Abstract
Purpose
To compare the relative efficacy of supportive therapies (inotropes, vasopressors, and mechanical circulatory support [MCS]) for adult patients with cardiogenic shock complicating acute myocardial infarction.
Source
We conducted a systematic review and network meta-analysis and searched six databases from inception to December 2021 for randomized clinical trials (RCTs). We evaluated inotropes, vasopressors, and MCS in separate networks. Two reviewers performed screening, full-text review, and extraction. We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework to rate the certainty in findings. The critical outcome of interest was 30-day all-cause mortality.
Principal findings
We included 17 RCTs. Among inotropes (seven RCTs, 1,145 patients), levosimendan probably reduces mortality compared with placebo (odds ratio [OR], 0.53; 95% confidence interval [CI], 0.33 to 0.87; moderate certainty), but primarily in lower severity shock. Milrinone (OR, 0.52; 95% CI, 0.19 to 1.39; low certainty) and dobutamine (OR, 0.67, 95% CI, 0.30 to 1.49; low certainty) may have no effect on mortality compared with placebo. With regard to MCS (eight RCTs, 856 patients), there may be no effect on mortality with an intra-aortic balloon pump (IABP) (OR, 0.94; 95% CI, 0.69 to 1.28; low certainty) or percutaneous MCS (pMCS) (OR, 0.96; 95% CI, 0.47 to 1.98; low certainty), compared with a strategy involving no MCS. Intra-aortic balloon pump use was associated with less major bleeding compared with pMCS. We found only two RCTs evaluating vasopressors, yielding insufficient data for meta-analysis.
Conclusion
The results of this systematic review and network meta-analysis indicate that levosimendan reduces mortality compared with placebo among patients with low severity cardiogenic shock. Intra-aortic balloon pump and pMCS had no effect on mortality compared with a strategy of no MCS, but pMCS was associated with higher rates of major bleeding.
Study registration
Center for Open Science (https://osf.io/ky2gr); registered 10 November 2020
Résumé
Objectif
Comparer l’efficacité relative des thérapies de soutien (inotropes, vasopresseurs et assistance circulatoire mécanique [ACM]) chez les patients adultes atteints d’un choc cardiogénique compliquant un infarctus aigu du myocarde.
Sources
Nous avons réalisé une revue systématique et une méta-analyse en réseau et effectué des recherches dans six bases de données depuis leur création jusqu’à décembre 2021 pour en tirer les études randomisées contrôlées (ERC). Nous avons évalué les inotropes, les vasopresseurs et les ACM dans des réseaux distincts. Deux réviseurs ont effectué la recherche, l’évaluation du texte intégral et l’extraction. Nous avons utilisé le système de notation GRADE (Grading of Recommendations Assessment, Development, and Evaluation) pour évaluer la certitude des résultats. Le critère d’évaluation d’intérêt était la mortalité toutes causes confondues à 30 jours.
Constatations principales
Nous avons inclus 17 ERC. Parmi les inotropes (sept ERC, 1145 patients), le lévosimendan a probablement réduit la mortalité par rapport au placebo (rapport de cotes [RC], 0,53; intervalle de confiance [IC] à 95 %, 0,33 à 0,87; certitude modérée), mais principalement en cas de choc de sévérité moindre. La milrinone (RC, 0,52; IC 95 %, 0,19 à 1,39; certitude faible) et la dobutamine (RC, 0,67, IC 95 %, 0,30 à 1,49; certitude faible) pourraient n’avoir aucun effet sur la mortalité par rapport au placebo. En ce qui concerne l’ACM (huit ERC, 856 patients), il pourrait n’y avoir aucun effet sur la mortalité avec un ballon intra-aortique (IABP) (RC, 0,94; IC 95 %, 0,69 à 1,28; certitude faible) ou un ACM percutané (ACMp) (RC, 0,96; IC 95 %, 0,47 à 1,98; certitude faible), par rapport à une stratégie sans ACM. L’utilisation d’un ballon intra-aortique était associée à moins de saignements majeurs par rapport à une ACMp. Nous n’avons trouvé que deux ERC évaluant les vasopresseurs, ce qui n’a pas fourni suffisamment de données pour la méta-analyse.
Conclusion
Les résultats de cette revue systématique et de la méta-analyse en réseau indiquent que le lévosimendan réduit la mortalité par rapport au placebo chez les patients présentant un choc cardiogénique de faible gravité. Le ballon intra-aortique et l’ACMp n’ont eu aucun effet sur la mortalité par rapport à une stratégie sans ACM, mais l’ACMp était associée à des taux plus élevés de saignements majeurs.
Enregistrement de l’étude
Center for Open Science (https://osf.io/ky2gr); enregistrée le 10 novembre 2020
Similar content being viewed by others
Cardiogenic shock is a clinical condition that is characterized by systemic hypoperfusion secondary to cardiac dysfunction.1,2,3 The clinical presentations of severe heart failure or cardiogenic shock can be heterogeneous, and patients may have various signs of end-organ dysfunction, with or without the presence of hypotension.2 Mortality from this condition has been reported to be between 30 and 80%, depending on the clinical context.4 Cardiogenic shock most commonly occurs secondary to acute myocardial infarction (MI), with incidence rates in the range of 3–13% of cases of acute MI.3 Patients with cardiogenic shock secondary to acute MI appear to have higher mortality, compared with other etiologies.5 Despite advancements in reperfusion therapies and regional systems of care, mortality from this condition remains substantial.6
The mainstay in the management of cardiogenic shock secondary to acute MI remains revascularization of culprit coronary lesions.7,8,9 Additional treatment is largely supportive and focused upon improving hemodynamics and end-organ perfusion, with various pharmacologic and mechanical therapies available. Pharmacologic treatments are divided into agents that are primarily vasopressors (e.g., norepinephrine, epinephrine, dopamine) and those that are primarily inotropes (e.g., dobutamine, milrinone, levosimendan, enoximone).10 While each of these agents has its own advantages and disadvantages, at present there are limited data related to their comparative efficacy in improving mortality, and there is substantial variation in their use among clinicians.11 Furthermore, therapy for cardiogenic shock has grown to include various forms of mechanical circulatory support (MCS), including the intra-aortic balloon pump (IABP), percutaneous MCS (pMCS, such as the Impella® [Abiomed®, Danvers, MA, USA] and the TandemHeart® [LivaNova PLC, London, UK]), and venoarterial extracorporeal membrane oxygenation (VA-ECMO).12,13 Mechanical circulatory support primarily serves as a bridge to recovery or transplant, with recommended use from contemporary guidelines,9 despite limited data on efficacy.
We conducted a systematic review and network meta-analysis of randomized clinical trials (RCTs), with the aim of evaluating the relative efficacy of available therapies for treatment of cardiogenic shock complicating acute MI. Previous conventional meta-analyses on the effectiveness of these treatments in cardiogenic shock have shown conflicting results,13,14 and new evidence has since emerged. Compared with conventional meta-analyses, network meta-analyses can harness the cumulative data from all trials in a particular condition and can generate indirect estimates of effect between treatments that have never previously been compared in a randomized trial. Of note, while RCTs of inotropes and vasopressors likely include patients with lower severity shock (Society for Cardiovascular Angiography and Interventions [SCAI] class A–C),15 RCTs for MCS often include patients with much higher severity of illness (SCAI class D–E). Because of these concerns regarding clinical heterogeneity between trials, our prespecified plan for analysis included three separate networks for each treatment category (vasopressors, inotropes, and MCS).
Methods
We followed the Preferred Reporting Items for Systematic Review and Meta-Analysis statement extension for network meta-analysis.16,17 We registered our protocol with the Center for Open Science (https://osf.io/ky2gr; November 10, 2020). No institutional review board approval was required because all study data had been published previously, and we did not include individual patient data.
Data sources and search strategy
We searched six databases (Medline, PubMed, EMBASE, Scopus, Web of Science, and the Cochrane Database of Systematic Reviews) from inception to 30 December 2021. An experienced health sciences librarian assisted in developing the search strategy. Electronic Supplementary Material (ESM) eFig. 1 provides details of our search strategy. We conducted further surveillance searches using the “related articles” feature.
Study selection
Two reviewers (S. M. F. and R. M.) independently screened titles and abstracts identified through the searches using Covidence (Melbourne, Vic, Australia) and assessed full texts of the selected articles from phase one. Reviewers resolved disagreements by discussion. We included RCTs (parallel, cluster, or crossover), without language restriction, meeting the following criteria: 1) enrolled adult patients (≥ 16 yr of age); 2) conducted primarily (≥ 70%) in patients with acute MI; 3) randomized patients to receive inotropes, vasopressors, MCS, or a combination of the above; 4) evaluated at least one of the outcomes of interest; and 5) included primarily (≥ 70%) patients with cardiogenic shock. Since there is no accepted definition of “cardiogenic shock,”2 we considered the SCAI definitions of cardiogenic shock, class A to E.15 While some studies have traditionally only included hypotensive patients, other trials have been more inclusive of any patients with evidence of hypoperfusion, or requiring vasoactive medications with relative hypotension. Therefore, we included trials of patients meeting any of the following criteria suggesting cardiogenic shock: 1) hypotension (defined by a systolic blood pressure < 100 mm Hg); 2) organ hypoperfusion (defined by cool extremities, altered mental status, elevated lactate, decreased urine output, or other end-organ dysfunction); or 3) severe heart failure requiring initiation of vasopressors or inotropes to maintain perfusion. We had originally sought to exclude patients with class A shock (“at risk” of cardiogenic shock, but without overt signs and symptoms), but many trials ultimately included these patients. We excluded trials that exclusively evaluated MI patients who were postoperative from cardiac surgery for revascularization because of concerns that shock in this population may not always be cardiogenic in nature.
The critical outcome of interest was 30-day all-cause mortality. We prespecified that in instances where 30-day mortality was unavailable, we would include mortality data that were closest to 30 days (minimum of 14 days). Other outcomes included acute kidney injury (as defined by study authors), initiation of renal replacement therapy, initiation of MCS (not relevant for the MCS network), duration of hemodynamic support, hospital length of stay, and major bleeding (as defined by study authors).
Data extraction
One investigator (S. M. F.) collected the following variables from included articles: author information, year of publication, eligibility criteria, and number of patients using a predesigned data extraction sheet (ESM eTable 1). Two investigators (S. M. F. and R. M.) independently collected data related to descriptions of interventions and outcomes. Where available, we only selected subgroups of patients from within RCTs that met our inclusion criteria. Disagreements were resolved through discussion.
Risk of bias assessment
Two reviewers (S. M. F. and R. M.) independently assessed the risk of bias of the included studies using a modified Cochrane Collaboration tool,18 which included sequence generation, allocation sequence concealment, blinding, missing outcome data, and other bias. Reviewers resolved disagreement through discussion.
Data synthesis and analysis
For each outcome and each pair of interventions, we calculated and reported odds ratios (ORs) and corresponding 95% confidence intervals (CIs). Initially, we performed a conventional pairwise meta-analysis using a DerSimonian and Laird random-effects model for all comparisons with two or more RCTs.19 We assessed heterogeneity between RCTs for each direct comparison with visual inspection of forest plots, and the I2 statistic. We evaluated the feasibility of conducting network meta-analysis by: 1) evaluating the availability of evidence (e.g., number of trials, number of interventions); 2) evaluating homogeneity of study designs, patients, and characteristics of interventions across the body of evidence (transitivity assumption); 3) evaluating the structural properties of the network of evidence (e.g., connectivity); and 4) evaluating the coherence in network (using the “design-by-treatment” model20), and in each closed loop of the network (using the side-splitting approach21,22).
We performed frequentist random-effects network meta-analysis using the methodology of multivariate meta-analysis assuming a common heterogeneity parameter,22,23 as performed previously.24,25 Coherence assumption in the entire network was confirmed using a “design-by-treatment” model (global test), as described by Higgins et al.20 We also used the node splitting method to assess the presence of incoherence between direct and indirect estimates of the effect.21,26 For each outcome, we also estimated ranking probabilities using surface under the cumulative ranking curve (SUCRA), and mean treatment rankings, and rankograms.27 We conducted all analyses using Stata 16 (StataCorp LLC, College Station, TX, USA). As inotrope trials included patients with varied severity of shock, we performed network metaregression to adjust for the percentage of patients with lower severity (SCAI A–B) shock included in each individual trial, to assess for possible effect modification by this variable.
Assessment of certainty of evidence
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the certainty of evidence for each comparison.28 The certainty assessment addresses the domains of risk of bias, imprecision, inconsistency, indirectness, intransitivity, publication bias, and incoherence. Imprecision for each comparison was assessed at the network level, and not at the level of the direct or indirect estimate. We used a minimally contextualized approach to evaluate certainty in outcomes.29 As recommended, we described our findings using the informative narrative statements (“probably,” “may”) that reflected our certainty in the effect estimates.30
Results
Search results and study characteristics
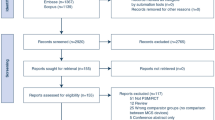
We identified 1,329 studies (Fig. 1). Following exclusion of duplicates, 1,193 studies were screened, and 48 underwent full-text review. We included 17 RCTs,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 with a total of 2,339 patients. For both the CAPITAL DOREMI39 and SURVIVE40 trials, we only included patients with acute MI, as published or obtained from study authors. All trials were parallel design. There were no trials that compared interventions from different classes (e.g., inotropes vs vasopressors).
Detailed study characteristics of the included RCTs are shown in Table 1, and risk-of-bias assessment is shown in ESM eTable 2. Forest plots depicting all direct estimates are shown in ESM eFig. 2, and statistical testing for incoherence is shown in ESM eTable 3.
Inotropes
Seven trials (1,145 patients) investigated the efficacy of inotropes in cardiogenic shock complicating acute MI.33,35,36,37,39,40,41 Importantly, five of the included trials primarily included patients with SCAI classification A–B and only partly C cardiogenic shock (“classic,” characterized by hypoperfusion requiring intervention with vasoactive support or MCS), and two primarily included patients with SCAI C class of shock. The relevant network plot is shown in Fig. 2a, with the GRADE summary of findings shown in Table 2. The associated SUCRA ranking is shown in ESM eTable 4.
Network plots for A) short-term mortality among inotrope agents; and B) short-term mortality among mechanical circulatory support treatments. The size of the node corresponds to the number of patients randomized to that intervention. The thickness of the line and the associated numbers correspond to the number of studies comparing the two linked interventions.
When compared with placebo, levosimendan probably reduced the odds of mortality (OR, 0.53; 95% CI, 0.33 to 0.87; moderate certainty). Importantly, the majority of trials included in the direct comparisons between levosimendan and placebo were those that enrolled patients with SCAI A-B shock. Milrinone (OR, 0.52; 95% CI, 0.19 to 1.39) and dobutamine (OR, 0.67; 95% CI, 0.30 to 1.49) may have no effect on mortality compared with placebo; however, this was based on low certainty evidence and limited by CIs that do not rule out the possibility of benefit or harm. Enoximone (OR, 1.58; 95% CI, 0.39 to 6.45) had an uncertain effect on mortality compared with placebo based on very low certainty evidence, and very wide confidence intervals that did not exclude important benefit and harm. Levosimendan may have no effect on mortality compared with dobutamine (OR, 0.80; 95% CI, 0.42 to 1.50), enoximone (OR, 0.34; 95% CI, 0.09 to 1.26), or milrinone (OR, 0.97; 95% CI, 0.41 to 2.28); however, conclusions related to these comparisons are all based on low certainty evidence and important imprecision. Milrinone may have no effect on mortality compared with dobutamine (OR, 0.77; 95% CI, 0.43 to 1.37); however, conclusions are limited by low-certainty evidence and large CIs. There were insufficient data in the included trials to investigate any of our prespecified secondary outcomes in either the network or conventional meta-analysis. We did not find statistically significant effect modification of our primary results when adjusting for the proportion of patients in each trial with SCAI A–B shock, though we were only able to include seven trials and a single closed loop in this analysis (ESM eTable 5).
Vasopressors
Only two RCTs evaluated the role of vasopressors,34,38 so network meta-analysis was not feasible. The major findings of these two trials are shown in ESM eTable 6. In a cardiogenic shock subgroup of the SOAP II trial, norepinephrine was associated with lower odds of mortality at 28 days compared with dopamine.34 The OptimaCC trial compared norepinephrine to epinephrine in patients with cardiogenic shock.38 This trial was terminated early due to higher incidence of a post hoc outcome of refractory shock in the patients receiving epinephrine, compared with those receiving norepinephrine (37% vs 7%, P = 0.008). The effect on 28-day mortality was not significantly different between the two groups (OR, 2.55; 95% CI, 0.84 to 7.72).
Mechanical circulatory support
In total, eight RCTs investigated MCS use in cardiogenic shock: three trials comparing IABP to a strategy of no MCS at all,42,44,47 four trials comparing IABP to pMCS (Impella or TandemHeart),32,43,45,46 and one trial comparing IABP with the combination of IABP and pMCS (Impella).31 The network plot is displayed in Fig. 2b, with the GRADE summary of findings shown in Table 3. The associated SUCRA ranking is shown in ESM eTable 7.
With regard to MCS, there may be no effect on mortality with the use of IABP (OR, 0.94; 95% CI, 0.69 to 1.28) or pMCS (OR, 0.96; 95% CI, 0.47 to 1.98) compared with a strategy involving no MCS; however, both comparisons are based on low-certainty evidence. There was an uncertain effect of combination of IABP and pMCS vs no MCS (OR, 5.56; 95% CI, 0.21 to 144.20; very low certainty). There may be no difference in mortality between IABP and pMCS (OR, 0.98; 95% CI, 0.51 to 1.88; low certainty). All other comparisons had uncertain effects (very low certainty evidence) because of very wide CIs.
Of the included MCS trials, five provided data on incidence of major bleeding.31,32,42,43,47 The associated network plot is depicted in ESM eFig. 3, with the GRADE summary of findings displayed in ESM eTable 8. Surface under the cumulative ranking curve (SUCRA) ranking is shown in ESM eTable 9. There may be no difference in major bleeding between IABP and no MCS (OR, 1.00; 95% CI, 0.69 to 1.45; low certainty), but IABP may be associated with lower incidence of major bleeding compared with pMCS (OR, 0.20; 95% CI, 0.06 to 0.69; low certainty). Finally, pMCS may be associated with higher incidence of major bleeding compared with a strategy of no MCS (OR, 4.91; 95% CI, 1.38 to 17.44; low certainty). Contribution matrices are shown in ESM eFig. 4.
Discussion
When acute MI is complicated by cardiogenic shock, patient mortality increases substantially.2,4 The use of inotropes can augment stroke volume and improve forward flow in a failing ventricle,10 and their use in shock associated with low cardiac output is endorsed by clinical practice guidelines.48 Nevertheless, the various inotropic agents have different mechanisms, and their relative use differs worldwide.11 Our work found that levosimendan was the only agent with evidence of possible benefit compared with placebo, though this was largely evident in patients without overt cardiogenic shock (SCAI A–B). That said, we did not see effect modification in our metaregression adjusting for the percentage of patients with SCAI A–B shock in the individual trials, though we were strongly limited by sample size in this analysis. Levosimendan has unique properties and is thought to improve myocardial efficiency without either increasing myocardial oxygen demand or improving ventricular relaxation.14,49 Use of this drug is not currently approved in North America, where clinicians may favor other inotropes, such as dobutamine or milrinone. Importantly, because these trials were primarily conducted in patients with lower severity of shock (SCAI A–B), we would caution clinicians on the application of these findings to patients with higher severity of shock, where the use of a single inotrope in isolation is uncommon.11 Our network meta-analysis also provides the first estimates of effect for both dobutamine and milrinone against placebo (as there has never been a direct, randomized comparison), and while low certainty evidence suggested no difference between these agents and placebo, we were limited by imprecision and could not rule out possible harm. Recent clinical practice guidelines recommend the use of inotrope therapy in patients with cardiogenic shock, despite a lack of randomized evidence to support this intervention.48 Nevertheless, given our findings and the known potential harms of these agents,50,51 whether inotropes truly provide benefit to justify routine use in cardiogenic shock is unclear.
Vasopressors represent an alternative type of vasoactive medication that might be used for the treatment of cardiogenic shock.52 Our search found only two RCTs investigating the efficacy of vasopressors, both evaluating the use of norepinephrine. Although conducted as a subgroup analysis as part of the SOAP II trial, norepinephrine was found to be associated with reduced mortality, compared with dopamine, in patients with cardiogenic shock.34 Similarly, the OptimaCC trial compared norepinephrine to epinephrine in patients with cardiogenic shock, and was stopped early because a significantly higher proportion of patients had refractory shock in the epinephrine group.38 Metabolic parameters (i.e., hyperlactatemia) were more deranged among patients receiving epinephrine. Thus, while not amenable to meta-analysis, these trials suggest that norepinephrine should likely remain the vasopressor of choice in cardiogenic shock, particularly when a rapid agent is required.3,10 Nevertheless, the large paucity of data highlights the need for further RCTs in this area.
Finally, we evaluated the relative efficacy of different types of MCS in the management of patients with cardiogenic shock. As stated, the primary goal of MCS is to provide temporary hemodynamic support in cardiogenic shock as a bridge to recovery or transplant,12 though evidence surrounding the efficacy of this strategy is limited. Our study did not find any important differences between IABP or pMCS (Impella or TandemHeart), compared with a strategy that did not include any MCS in patients with cardiogenic shock. These findings are in keeping with existing evidence surrounding pMCS.53 Importantly, the use of pMCS may be associated with higher incidence of major bleeding, compared with the use of IABP or a strategy of no MCS. Use of MCS has been increasing over time,12 and the use of this technology has outpaced the evidence. Given the costs and resources associated with MCS,54,55 additional trials are necessary to evaluate the safety, efficacy, and optimal patient selection in cardiogenic shock. Importantly, our meta-analysis did not include any studies involving VA–ECMO, and randomized trials evaluating its efficacy in cardiogenic shock are ongoing.
Our study has important limitations. First, in our attempts to reduce heterogeneity, our individual meta-analyses included a relatively small number of trials and patients. This resulted in imprecision, which was accounted for in GRADE assessments and conclusions. Although we did not find any statistical evidence of incoherence, our treatment networks contained only a few closed loops per outcomes, with a relatively small number of included studies, and as such we cannot exclude the existence of potentially important incoherence. Several therapies (including vasopressin, phenylephrine, and VA-ECMO) have not been tested in RCTs, so could not be included in this analysis. Furthermore, despite our attempts to focus largely on patients with cardiogenic shock secondary to acute MI, there is inherent heterogeneity within that population. Some patients might have refractory left ventricular, right ventricular, or biventricular failure, some may be amenable to percutaneous coronary intervention, while others may require cardiac surgery for revascularization. Severity of illness likely differs across trial populations and data from the differing stages of cardiogenic shock need to be studied to identify key time periods to intervene.15 All of these factors are likely to influence prognosis and were not easily accounted for in our study. That being said, statistical measures of heterogeneity (such as incoherence) were not significant, suggesting that while clinical heterogeneity may exist, its impact on our effect estimates is unclear. Finally, while we sought to study various secondary outcomes, there was insufficient data to do so; therefore, we cannot rule out differences in efficacy between these therapies with regard to such outcomes.
Conclusion
This systematic review and network meta-analyses evaluated different supportive therapies for cardiogenic shock complicating acute MI. With regard to inotropes, levosimendan was the only agent showing possible reduction in mortality with moderate certainty when compared with placebo, but did not show benefit compared with any other inotrope, and our analysis was largely limited to trials with at-risk or evolving cardiogenic shock. Little randomized data exist on vasopressors, but the available evidence suggests that norepinephrine may be associated with reduced mortality, compared with dopamine or epinephrine. Finally, neither IABP or pMCS provided benefit compared with a strategy with no MCS, but likely higher incidence of major bleeding was seen with pMCS. Taken together, our study summarizes the available evidence for supportive treatment of cardiogenic shock, while also highlighting important areas for further investigation.
References
van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017; 136: e232–68. https://doi.org/10.1161/cir.0000000000000525
Mebazaa A, Combes A, van Diepen S, et al. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med 2018; 44: 760–73. https://doi.org/10.1007/s00134-018-5214-9
Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J 2019; 40: 2671–83. https://doi.org/10.1093/eurheartj/ehz363
Goldberg RJ, Samad NA, Yarzebski J, Gurwitz J, Bigelow C, Gore JM. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med 1999; 340: 1162–8. https://doi.org/10.1056/nejm199904153401504
Jung RG, Di Santo P, Mathew R, et al. Implications of myocardial infarction on management and outcome in cardiogenic shock. J Am Heart Assoc 2021; 10: e021570. https://doi.org/10.1161/jaha.121.021570
Aissaoui N, Puymirat E, Delmas C, et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur J Heart Fail 2020; 22: 664–72. https://doi.org/10.1002/ejhf.1750
Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 1999; 341: 625–34. https://doi.org/10.1056/nejm199908263410901
Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med 2017; 377: 2419–32. https://doi.org/10.1056/nejmoa1710261
Henry TD, Tomey MI, Tamis-Holland JE, et al. Invasive management of acute myocardial infarction complicated by cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2021; 143: e815–29. https://doi.org/10.1161/cir.0000000000000959
Levy B, Buzon J, Kimmoun A. Inotropes and vasopressors use in cardiogenic shock: when, which and how much? Curr Opin Crit Care 2019; 25: 384–90. https://doi.org/10.1097/mcc.0000000000000632
Scheeren TW, Bakker J, Kaufmann T, et al. Current use of inotropes in circulatory shock. Ann Intensive Care 2021; 11: 21. https://doi.org/10.1186/s13613-021-00806-8
Combes A, Price S, Slutsky AS, Brodie D. Temporary circulatory support for cardiogenic shock. Lancet 2020; 396: 199–212. https://doi.org/10.1016/s0140-6736(20)31047-3
Ni hici T, Boardman HM, Baig K, et al. Mechanical assist devices for acute cardiogenic shock. Cochrane Database Syst Rev 2020; 6: CD013002. https://doi.org/10.1002/14651858.cd013002.pub2
Uhlig K, Efremov L, Tongers J, et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev 2020; 11: CD009669. https://doi.org/10.1002/14651858.cd009669.pub4
Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019; 94: 29–37. https://doi.org/10.1002/ccd.28329
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. https://doi.org/10.1136/bmj.n71
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–84. https://doi.org/10.7326/m14-2385
Higgins JP, Savović, Page MJ, Sterne JA. Revised Cochrane risk of bias tool for randomized trials (ROB 2.0), 2016. Available from URL: https://www.unisa.edu.au/contentassets/72bf75606a2b4abcaf7f17404af374ad/rob2-0_indiv_main_guidance.pdf (accessed July 2022).
DerSimonian R, Laìrd N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. https://doi.org/10.1016/0197-2456(86)90046-2
Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012; 3: 98–110. https://doi.org/10.1002/jrsm.1044
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. https://doi.org/10.1136/bmj.327.7414.557
White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012; 3: 111–25. https://doi.org/10.1002/jrsm.1045
White IR. Network meta-analysis. Stata J 2015; 15: 951–85.
Fernando SM, Di Santo P, Sadeghirad B, et al. Targeted temperature management following out-of-hospital cardiac arrest: a systematic review and network meta-analysis of temperature targets. Intensive Care Med 2021; 47: 1078–88. https://doi.org/10.1007/s00134-021-06505-z
Fernando SM, Tran A, Sadeghirad B, et al. Noninvasive respiratory support following extubation in critically ill adults: a systematic review and network meta-analysis. Intensive Care Med 2022; 48: 137–47. https://doi.org/10.1007/s00134-021-06581-1
Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006; 101: 447–59. https://doi.org/10.1198/016214505000001302
Yepes-Nuñez JJ, Li SA, Guyatt G, et al. Development of the summary of findings table for network meta-analysis. J Clin Epidemiol 2019; 115: 1–13. https://doi.org/10.1016/j.jclinepi.2019.04.018
Brignardello-Petersen R, Bonner A, Alexander PE, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018; 93: 36–44. https://doi.org/10.1016/j.jclinepi.2017.10.005
Brignardello-Petersen R, Florez ID, Izcovich A, et al. GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ 2020; 371: m3900. https://doi.org/10.1136/bmj.m3900
Santesso N, Glenton C, Dahm P, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 2020; 119: 126–35. https://doi.org/10.1016/j.jclinepi.2019.10.014
Bochaton T, Huot L, Elbaz M, et al. Mechanical circulatory support with the Impella® LP5.0 pump and an intra-aortic balloon pump for cardiogenic shock in acute myocardial infarction: the IMPELLA-STIC randomized study. Arch Cardiovasc Dis 2020; 113: 237–43. https://doi.org/10.1016/j.acvd.2019.10.005
Burkhoff D, Cohen H, Brunckhorst C, O'Neill WW, TandemHeart Investigators Group. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J 2006; 152: e1–8. https://doi.org/10.1016/j.ahj.2006.05.031
Caldicott LD, Hawley K, Heppell R, Woodmansey PA, Channer KS. Intravenous enoximone or dobutamine for severe heart failure after acute myocardial infarction: a randomized double-blind trial. Eur Heart J 1993; 14: 696–700. https://doi.org/10.1093/eurheartj/14.5.696
De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362: 779–89. https://doi.org/10.1056/nejmoa0907118
Fuhrmann JT, Schmeisser A, Schulze MR, et al. Levosimendan is superior to enoximone in refractory cardiogenic shock complicating acute myocardial infarction. Crit Care Med 2008; 36: 2257–66. https://doi.org/10.1097/ccm.0b013e3181809846
Husebye T, Eritsland J, Müller C, et al. Levosimendan in acute heart failure following primary percutaneous coronary intervention-treated acute ST-elevation myocardial infarction. Results from the LEAF trial: a randomized, placebo-controlled study. Eur J Heart Fail 2013; 15: 565–72. https://doi.org/10.1093/eurjhf/hfs215
Jia Z, Guo M, Zhang YQ, Liang HQ, Zhang LY, Song Y. Efficacy of intravenous levosimendan in patients with heart failure complicated by acute myocardial infarction. Cardiology 2014; 128: 195-201. https://doi.org/10.1159/000357864
Levy B, Clere-Jehl R, Legras A, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2018; 72: 173–82. https://doi.org/10.1016/j.jacc.2018.04.051
Mathew R, Di Santo P, Jung RG, et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N Engl J Med 2021; 385: 516–25. https://doi.org/10.1056/nejmoa2026845
Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA 2007; 297: 1883–91. https://doi.org/10.1001/jama.297.17.1883
Moiseyev VS, Põder P, Andrejevs N, et al. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J 2002; 23: 1422–32. https://doi.org/10.1053/euhj.2001.3158
Ohman EM, Nanas J, Stomel RJ, et al. Thrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: results of the TACTICS Trial. J Thromb Thrombolysis 2005; 19: 33–9. https://doi.org/10.1007/s11239-005-0938-0
Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017; 69: 278–87. https://doi.org/10.1016/j.jacc.2016.10.022
Prondzinsky R, Lemm H, Swyter M, et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med 2010; 38: 152–60. https://doi.org/10.1097/ccm.0b013e3181b78671
Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008; 52: 1584–8. https://doi.org/10.1016/j.jacc.2008.05.065
Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005; 26: 1276–83. https://doi.org/10.1093/eurheartj/ehi161
Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287–96. https://doi.org/10.1056/nejmoa1208410
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2022; 145: e895–1032. https://doi.org/10.1161/cir.0000000000001063
Cholley B, Levy B, Fellahi JL, et al. Levosimendan in the light of the results of the recent randomized controlled trials: an expert opinion paper. Crit Care 2019; 23: 385. https://doi.org/10.1186/s13054-019-2674-4
Felker GM, Benza RL, Chandler AB, et al. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol 2003; 41: 997–1003. https://doi.org/10.1016/s0735-1097(02)02968-6
Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J 2002; 144: 1102–8. https://doi.org/10.1067/mhj.2002.125620
Squara P, Hollenberg S, Payen D. Reconsidering vasopressors for cardiogenic shock: everything should be made as simple as possible, but not simpler. Chest 2019; 156: 392–401. https://doi.org/10.1016/j.chest.2019.03.020
Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 2017; 38: 3523–31. https://doi.org/10.1093/eurheartj/ehx363
Fernando SM, Qureshi D, Tanuseputro P, et al. Mortality and costs following extracorporeal membrane oxygenation in critically ill adults: a population-based cohort study. Intensive Care Med 2019; 45: 1580–9. https://doi.org/10.1007/s00134-019-05766-z
Fernando SM, Qureshi D, Tanuseputro P, et al. Long-term mortality and costs following use of Impella® for mechanical circulatory support: a population-based cohort study. Can J Anesth 2020; 67: 1728–37. https://doi.org/10.1007/s12630-020-01755-9
Author contributions
Shannon M. Fernando, Rebecca Mathew, Benjamin Hibbert, and Bram Rochwerg conceived the study idea. Shannon M. Fernando, Rebecca Mathew, and Bram Rochwerg coordinated the systematic review. Shannon M. Fernando and Rebecca Mathew designed the search strategy. Shannon M. Fernando and Rebecca Mathew screened abstracts and full-texts. Shannon M. Fernando and Rebecca Mathew acquired the data and judged risk of bias in the studies. Behnam Sadeghirad verified the data and performed the analyses. Bram Rochwerg created the GRADE evidence profiles. All authors interpreted the data analyses. All authors co-wrote and revised the manuscript for intellectual content. All authors provided their final approval for manuscript submission. Benjamin Hibbert and Bram Rochwerg contributed equally as co-senior authors. All authors agree to be accountable for all aspects of the work.
Disclosures
Dr. Behnam Sadeghirad reports receiving funding from PIPRA AG (www.pipra.ch), outside of the submitted work. Dr. Daniel Brodie reports receiving research support from ALung Technologies, outside of the submitted work, and was previously on their medical advisory board. He has been on the medical advisory boards for Abiomed, Xenios, Medtronic, Inspira, and Cellenkos. Dr. Emilie P. Belley-Côté is supported by the E.H. Moran Campbell McMaster Career Research Award. Dr. Eddy Fan reports receiving personal fees from ALung Technologies, Aerogen, Baxter, Boehringer-Ingelheim, GE Healthcare, Inspira, and Vasomune, outside of the submitted work. Dr. Alain Combes reports receiving personal fees from Maquet, Xenios AG, and Baxter International Inc., outside of the submitted work. None of the other authors report any conflict of interest.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernando, S.M., Mathew, R., Sadeghirad, B. et al. Inotropes, vasopressors, and mechanical circulatory support for treatment of cardiogenic shock complicating myocardial infarction: a systematic review and network meta-analysis. Can J Anesth/J Can Anesth 69, 1537–1553 (2022). https://doi.org/10.1007/s12630-022-02337-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02337-7