l’électroencéphalogramme (EEG) au niveau du scalp cortical après l’interruption des thérapies de maintien des fonctions vitales et a montré que l’activité EEG tombait en dessous de 2 μV lorsque la pression pulsée atteignait 8 mm Hg. Ces données probantes indirectes suggèrent qu’il existe une possibilité d’activité cérébrale persistante à des pressions pulsées artérielles > 5 mm Hg.
Conclusion
Des données probantes indirectes suggèrent que les cliniciens pourraient diagnostiquer à tort un décès selon des critères circulatoires s’ils appliquent un seuil de pression pulsée artérielle supérieur à 5 mm Hg. De plus, il n’y a pas suffisamment de données probantes pour déterminer que tout seuil de pression pulsée supérieur à 0 et inférieur à 5 peut permettre de déterminer en toute sécurité un décès cardiocirculatoire.
Enregistrement de l’étude
PROSPERO (CRD42021275763); soumis pour la première fois le 28 août 2021.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ongoing shortages of deceased donor organs for transplantation and limited numbers of brain-dead donors have led to increasing interest in the recovery of transplantable organs after death determined based on circulatory criteria known as donation after circulatory determination of death (DCD).1,2 This has also been referred to as donation after circulatory death, donation after death determination by circulatory criteria, non-heart-beating organ donation, or donation after cardiac death.3
The dead donor rule (DDR) serves as the fundamental ethical and legal foundation for organ donation/transplantation.4 The DDR holds that no patient should be killed by organ recovery and that patients must be dead before vital organs are recovered.3,4,5,6 While there are well-defined neurologic criteria for death determination, inconsistency in DCD protocols suggests that there is no consensus on the criteria for death determination by circulatory criteria.7,8 Current Canadian protocols for DCD recommend five minutes of observation of pulselessness based on an invasive arterial blood pressure (ABP) tracing, although practices vary from two to ten minutes.9,10,11 Without spontaneous resumption or resuscitation attempting to restart circulation, at the end of the observation period, loss of circulation is considered permanent and organ recovery ensues.
Current DCD protocols assume that brain function has ceased during the period of observation following pulselessness. While this is a reasonable assumption based on what is known regarding cerebral neurophysiology, it remains an assumption. There are concerns that brain function, and therefore conscious awareness as well as the capacity to feel pain, may persist even after cessation of anterograde circulation. In the setting of DCD, proceeding with organ procurement while the brain is still functioning would be harmful and would risk breaching the DDR. There is an emerging body of evidence describing surges of cerebral electrical activity after circulatory arrest,12,13,14 which have highlighted the need for further scientific exploration of cerebral function during a low flow state and at the time of circulatory arrest.
A primary concern with death determination by circulatory criteria is that the currently recommended thresholds for cessation of circulation are based on insufficient data relating to cerebral function.5 There is no universally accepted definition of or criteria for pulselessness in this context, and scant evidence to support the exact arterial pulse pressure threshold, as measured using an indwelling arterial transducer, at which cessation of circulation should be determined. A lack of precision in the pulse pressure threshold for DCD practices could threaten adherence to the DDR.4,6 Therefore, a key question surrounding DCD is: How does one define pulselessness based on invasive arterial pulse pressure measurements?
Using systematic review methodology, we sought to determine whether one should use an arterial pulse pressure of 0 mm Hg vs more than 0 (5, 10, 20, and 40) mm Hg to confirm permanent cessation of circulation for DCD donors. Our aim was to summarize and synthesize evidence supporting the minimum arterial pulse pressure required for confirming permanent cessation of circulation in the context of death determination by circulatory criteria.
Methods
This systematic review was conducted as part of a larger project in collaboration with the Canadian Critical Care Society, Canadian Medical Association, and Canadian Blood Service to develop a clinical practice guideline for death determination after arrest of circulatory or neurologic function, as well as a medical, brain-based definition of death.15,16
Search strategy and selection criteria
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)17 and Synthesis Without Meta-analysis in systematic reviews (SWiM)18 guidelines. The review protocol was registered with PROSPERO (CRD42021275763; first submitted 28 August 2021). No amendments were made to the registered protocol. Research ethics approval was not required for this systematic review.
Our primary objective was to compare studies that used an arterial pulse pressure of more than 0 (5, 10, 20, and 40) mm Hg for death determination by circulatory criteria in patients undergoing DCD with studies that used an arterial pulse pressure of 0 mm Hg serving as comparators/controls. Our secondary objective was to collect indirect evidence by including studies in all age groups reporting invasive ABP measurement during low- or no flow states in conjunction with measures of cerebral circulation, electrical activity of the brain (electroencephalography, Bispectral Index™ [BIS; Medtronic/Covidien, Boulder, CO, USA], SedLine® [Masimo Corporation, Irvine, CA, USA], evoked potentials, etc.), or brain function (clinical neurologic assessments). An information specialist (R. F.) designed and executed a comprehensive search that was verified by content experts and reviewed by a second information specialist (D. C.). The search strategy comprised text words and controlled vocabulary terms (e.g., MeSH) combining concepts for cardiac arrest or circulatory death or autoresuscitation,19,20 vital signs monitoring, and arterial pressure. Search filters were applied to exclude animal studies. No limits on language, publication date, or publication type were applied to the search. See Electronic Supplementary Material (ESM) eAppendix 1 for the complete search strategy.
We searched Ovid MEDLINE, Ovid Embase, the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Library, and Web of Science (Science Citation and Conference Proceedings Citation Indexes). Conference proceedings were retrieved from Embase, CENTRAL, and Web of Science searches, and trial registry records from ClinicalTrials.gov and the World Health Organization’s International Clinical Trials Registry Platform were retrieved by the CENTRAL search. Databases were searched for papers published from the date of database inception to 18 April 2021, and the search was updated on 21 August 2021. References were managed and duplicates removed in EndNote® X9 (Clarivate, London, UK) and subsequently uploaded to Covidence® systematic review software (Veritas Health Innovation, Melbourne, VIC, Australia; available at URL: https://www.covidence.org/ [accessed January 2023]) for primary (title/abstract) screening. The bibliographies of relevant articles and personal literature libraries of the authors were examined for additional published data that met the inclusion criteria.
We included studies related to arterial pulse pressure as monitored by an indwelling arterial pressure transducer around circulatory arrest or determination of death with either direct context-specific (organ donation) or indirect (outside of organ donation context) data. We defined a priori subgroups of interest based on age (neonates, pediatrics, adults), setting—both controlled DCD (i.e., following withdrawal of life-sustaining measures [WLSM]) and uncontrolled DCD21 (i.e., following an unexpected cardiac arrest with failed resuscitation) cases including organ donation following medical assistance in dying (MAID),22,23 and presence of mechanical circulatory assistance (extracorporeal membrane oxygenation, ventricular assist device, pacemaker, etc.).
We included all types of peer-reviewed original research publications without secondary reporting as well as randomized controlled trials, observational studies, case control studies, cohort studies, case series, and qualitative studies. For the initial title/abstract screening, we also included review articles (scoping, narrative, etc.) to review their references so that no relevant study would be missed. We excluded non-peer-reviewed articles, conference abstracts, study protocols, surveys, editorials, ethical reviews, commentaries and books or publications in a language other than French or English, for which we could not obtain translation.
Data extraction and analysis
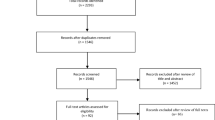
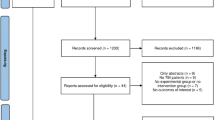
Each abstract was screened in duplicate by a team of eight reviewers, who independently screened and selected studies (Figure) for full-text review. Disagreements between reviewers were resolved through discussion. The full texts of articles were independently reviewed by two reviewers to assess for study eligibility. In addition, the reference lists of these articles were independently examined to identify additional relevant articles. All disagreements were resolved by discussion with a third reviewer. Studies that were excluded were tracked and the reason for exclusion recorded. Extracted data included the number of patients/participants reported, age, study design, population, method for measurement of circulation, and assessment of cerebral blood flow or electrical activity in relation to circulatory arrest. Data were extracted independently by three authors (L. H., T. G., and S. L. G.) and discrepancies were solved through discussion with the research team.
We were aware of and wanted to overcome the challenges posed by the paucity of studies evaluating the question of interest. The grouping of studies into direct vs indirect evidence was prespecified in the systematic review protocol. Indirect evidence to explore the evidence for brain perfusion or electrical activity at the time of circulatory arrest (outside the context of organ donation) could help inform future research in this population. Given the variability within the indirect evidence, it was not possible to combine the data quantitatively for statistical comparison. Narrative synthesis and tabular format were used to analyze and present results. Studies included for narrative synthesis were selected based on predetermined criteria. We followed SWiM reporting guidelines18 for the qualitative review of the indirect evidence.
Risk of bias and quality appraisal
For the entire body of evidence identified (i.e., observational studies, randomized and nonrandomized trials, and excluding case reports/series), we planned to perform a risk of bias and certainty of evidence assessment. Nevertheless, the literature search did not identify any cohort studies comparing different thresholds of ABP for determining circulatory arrest. In fact, none of the identified studies used a comparison group. All relevant studies constituted small to large case series or case reports providing indirect evidence only or larger cohorts without a comparison group. Therefore, a risk of bias assessment could not be performed. We assessed studies for clinical and methodological heterogeneity24 considering participant characteristics, types, and timing of outcome measurements as well as variability in study methodology. Case reports and case series were included consistent with the Joanna Briggs Institute checklist for case reports and series.25
We assessed the certainty of evidence for the studies included in the evidence profile using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.26 This framework classifies evidence as very low to high based on risk of bias, inconsistency, indirectness, imprecision, and publication bias. We assessed the certainty of the following outcomes of interest: 1) declaring someone dead who is not yet dead; 2) association of a pulse pressure value of more than 0 mm Hg with brain activity/function (i.e., false positive); 3) missing someone dead who is dead; and 4) no association of a pulse pressure value of more than 0 mm Hg with brain activity/function (i.e., false negative). In this review, we included additional studies that were not included in the evidence profile for the larger project to develop a clinical practice guideline for death determination after circulatory arrest. The additional studies do not provide sufficient certainty of evidence and have a high risk of bias, but they nonetheless contribute to scientific knowledge relevant to the research question.
Results
A total of 3,289 abstracts were identified through our search and after removal of duplicates, were screened for eligibility. Based on title/abstract screening, 3,186 abstracts were excluded. Ninety-six studies underwent full-text review, 11 of which were included for data extraction. Three further studies27,28,29 came from citation searching and the authors’ personal libraries that were not found in the initial search. Details on reasons for exclusion at the full-text review stage are outlined in the PRISMA flow diagram (Figure). Ultimately, only five27,28,29,30,31 of the 14 studies were considered adequate for use in the evidence profile supporting the clinical practice guideline statement. The remaining nine studies12,32,33,34,35,36,37,38,39 are included herein because they provide additional contextual information relevant to the research question. PRISMA and SWiM checklists for reporting items are provided in ESM eAppendices 2 and 3.
Pulse pressure thresholds
We did not find any study that compared zero vs non-zero pulse pressure thresholds for determination of cessation of circulation in the context of DCD or otherwise. Because there were no publications available providing direct evidence for the research question, heterogeneity could not be examined. We found two studies that clearly defined pulselessness38,39 and we found 12 studies that measured brain function clinically or using electroencephalography during sudden cardiac arrest and/or progressive loss of cerebral blood flow.12,27,28,29,30,31,32,33,34,35,36,37 The studies were included as indirect evidence, but there was substantial clinical heterogeneity precluding statistical synthesis of the studies. The clinical heterogeneity stemmed from variability of patient characteristics, clinical monitoring techniques, and monitoring conditions (e.g., during cardiopulmonary resuscitation [CPR] vs during WLSM with no attempts at resuscitation). Methodological heterogeneity was also present because there was no standardized method for reporting brain activity alongside persistent circulation under conditions of low arterial pressures, during CPR or at end of life. Therefore, we concluded that the studies providing indirect evidence could not be combined statistically.
Definitions of pulselessness
Two studies clearly defined criteria for pulselessness (Table 1). Dhanani et al.38 conducted the largest prospective observational study (n = 631; 32/631 were DCD patients) to date of adults dying after planned WLSM. In this study, the authors designated a period of pulse pressure less than 5 mm Hg for at least 60 sec as “cessation of circulation.” This definition was developed by an expert clinical advisory committee consisting of intensive care physicians and cardiac physiology experts, based on a thorough review of literature and a thoughtful application of principles of cerebral physiology. This study has good generalizability given the large number of study participants and the prospective multicentred observational study design.
Morgan et al.39 provided the only pediatric report of hemodynamics during pediatric CPR. Pulselessness in this study was defined by invasive arterial pulse pressure as less than 10 mm Hg and systolic pressure as less than 50 mm Hg (≥ one year) or less than 40 mm Hg (< one year). The study was a prospective multicentred observational study, but the definition of pulselessness was arbitrary.
Brain function at circulatory arrest
Twelve studies reported cerebral function at the time of circulatory arrest (Table 2). The studies used various modalities to monitor cerebral function or perfusion including clinical examination,33,34 transcranial doppler,35,36 BIS/SedLine,12 simplified electroencephalogram (EEG) montage,30 10–20 system continuous EEG,27,28,29,31 invasive multimodal neuromonitoring,32,37 and somatosensory evoked potentials.28
Clinical examination
Two case reports reported preserved consciousness in a total of two adult patients undergoing CPR. In the first report, invasive ABP and electrocardiogram (ECG) were monitored.33 The patient was reported to retain a high level of awareness throughout CPR if the mean ABP was maintained > 50 mm Hg. Below 50 mm Hg, the patient became unresponsive. Similarly, in a second report, a patient regained awareness during CPR after cardiac arrest.34 The patient was able to obey commends (wiggle toes, give “thumbs up”) with a mean arterial pressure (MAP) > 50 mm Hg, but not with a MAP < 50 mm Hg. While these are only case reports, the consistency of the data reported is interesting. These results suggest that mean arterial blood pressures below 50 mm Hg in the context of CPR may have a negative impact on cerebral blood flow resulting in dysfunction.
Transcranial Doppler
Two case reports with a total of two patients measured cerebral blood flow velocity (CBFV) using transcranial doppler during CPR.35,36 Both reported similar findings. In the first report, optimal CPR maintained a CBFV of 20 cm·sec-1 in the middle cerebral artery, which corresponded to a MAP of 33 mm Hg.35 In the second report,36 the MAP dropped to 22 mm Hg and the cardiac output was 0.0 L·min-1 at the onset of a pulseless electrical activity (PEA) arrest. Initiation of CPR resulted in a mean flow velocity of 51 cm·sec-1, which corresponded to a MAP of 33 mm Hg. Cerebral blood flow velocity increased sharply to 60% of precirculatory collapse levels after the institution of chest compressions and was at prearrest baseline after 100 sec of CPR before normalization of MAP and return of spontaneous circulation.
Bispectral index/sedline/simplified EEG montage
Chawla et al. reported a case series of patients showing surges of Patient State Index (PSi; Masimo Corporation, Irvine, CA, USA) activity at the time of death occurring up to 300 sec after loss of measurable blood pressure.12
Norton et al. reported electrocortical activity in relation to cardiac function after withdrawal of life-sustaining therapy in four adults.30 Absence of electrocerebral activity was defined as amplitude of less than 2 μV. Subhairline EEG activity ceased before cessation of cardiac rhythm and invasive ABP in three out of four patients while single delta wave bursts persisted following cessation of both cardiac rhythm and ABP in one patient. In the fourth patient, these delta bursts continued for 10 min 38 sec following ECG cessation with a mean amplitude of 4.5 μV in the last burst of delta activity. In the three patients who developed isoelectric EEG before cessation of cardiac rhythm, loss of EEG activity occurred at blood pressure values of 40/22, 59/34, and 123/59 mm Hg, respectively, corresponding to arterial pulse pressure values of 18, 25, and 64 mm Hg, respectively. Based on their comparison of EEG mean amplitude and mean spectral power, the authors report that analysis of amplitude may be more sensitive than examination of frequency in detecting changes in the EEG during the dying process because of diminished cerebral blood flow.
10–20 system continuous EEG
A subgroup of patients in the Dhanani study38 underwent continuous EEG at the time of WLSM. This substudy was reported by Gofton et al.29 and included eight adults (median age, 68 yr) with continuous monitoring of invasive blood pressure, EEG, heart rate, and oxygen saturation starting 30 min before initiation of WLSM and continuing up to 30 min after asystole. This study reported that EEG stops at a median [interquartile range (IQR)] of 78 [-387 to 111] sec before asystole. At the time of EEG cessation, the median [IQR] MAP was 31.1 [24.5–36.2] mm Hg and the median [IQR] pulse pressure was 8.2 [5.3–25] mm Hg. Given the small number of patients reported in this case series, the precision was low with respect to the calculated time of EEG cessation compared with circulatory arrest.
Matory et al.31 reported findings from 19 adults who died from different causes while undergoing EEG monitoring. They defined cessation of circulation to the brain as a MAP of < 20 mm Hg, a systolic pressure of < 40 mm Hg, or a diastolic pressure of < 20 mm Hg based on previous reports.40,41 Electroencephalogram amplitude permanently dropped below 2 µV at a median [IQR] of 2 [8 before to 0 after] min before the last QRS complex (range, 80 min before to 2 min after). Similarly, the EEG amplitude dropped below 2 µV for a median [IQR] of 2 [1.5 min before to 6 min after] min after cessation of circulation to the brain (range, 33 min before to 34 min after). The retrospective nature of this study introduces the possibility of bias; however, its larger cohort size is a strength.
Hughes et al.27 reported a 57-yr-old male with multifocal brain infarcts who had a cardiac arrest during EEG recording. In this case, the EEG became isoelectric around 60 sec prior to asystole. The authors suggested that this patient likely had a neurologic deterioration leading to death and that the EEG may therefore have become isoelectric prior to asystole. No blood pressure information was reported. There was a high risk of bias in this study given that it was a single case report.
Invasive multimodal neuromonitoring
Imberti et al.32 reported multimodal neurologic data around sudden cardiac arrest in a head-injured 17-yr-old. The first figure in their report showed a precipitous drop in cerebral tissue oxygen to below 8 mm Hg when the MAP dropped below ~20 mm Hg. There was a high risk of bias given that this was a single case report.
Dreier et al.37 reported findings from multimodal neuromonitoring systems including subdural (n = 4) or intraparenchymal (n = 5) electrical recordings in patients undergoing WLSM. In one of these patients, when the electrocorticography became isoelectric, the MAP had fallen to 18 mm Hg. Also, at the onset of non-spreading depression, the median [IQR] MAP was 25 [23–37] mm Hg. While this study had a slightly higher number of reported cases than some studies, there was variability in the monitoring modalities (subdural vs intraparenchymal), which should be considered.
Evoked potentials
Stecker et al.28 reported the time course of neurophysiologic changes in two patients (compared with a group of patients undergoing deep hypothermic arrest): one who experienced acute global hypoxia/ischemia at normothermia and one who experienced acute global ischemia at moderate hypothermia. During deep hypothermic circulatory arrest, the somatosensory evoked potentials (N13, N18) took five times longer to drop to 50% of their value compared with circulatory arrest during moderate hypothermia. The peak-to-peak EEG amplitude dropped to less than 2 µV by about two minutes after the onset of ischemia in the patient who underwent circulatory arrest during normothermia. In the same patient, EEG decreased to half its baseline amplitude in 1.1 ± 0.02 min, while in the patient with circulatory arrest during deep hypothermia (30.9 ºC), the corresponding value was 0.66 ± 0.06 min. Also, the EEG τ50 for circulatory arrest at moderate hypothermia was longer than the times expected for EEG inactivity at normothermia. This study was limited by the failure to report EEG findings in relation to ABP values.
Subgroup considerations
A priori subgroups were considered. No direct evidence exists in pediatric populations. Morgan et al.39 defined pulselessness during pediatric in-hospital CPR. as a pulse pressure of < 10 mm Hg and a systolic blood pressure (SBP) of < 50 mm Hg (≥ one year) or < 40 mm Hg (< one year) based on previous reports in the literature.42,43 There is no direct or indirect evidence in neonates. In uncontrolled DCD in the context of abrupt cessation of circulation, indirect evidence suggests that brain perfusion can still be maintained at very low SBP; a recent case report by de Wilde et al. (2017)36 reported that at onset of PEA arrest, the MAP dropped to 22 mm Hg and the cardiac output was 0.0 L·min-1. During compressions, CBFV was 51 cm·sec-1 and peak systolic ABP was 33 mm Hg.
Grading of evidence
The level of evidence using the GRADE approach for the studies included in the clinical practice guideline evidence profile is shown in Table 3.
Discussion
Ensuring public trust for deceased organ donation relies on adherence to the DDR. It is important that adherence to the DDR is ensured and that the community trusts health care providers to define death as precisely as possible based on robust evidence. At the same time, determining death in a timely fashion is important for family members who are present at the bedside and is imperative in the case of DCD donors to minimize warm ischemia time. Cardiac electrical asystole should not be used to determine circulatory arrest, as recent research has shown that cardiac electrical activity can persist for more than 30 min after loss of ABP.29 With the understanding that consciousness and awareness rests within the brain, before proceeding with declaring an individual dead in the setting of DCD organ procurement, it is important that any threshold for blood-pressure based circulatory arrest declaration coincides with or follows complete loss of brain function associated with conscious awareness. Nevertheless, the minimal pulse pressure that ensures permanent cessation of anterograde arterial circulation for death determination and the absence of cerebral function is unknown. Our work highlights important gaps in identifying a brain-based arterial pulse pressure threshold for circulatory death. In this systematic review of the literature, we were unable to identify any existing studies that directly address whether one should use an arterial pulse pressure of 0 mm Hg vs any other pulse pressure for determining pulselessness for the purpose of death determination in organ donors.
Indirect evidence suggests that an arterial pulse pressure of 5 mm Hg or below, as measured by invasive arterial monitoring, should be used for death determination in controlled DCD. In situations of progressive hypotension and hypoxia (following WLSM), cerebral activity ceases at or above pulse pressures of 5 mm Hg29). This was a small cohort, but this is the only study we found in humans that provides information about what the pulse pressures were when EEG became isoelectric. Given the implications for determining death in someone who may have some brain activity, it provides important information. Additionally, these data suggest that Dhanani et al.’s consensus-based criteria for cessation of circulation was appropriate.38 Further, consciousness could be potentially lost at an SBP of < 50 mm Hg, as reported in a few case reports.33 While no objective neuromonitoring was completed in these studies, it may be reasonable to infer that an SBP of > 50 mm Hg is required to maintain a high level of awareness.
Four studies27,28,30,31 (one observational with n = 19 adults and three case studies/series with n = 7 adults) reported on timing of isoelectric EEG compared with arrest of circulation. In all studies but one (n = 1/25), EEG was reported to stop from 80 min before to two minutes after asystole. Norton et al. reported that 1/4 patients had single delta wave bursts that persisted following the cessation of both the cardiac rhythm and ABP; it was uncertain if these were cerebrally originating or artefacts, but artefact was favoured.30
It is also important to consider that there are several reports of surges of brain activity seen after sudden circulatory arrest.12,13 The PSi is a quantitative parameter intended to measure depth of sedation and is derived using a proprietary multivariate algorithm from 4-channel frontal EEG. Chawla et al. recently reported a 44.6% increase in PSi incidence of greater than 50% above immediate baseline after death in critically ill patients.13 Such electroencephalographic surges occurred 180–360 sec after complete loss of blood pressure and were characterized by high frequency non-epileptiform signals. Notably, their work is consistent with several animal studies that have shown evidence of similar electroencephalographic surges (cohesive gamma wave activity) 30–180 sec after cessation of cardiac activity.44,45,46 Nevertheless, these are reported in studies using BIS/SedLine, which is more difficult to interpret because the algorithm is proprietary. It is unknown if the reported surges can be attributed solely to changes in EEG or whether there could be a change noted in PSi due to changes in other channels such as muscle activity.
Other studies also reported brain activity outlasting circulation, but these reports occurred in acute cardiac arrest as opposed to progressive hypoxia and hypotension.31 In the Dhanani et al. study,38 a pulse pressure of less than 5 mm Hg sustained for 60 sec was required to determine pulselessness in the context of WLSM in the intensive care unit. With these parameters, there were resumptions of cardiac activity (defined as any pulse pressure ≥ 5 mm Hg for one beat) in 14% of patients. Nevertheless, none of these resumptions were prolonged and all occurred within five minutes of meeting the initial criteria for pulselessness. A comprehensive review by Pana et al.47 described seven studies that reported the loss of EEG after circulatory arrest in humans. Four studies reported loss of monitored EEG activity between ten and 30 sec during general anesthesia and intraoperative asystole41,48,49,50 while two studies reported that EEG activity was lost within 10–15 sec of cardiac arrest (ventricular fibrillation).51,52 In the absence of anesthesia, however, the EEG tracing became isoelectric after ten seconds of asystole.53 No direct or indirect evidence exists for either extracorporeal membrane oxygenation or MAID; however, both situations are likely similar to reported cases with an abrupt loss of circulation. Brain activity after a sudden cardiac arrest (such as in uncontrolled DCD) could persist longer than that after a gradual decline in respiration (oxygenation) progressing to a cardiac arrest which often happens in WLSM or controlled DCD settings. Future research should evaluate these two subgroups separately. In addition, our suggested arterial pulse pressure threshold is based on a relatively small study in adults. With children and neonates having a lower blood pressure during health, cerebral perfusion and cerebral electrical activity could persist below 5 mm Hg pulse pressure and we could not find any evidence to support or refute this conclusion. Similarly, in the context of MAID, under controlled settings, when sudden cardiac arrest occurs with an intact non-injured brain as happens with the administration of intravenous potassium, our findings may not be applicable. Cerebral perfusion and electrical activity need to be studied specifically in the context of MAID.
Our work has important strengths and limitations. Firstly, our work is timely as advances in medicine have allowed for restarting of organ perfusion through technological assistance and reanimation of organ function outside (ex situ) or inside the body (in situ). Such in situ normothermic regional perfusion (NRP), which involves reperfusion of organs in the donor’s body after death determination by circulatory criteria, is undertaken to improve function of the transplantable organs.40 Even though in both abdominal and thoracoabdominal NRP, the cardiopulmonary function in the donor would not persist without machine assistance, and even though our question does not directly concern NRP, having an evidence-based brain-guided threshold for circulatory determination of death would allow us to better navigate ethical debates surrounding NRP. Secondly, our work evaluates both direct and indirect evidence to answer this important question. One of the most important limitations of our work is that we had to rely most often on small, single-centre studies and case reports to draw these conclusions. Given the clinical implications of declaring someone dead, this should be considered insufficient evidence and should serve as an urgent call for more research in these areas to ensure clarity, transparency, and public trust in donation. Secondly, it is possible that clinical monitoring equipment may lose its accuracy at lower pulse pressures.54 The precision and accuracy of clinical monitoring equipment at very low (subphysiologic) arterial pulse pressure ranges for young children are not well known or have not been reported. It would be helpful for clinical monitoring equipment manufacturers to determine these factors and make this information available in the public domain for individual clinical monitoring systems including invasive arterial pressure transducers.
Future prospective studies should quantify cerebral electrical activity, brainstem neuronal activity, and behavioural assessments of level of consciousness in relation to arterial pulse pressure in specific clinical contexts across the age range to better delineate thresholds for determining cessation of circulation. These thresholds should also be determined by considering the accuracy or limitations of clinical monitoring equipment at very low pulse pressures.
Conclusions
In our systematic review, we did not find any direct evidence that could help define an arterial pulse pressure threshold for determining cessation of circulation. Nevertheless, based on indirect evidence from studies reporting cerebral electrical activity around the time of cardiac arrest, an arterial pressure not exceeding 5 mm Hg should be required to confirm the absence of electrical activity of the brain and cessation of circulation for DCD until further direct evidence is acquired. Further research is required to confirm if the same threshold is applicable to specific subgroups such as children, neonates, or adults dying in the context of sudden cardiac arrest or MAID.
References
Smith M, Dominguez-Gil B, Greer DM, Manara AR, Souter MJ. Organ donation after circulatory death: current status and future potential. Intensive Care Med 2019; 45: 310–21. https://doi.org/10.1007/s00134-019-05533-0
Manyalich M, Nelson H, Delmonico FL. The need and opportunity for donation after circulatory death worldwide. Curr Opin Organ Transplant 2018; 23: 136–41. https://doi.org/10.1097/mot.0000000000000486
Murphy N, Weijer C, Smith M, et al. Controlled donation after circulatory determination of death: a scoping review of ethical issues, key concepts, and arguments. J Law Med Ethics 2021; 49: 418–40. https://doi.org/10.1017/jme.2021.63
Truog R, Robinson WM. Role of brain death and the dead-donor rule in the ethics of organ transplantation. Crit Care Med 2003; 31: 2391–6. https://doi.org/10.1097/01.ccm.0000090869.19410.3c
Truog R, Miller FG, Halpern SD. The dead-donor rule and the future of organ donation. N Engl J Med 2013; 369: 1287–9. https://doi.org/10.1056/nejmp1307220
Gardiner D, Sparrow R. Not dead yet: controlled non-heart-beating organ donation, consent, and the Dead Donor Rule. Camb Q Healthc Ethics 2010; 19: 17–26. https://doi.org/10.1017/s0963180109990211
DeVita MA. The death watch: certifying death using cardiac criteria. Prog Transplant 2001; 11: 58–66. https://doi.org/10.1177/152692480101100109
Wall AE, Shabbir R, Chebrolu S, et al. Variation in donation after circulatory death hospital policies in a single donor service area. Am J Surg 2022; 224: 595–601. https://doi.org/10.1016/j.amjsurg.2022.03.043
Dhanani S, Hornby L, Ward R, Shemie S. Variability in the determination of death after cardiac arrest: a review of guidelines and statements. J Intensive Care Med 2012; 27: 238–52. https://doi.org/10.1177/0885066610396993
Weiss MJ, Hornby L, Rochwerg B, et al. Canadian guidelines for controlled pediatric donation after circulatory determination of death-summary report. Pediatr Crit Care Med 2017; 18: 1035–46. https://doi.org/10.1097/pcc.0000000000001320
Shemie SD, Baker AJ, Knoll G, et al. National recommendations for donation after cardiocirculatory death in Canada: donation after cardiocirculatory death in Canada. CMAJ 2006; 175: S1. https://doi.org/10.1503/cmaj.060895
Chawla LS, Akst S, Junker C, Jacobs B, Seneff MG. Surges of electroencephalogram activity at the time of death: a case series. J Palliat Med 2009; 12: 1095–100. https://doi.org/10.1089/jpm.2009.0159
Chawla LS, Terek M, Junker C, et al. Characterization of end-of-life electroencephalographic surges in critically ill patients. Death Stud 2017; 41: 385–92. https://doi.org/10.1080/07481187.2017.1287138
Auyong DB, Klein SM, Gan TG, Roche AM, Olson D, Habib AS. Processed electroencephalogram during donation after cardiac death. Anesth Analg 2010; 110: 1428–32. https://doi.org/10.1213/ane.0b013e3181d27067
Organ Donation and Transplantation Collaboration. Developing a brain-based definition of death and evidence-based criteria for its determination after arrest of circulation or neurologic function in Canada. Available from URL: https://professionaleducation.blood.ca/sites/default/files/odtc_death_determination_guideline_project_snapshot.pdf (accessed December 2022).
Shemie SD, Wilson LC, Hornby L, et al. A brain-based definition of death and criteria for its determination after arrest of circulation or neurologic function in Canada: a 2023 Clinical Practice Guideline. Can J Anesth 2023; https://doi.org/10.1007/s12630-023-02431-4.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. https://doi.org/10.1136/bmj.n71
Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020; 368: I6890. https://doi.org/10.1136/bmj.l6890
Dhanani S, Hornby L, Ward R, et al. Vital signs after cardiac arrest following withdrawal of life-sustaining therapy: a multicentre prospective observational study. Crit Care Med 2014; 42: 2358–69. https://doi.org/10.1097/ccm.0000000000000417
Gordon L, Pasquier M, Brugger H, Paal P. Autoresuscitation (Lazarus phenomenon) after termination of cardiopulmonary resuscitation - a scoping review. Scand J Trauma Resusc Emerg Med 2020; 28: 14. https://doi.org/10.1186/s13049-019-0685-4
Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int 2016; 29: 749–59. https://doi.org/10.1111/tri.12776
Government of Canada. Medical assistance in dying. Available from URL: https://www.canada.ca/en/health-canada/services/medical-assistance-dying.html (accessed December 2022).
Government of Canada. Criminal Code (R.S.C., 1985, c. C-46). PART VIII: offences against the person and reputation (continued): murder, manslaughter and infanticide (continued). Available from URL: https://laws-lois.justice.gc.ca/eng/acts/c-46/page-33.html (accessed December 2022).
Gagnier J, Moher D, Boon H, Beyene J, Bombardier C. Investigating clinical heterogeneity in systematic reviews: a methodologic review of guidance in the literature. BMC Med Res Methodol 2012; 12: 111. https://doi.org/10.1186/1471-2288-12-111
JBI. Critical appraisal tools. Available from URL: https://jbi.global/critical-appraisal-tools (accessed December 2022).
Balshem H, Helfand M, Schünemann H, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–6. https://doi.org/10.1016/j.jclinepi.2010.07.015
Hughes J, Uppal H. The EEG changes during cardiac arrest: a case report. Clin Electroencephalogr 1998; 29: 16–8. https://doi.org/10.1177/155005949802900108
Stecker MM, Escherich A, Patterson T, Bavaria JE, Cheung AT. Effects of acute hypoxemia/ischemia on EEG and evoked responses at normothermia and hypothermia in humans. Med Sci Monit 2002; 8: CR223–8.
Gofton TE, Norton L, Laforge G, et al. Cerebral cortical activity after withdrawal of life-sustaining measures in critically ill patients. Am J Transplant 2022; 22: 3120–9. https://doi.org/10.1111/ajt.17146
Norton L, Gibson R, Gofton T, et al. Electroencephalographic recordings during withdrawal of life-sustaining therapy until 30 minutes after declaration of death. Can J Neurol Sci 2017; 44: 139–45. https://doi.org/10.1017/cjn.2016.309
Matory AL, Alkhachroum A, Chiu WT, et al. Electrocerebral signature of cardiac death. Neurocrit Care 2021; 35: 853–61. https://doi.org/10.1007/s12028-021-01233-0
Imberti R, Bellinzona G, Riccardi F, Pagani M, Langer M. Cerebral perfusion pressure and cerebral tissue oxygen tension in a patient during cardiopulmonary resuscitation. Intensive Care Med 2003; 29: 1016–9. https://doi.org/10.1007/s00134-003-1719-x
Bihari S, Rajajee V. Prolonged retention of awareness during cardiopulmonary resuscitation for asystolic cardiac arrest. Neurocrit Care 2008; 9: 382–6. https://doi.org/10.1007/s12028-008-9099-2
Tobin JM, Mihm FG. A hemodynamic profile for consciousness during cardiopulmonary resuscitation. Anesth Analg 2009; 109: 1598–9. https://doi.org/10.1213/ane.0b013e3181b89432
Blumenstein J, Kempfert J, Walther T, et al. Cerebral flow pattern monitoring by transcranial Doppler during cardiopulmonary resuscitation. Anaesth Intensive Care 2010; 38: 376–80. https://doi.org/10.1177/0310057x1003800223
de Wilde RB, Helmerhorst HJ, van Westerloo DJ. Cerebral blood flow velocity during chest compressions in cardiac arrest. Neth J Crit Care 2017; 25: 137–9.
Dreier JP, Major S, Foreman B, et al. Terminal spreading depolarization and electrical silence in death of human cerebral cortex. Ann Neurol 2018; 83: 295–310. https://doi.org/10.1002/ana.25147
Dhanani S, Hornby L, van Beinum A, et al. Resumption of cardiac activity after withdrawal of life-sustaining measures. New Engl J Med 2021; 384: 345–52. https://doi.org/10.1056/nejmoa2022713
Morgan RW, Reeder RW, Meert KL, et al. Survival and hemodynamics during pediatric cardiopulmonary resuscitation for bradycardia and poor perfusion versus pulseless cardiac arrest. Crit Care Med 2020; 48: 881–9. https://doi.org/10.1097/ccm.0000000000004308
Trojaborg W, Boysen G. Relation between EEG, regional cerebral blood flow and internal carotid artery pressure during carotid endarterectomy. Electroencephalogr Clin Neurophysiol 1973; 34: 61–9. https://doi.org/10.1016/0013-4694(73)90151-x
Moss J, Rockoff M. EEG monitoring during cardiac arrest and resuscitation. JAMA 1980; 244: 2750–1.
Tibballs J, Russell P. Reliability of pulse palpation by healthcare personnel to diagnose paediatric cardiac arrest. Resuscitation 2009; 80: 61–4. https://doi.org/10.1016/j.resuscitation.2008.10.002
Morgan RW, Landis WP, Marquez A, et al. Hemodynamic effects of chest compression interruptions during pediatric in-hospital cardiopulmonary resuscitation. Resuscitation 2019; 139: 1–8. https://doi.org/10.1016/j.resuscitation.2019.03.032
Li D, Mabrouk OS, Liu T, et al. Asphyxia-activated corticocardiac signaling accelerates onset of cardiac arrest. Proc Natl Acad Sci U S A 2015; 112: E2073–82. https://doi.org/10.1073/pnas.1423936112
van Rijn CM, Krijnen H, Menting-Hermeling S, Coenen AM. Decapitation in rats: latency to unconsciousness and the 'wave of death'. PLoS One 2011; 6: e16514. https://doi.org/10.1371/journal.pone.0016514
Borjigin J, Lee U, Liu T, et al. Surge of neurophysiological coherence and connectivity in the dying brain. Proc Natl Acad Sci U S A 2013; 110: 14432–7. https://doi.org/10.1073/pnas.1308285110
Pana R, Hornby L, Shemie SD, Dhanani S, Teitelbaum J. Time to loss of brain function and activity during circulatory arrest. J Crit Care 2016; 34: 77–83. https://doi.org/10.1016/j.jcrc.2016.04.001
Cullen SC. Cardiac arrest during chloroform monitored by EEG and EKG. Anesthesiology 1957; 18: 504–5. https://doi.org/10.1097/00000542-195705000-00018
Young WL, Ornstein E. Compressed spectral array EEG monitoring during cardiac arrest and resuscitation. Anesthesiology 1985; 62: 535–8. https://doi.org/10.1097/00000542-198504000-00033
Losasso TJ, Muzzi DA, Meyer FB, Sharbrough FW. Electroencephalographic monitoring of cerebral function during asystole and successful cardiopulmonary resuscitation. Anesth Analg 1992; 75: 1021–4. https://doi.org/10.1213/00000539-199212000-00025
Aminoff MJ, Scheinman MM, Griffin JC, Herre JM. Electrocerebral accompaniments of syncope associated with malignant ventricular arrhythmias. Ann Intern Med 1988; 108: 791–6. https://doi.org/10.7326/0003-4819-108-6-791
de Vries LS, Toet MC. Amplitude integrated electroencephalography in the full-term newborn. Clin Perinatol 2006; 33: 619–32. https://doi.org/10.1016/j.clp.2006.06.002
Ziller MG, Natola MA. EEG findings during tilt-table induced asystole: a case report. Am J Electroneurodiagn Technol 2011; 51: 26–30.
Romagnoli S, Ricci Z, Quattrone D, et al. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care 2014; 18: 644. https://doi.org/10.1186/s13054-014-0644-4
Author contributions
Teneille Gofton, Laura Hornby, Matthew Weiss, Kirk Dawe, and Sonny Dhanani designed the study. Teneille Gofton, Saptharishi Lalgudi Ganesan, Laura Hornby, Matthew Weiss, Kirk Dawe, Chelsea Lanos, Krista Wollny, and Sonny Dhanani assessed citations for eligibility. Teneille Gofton, Saptharishi Lalgudi Ganesan, and Laura Hornby abstracted data, assessed risk of bias, and checked data for accuracy. Saptharishi Lalgudi Ganesan, Teneille Gofton, Laura Hornby, and Sonny Dhanani conducted analyses. All authors contributed to interpretation of the data. All authors read the manuscript and provided feedback.
Acknowledgements
The authors would like to express their appreciation to Robin Featherstone, MLIS, for her help in updating our search strategy; Dagmara Chojecki, MLIS, for providing peer review of our search strategy; and Dr. Michael Hickey for assistance with submission of the systematic review protocol to PROSPERO.
Disclosures
Laura Hornby is a paid research consultant for Canadian Blood Services, and Matthew Weiss is a paid consultant as medical director of donation at Transplant Québec. The remaining authors have no conflicts to declare.
Funding statement
This work was conducted as part of the project entitled, A Brain-Based Definition of Death and Criteria for its Determination After Arrest of Circulation or Neurologic Function in Canada made possible through a financial contribution from Health Canada through the Organ Donation and Transplantation Collaborative and developed in collaboration with the Canadian Critical Care Society, Canadian Blood Services, and the Canadian Medical Association. The views expressed herein do not necessarily represent the views of Health Canada, the Canadian Critical Care Society, Canadian Blood Services, or the Canadian Medical Association.
Data availability statement
The systematic review protocol is publicly available at the following PROSPERO registry link: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=275763. The data extracted from included studies and data used for analyses can be obtained by contacting the corresponding author.
Prior conference presentations
Some findings from this work were shared at the 2022 Critical Care Canada Forum (Toronto, ON, Canada) as part of the 24 November 2022 Keynote address titled, Deceased Donation Stream: The Paradigm Shift to Brain-Based Definition and Determination of Death.
Editorial responsibility
This submission was handled by Dr. Maureen Meade, Guest Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lalgudi Ganesan, S., Hornby, L., Weiss, M. et al. Brain-based arterial pulse pressure threshold for death determination: a systematic review. Can J Anesth/J Can Anesth 70, 685–698 (2023). https://doi.org/10.1007/s12630-023-02425-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02425-2