Abstract
Purpose
Double-lumen endotracheal tubes (DL-ETT) and bronchial blockers (BB) are frequently used to allow one-lung ventilation (OLV) during video-assisted thoracic surgery (VATS). Recently, faster lung collapse has been documented with a BB than with a DL-ETT. The physiologic mechanisms behind this faster collapse remained unknown. We aimed to measure ambient air absorption (Vresorb) and intra-bronchial pressure (Pairway) into the non-ventilated lung during OLV using DL-ETT and BB.
Methods
Patients undergoing VATS and OLV for lung resection were randomly assigned to have measurements made of Vresorb or Pairway within the non-ventilated lung using either a DL-ETT or BB.
Results
Thirty-nine patients were included in the analyses. The mean (standard error of the mean [SEM]) Vresorb was similar in the DL-ETT and BB groups [504 (85) vs 630 (86) mL, respectively; mean difference, 126; 95% confidence interval [CI], -128 to 380; P = 0.31]. The mean (SEM) Pairway became progressively negative in the non-ventilated lung in both the DL-ETT and the BB groups reaching [-20 (5) and -31 (10) cmH2O, respectively; mean difference, -11; 95% CI, -34 to 12; P = 0.44] at the time of the pleural opening.
Conclusions
During OLV before pleural opening, entrainment of ambient air into the non-ventilated lung occurs when the lumen of the lung isolation device is kept open. This phenomenon is prevented by occluding the lumen of the isolation device before pleural opening, resulting in a progressive build-up of negative pressure in the non-ventilated lung. Future clinical studies are needed to confirm these physiologic results and their impact on lung collapse and operative outcomes.
Trial registration
www.clinicaltrials.gov (NCT02919267); registered 28 September 2016.
Résumé
Objectif
Les tubes endotrachéaux à double lumière (TET-DL) et les bloqueurs bronchiques (BB) sont fréquemment utilisés pour l’isolation pulmonaire pendant une chirurgie thoracique assistée par vidéoscopie. Récemment, un affaissement pulmonaire plus rapide avec un BB qu’avec un TET-DL a été documenté. Les mécanismes physiologiques derrière cet affaissement plus rapide demeurent inconnus. Notre objectif était de mesurer l’absorption de l’air ambiant (Vresorb) et la pression intra-bronchique (Paérienne) dans le poumon non ventilé pendant la ventilation à un poumon en utilisant un TET-DL et un BB.
Méthode
Les patients subissant une chirurgie thoracique assistée par vidéoscopie et recevant une ventilation unipulmonaire à l’aide d’un TET-DL ou d’un BB pour une résection pulmonaire ont été aléatoirement assignés à des mesures de Vresorb ou Paérienne dans le poumon non ventilé.
Résultats
Trente-neuf patients ont été inclus dans les analyses. La Vresorb moyenne (erreur-type sur la moyenne) était similaire dans les groupes TET-DL et BB [504 (85) vs 630 (86) mL, respectivement; différence moyenne, 126; intervalle de confiance [IC] 95 %, -128 à 380; P = 0,31]. La Paérienne moyenne (erreur-type sur la moyenne) est devenue progressivement négative dans le poumon non ventilé dans les groupes TET-DL et BB en atteignant [-20 (5) et -31 (10) cmH2O, respectivement; différence moyenne, -11; IC 95 %, -34 à 12; P = 0,44] au moment de l'ouverture de la plèvre.
Conclusion
Pendant la ventilation unipulmonaire avant l’incision de la plèvre, un appel d’air ambiant dans le poumon non ventilé se produit quand la portion du dispositif d’isolation pulmonaire est maintenue ouverte. Ce phénomène peut être évité en occluant la lumière du dispositif d’isolation pulmonaire avant l’ouverture de la plèvre, ce qui entraînera une accumulation progressive de pression négative dans le poumon non ventilé. De futures études cliniques sont nécessaires pour confirmer ces résultats physiologiques et leur impact sur l'affaissement pulmonaire et les devenirs opératoires.
Enregistrement de l’étude
www.clinicaltrials.gov (NCT02919267); enregistrée le 28 septembre 2016.
Similar content being viewed by others
Video-assisted thoracoscopic surgery (VATS) has progressively increased over the last two decades, becoming the standard approach for the majority of thoracic surgical procedures.1,2 As a consequence, effective lung isolation and collapse have become increasingly essential to ensure optimal surgical exposure. While many authors still recommend the use of a double-lumen endotracheal tube (DL-ETT) for one-lung ventilation (OLV), the optimal method to achieve efficient lung collapse remains unknown, particularly with the use of the most recent bronchial blocker (BB) designs.3,4,5,6,7
Contrary to many earlier studies,6 we previously documented that using a BB was associated with a significantly faster time to complete lung collapse than with using a DL-ETT during VATS.8 Importantly, the technique used in that study significantly differed from previous reports in that the internal channel of the BB was occluded at the beginning of OLV.9 Whether the improvement in complete lung collapse was related to the BB’s internal channel occlusion is not known, highlighting the need to better understand the physiology of lung collapse during OLV.10
Therefore, we aimed to determine the physiologic changes in ambient air absorption (Vresorb) and airway pressure (Pairway) in the non-ventilated lung during OLV with the use of either a DL-ETT or BB. We hypothesized that OLV would be associated with the development of a negative Pairway within the non-ventilated lung (i.e,, from absorption atelectasis) resulting in entrainment of ambient air11 that could explain delayed lung collapse when the lumen of the non-ventilated lung is kept open. As the BB is longer and has a much smaller internal channel compared with the DL-ETT, we further hypothesized that ambient air absorption (Vresorb) would be greater with DL-ETT than with BB.
Methods
We performed a prospective unblinded randomized-controlled trial in patients requiring OLV for elective unilateral lung resection using VATS (NCT02919267). The study protocol was approved on 8 September 2016 by the local Research Ethics Board (CER 21299) and written informed consent was obtained from all participants. We excluded patients with previous or anticipated difficult intubation, prior chemotherapy, prior thoracic radiotherapy, ipsilateral thoracic surgery, sternotomy, forced expiratory volume in one second (FEV1) < 50% predicted, pulmonary infection, endobronchial mass, pleural and/or interstitial pathology, and tracheostomy.
Before induction of anesthesia, patients were assigned to one of the four groups based on the variable to be measured and the method of OLV: VResorb with either a DL-ETT (#1) or a BB (#2), and Pairway with either a DL-ETT (#3) or a BB (#4). The software minim.exe (York, United Kingdom) was used for the computer-generated randomized allocation using minimization procedures, controlling for age, sex, body mass index, FEV1, and side of surgery to ensure balance between groups.12 Post-randomization exclusion criteria defined a priori included bronchoscopic findings precluding the use of the specific randomized devices, severe oxygen desaturation necessitating ventilation of the non-dependent lung, and air leaks occurring at the level of the bronchial isolation. All patients excluded after randomization were replaced by another randomized patient.
Anesthesia and lung isolation
Anesthesia management consisted of routine monitoring,13 standardized induction of anesthesia with propofol, sufentanil and rocuronium, and maintenance with 1 minimum alveolar concentration volatile anesthesia.8 Patients were intubated with either a left-sided DL-ETT (Mallinckrodt™ left endobronchial tube; Mallinckrodt Medical, Cornamaddy, Athlone, Westmeath, Ireland) with size determined according to recommendations,14 or a 8.0-mm internal diameter single-lumen endotracheal tube. Lung isolation was confirmed with flexible bronchoscopy (FB) to ensure that the distal tip of the bronchial lumen of the DL-ETT did not obstruct the interlobar carina.15 The BB (Fuji Uniblocker; Fuji Systems, Tokyo, Japan) was introduced in the endotracheal tube using FB guidance and positioned in the main bronchus with its cuff deflated.
Bilateral lung ventilation was provided with a tidal volume of 6–8 mL·kg−1 of ideal body weight, a fraction of inspired oxygen (FiO2) of 1.0, and a positive end-expiratory pressure (PEEP) of 5 cmH2O to obtain an end-tidal carbon dioxide between 35 and 45 mmHg16,17 Once the patient was placed in lateral decubitus position, the correct position of the lung isolation device was re-confirmed with FB. The experimental protocol was initiated at the beginning of OLV with the chest closed, as long as the hemodynamics and ventilation were stable.
Experimental protocol
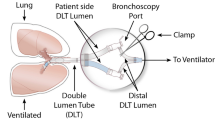
After an apneic period of one minute in both groups, and with the airway in direct communication with the ambient atmospheric pressure, lung isolation was initiated by inflating the cuff of the BB or by clamping the non-ventilated lung lumen of the DL-ETT to interrupt the ventilation (Fig. 1). One-lung ventilation was provided with tidal volumes of 4–6 mL·kg−1 of ideal body weight, PEEP of 5 cmH2O and FiO2 was gradually tapered to 0.50 if tolerated (oxygen saturation ≥ 92%).
For patients randomized to the Vresorb groups (Fig. 2), a 2-L bag (Roxon, Etobicoke, ON, Canada) was filled using a 1,000-mL calibrated syringe (Hans Rudolph Inc., Shawnee, KS, USA) via a three-way valve (Hans Rudolph Inc., Shawnee, KS, USA) and using a pneumotachometer (Hans Rudolph Inc., Shawnee, KS, USA) to precisely measure 1,000 mL of air (FiO2 0.21) (Fig. 2 A–C). This half-filled 2-L bag was used to ensure that there was no restriction in the movement of air between the lung and the bag, reproducing the situation of opening the non-ventilated lung to ambient air. The pneumotachometer signal was amplified with Pneumotach Amplifier 1 (Series 1110, Hans Rudolph Inc., Shawnee, KS, USA) and digitized at 200 Hz using a MP100 analogic/numeric system (BIOPAC Systems, Goleta, CA, USA). Volumes were measured with ACQKnowledge (BIOPAC Systems, Goleta, CA, USA) by integration of flows measured with the pneumotachometer.
Volume change measurement setup. A–B) The respiratory bag and three-way valve were connected to the non-ventilated lung lumen of the double-lumen tube using a 13-mm tube. C–D) The respiratory bag and three-way valve were connected to the internal channel of the bronchial blocker with the use of a 15–4-mm adaptor
During the one minute apneic period at the beginning of OLV, the three-way valve was connected to the non-ventilated lumen of the DL-ETT or to the internal channel of the BB through an adaptor to ensure that any air flow that might occur during OLV would take place between the non-ventilated lung and the bag (Fig. 2 B–D). Immediately prior to pleural opening, the bag was closed and an apnea period of one minute was provided during which we temporarily deflated the BB cuff or disconnected the breathing circuit from the DL-ETT. After pleural opening, the bag was reopened for a total of 60 min of OLV. At the end of the observation period, the bag was emptied with the 1-L syringe through the pneumotachometer to measure its residual volume. Vresorb was calculated as initial minus final volume. After observing that most air absorption was occurring while the chest was closed, we modified the protocol for the final ten patients by adding a second bag to the three-way valve to specifically measure Vresorb before and after pleural opening using the methods described above. The time to pleural opening was defined as the period between beginning of OLV and surgical pneumothorax in minutes.
For patients randomized to Pairway groups, a pressure-tubing catheter was connected to the Luer lock adaptor located on the side of the occluding system mounted at the extremity of the DL-ETT (Fig. 3A) or to the Luer lock adaptor of the BB (Fig. 3B) during the apneic period of one minute at the beginning of OLV. The catheter was connected to a differential pressure transducer (AD Instruments, Colorado Springs, CO, USA) and Pairway were continuously monitored. Signals were amplified with a CD15 carrier demodulator (Validyne Enginereing, CA, USA) then digitized at 5 Hz and sampled using an MP100 analogic/numeric system. Immediately prior to pleural opening, an apnea period was re-established as described above, and thereafter measurements resumed until 60 min of OLV. Tracings were recorded and off-line analyses were accomplished using ACQKnowledge. Data were exported in Excel (Microsoft, WA, USA) and pressures were averaged every 30 sec, excluding aberrant measures (above and below 2 standard deviations [SD] of the mean). As there was no flow of air in the Pairway measurements groups, the pressure measured at the extremity of the DL-ETT or BB was assumed to be equal to the pressure in the distal airways of the non-ventilated lung.
Airway pressure measurement setup for double-lumen endotracheal tubes and bronchial blockers. A) For double-lumen endotracheal tubes, the pressure transducer was connected to the non-ventilated lung lumen with the use of a connecting piece occluded to the ambient air and stiff arterial pressure tubing (black arrow). B) For bronchial blockers, the pressure transducer was connected on the Luer lock adaptor of the internal channel
Statistical analysis
Continuous and categorical variables were respectively expressed using mean (standard error of the mean [SEM]) or frequency, unless otherwise specified. Characteristic variables expressed in percentage were analyzed using Chi square or Fisher’s exact test. VResorb was analyzed using one-way analysis of variance (ANOVA) using separate residual variances in each group as effect that specifies heterogeneity in the covariance structure was significant (heteroscedasticity) compared with the same variance between groups (i.e., the assumption of equality of variances was rejected when performing the Brown and Forsythe test for homogeneity of variance). The Satterthwaite’s degree of freedom statement was added for unequal variance structures. Pairway measured from initiation of OLV to pleural opening were similarly analyzed using a two-way ANOVA. Two experimental factors were defined: one associated with the comparison between two groups (DL-ETT vs BB) and factor fixed, and one associated with the comparison among results from the time periods (0–10 min) and factor fixed. Interaction terms between the fixed factors were also defined. The data were analyzed using a repeated mixed model. An autoregressive covariance structure was used to consider the dependency among repeated measurements. The same statistical approach was used to analyze Pairway measured from pleural opening to 30 min after. The normality assumptions were verified with the Shapiro–Wilk test after a Cholesky factorization on residuals.
The primary endpoint was Vresorb, whereas Pairway was measured to provide a physiologic explanation of air inflow. Assuming a net mean (SD) air influx of 300 (110) mL with the DL-ETT (11) vs 150 (110) mL with the BB after initiation of OLV, with an alpha of 0.05 and a power of 0.80, we estimated that we would require a sample size of nine patients per group. We thus randomized 40 patients (ten subjects per group). The results were considered significant with a P < 0.05. All analyses were conducted using the statistical package SAS, version 9.4 (SAS Institute Inc, Cary, NC, USA) and R (R Core Team (2016), Foundation for Statistical Computing, Vienna, Austria.).
Results
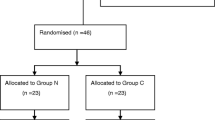
Seventy-three patients requiring OLV for VATS were screened (Fig. 4) and 49 patients met eligibility. Ten patients were excluded after randomization because the lung could not be isolated with the assigned device (n = 3), air leaked persistently during lung isolation (n = 4), or the operative lung needed ventilating before the end of the observation period (n = 3). A total of 39 patients were included in the final statistical analysis. Patient characteristics and the side of the surgery were similar between the four groups (Table).
Analysis of air entrainment during OLV showed that mean (SEM) Vresorb was similar for DL-ETT and BB [504 (85) and 630 (86) mL, respectively; mean difference, 126 mL; 95% confidence interval (CI), -128 to 380; P = 0.31] (Fig. 5A). The majority of the resorbed volume was noted during the period between initiation of OLV and pleural opening (Fig. 5B) and became negligible thereafter. Analysis of the Pairway during closed chest OLV showed that mean (SEM) Pairway became progressively negative over time with both isolation devices (P < 0.001 compared with initiation of OLV), reaching comparable levels with DL-ETT and BB before pleural opening (-20 [5] vs -31 [10] cmH2O, respectively; mean difference, -11 cmH2O; 95% CI, -33 to 12; P = 0.44) (Fig. 6A). After surgical pneumothorax, Pairway approached atmospheric pressure in both DL-ETT and BB groups (P = 0.84) (Fig. 6B).
Gas volume change quantification. A) Amount of ambient air entrained into the non-ventilated lung throughout the observation period according to lung isolation device. The mean (SEM) Vresorb was 504 (85) mL for DL-ETT and 630 (86) mL for BB (P = 0.31). (B) Amount of ambient air absorbed in the non-ventilated lung before and after pleural opening (includes the last ten patients); P = 0.61 before pleural opening and P = 0.58 after pleural opening. BB = bronchial blocker; DL-ETT = double-lumen tube. Data presented as mean ± standard error of the mean
Intra-bronchial pressures according to pleural opening. A) Intra-bronchial pressures measured from initiation of one-lung ventilation to pleural opening; P < 0.001 for change in intra-bronchial pressure over time whereas; P = 0.44 for double-lumen endotracheal tube (DL-ETT) vs bronchial blocker (BB). B) Intra-bronchial pressures measured from pleural opening to 30 min after; P < 0.05 for change in intra-bronchial pressures over time, and P = 0.84 for Double-lumen endotracheal tube vs bronchial blocker. PO = pleural opening. Data presented as mean ± standard error of the mean
Discussion
The present study documented that during OLV (and when exposed to the same protocol), DL-ETT and BB present similar physiology with a significant entrainment of ambient air within the non-ventilated lung. From the beginning of OLV until surgical pneumothorax, we observed the build-up of negative pressure within the non-ventilated lung, with Pairway averaging -20 to -30 cmH2O. When the non-ventilated lumen of the DL-ETT or the internal channel of the BB was open, this resulted in the aspiration of 500–600 mL of ambient air. These phenomena were predominantly observed before pleural opening with minimal air movement or pressure gradient thereafter. We believe that the entrainment of ambient air before pleural opening, can delay lung collapse during OLV and that occluding the non-ventilated lung may fasten lung collapse. Nevertheless, further studies are needed before recommending clamping the BB internal channel or the DL-ETT lumen during thoracic surgeries.
Many of the previous studies describing the physiology of lung collapse during OLV focused on two distinct phases that occur after pleural opening.16,17 The first phase occurs early after the opening of the pleural cavity and corresponds to a quick partial collapse of the lung due to its intrinsic recoil, until it reaches its closing volume.18 The second phase corresponds to the reabsorption by the capillary bed of the gas remaining within the alveoli. The speed of reabsorption depends, among other factors, on the solubility of the alveolar gas.19,20 Nevertheless, no studies specifically assessed both Vresorb and Pairway before pleural opening with the use of DL-ETT and BB.11,18,21 Importantly, we believe that lung collapse might begin before pleural opening. Therefore, a better understanding of the physiology of lung collapse during OLV is essential to optimize lung collapse. To the best of our knowledge, this is the first study to extensively describe the changes in Vresorb and Pairway, using DL-ETT and BB, during OLV before and after pleural opening for VATS.
Following two-lung ventilation with an FiO2 of 1.0, the alveoli contain a high oxygen partial pressure (PAO2). When initiating OLV, the non-dependent lung is separated from the ventilator and it presumably reaches its functional residual capacity. Thereafter, the oxygen-rich content of alveoli is expected to be absorbed by the capillaries.21,22 Nevertheless, this theory has never adequately be shown experimentally. This study documented that the occlusion of the non-ventilated lung in the presence of an intact pleural interface creates a sub-atmospheric airway pressure, probably caused by O2 absorption by the alveoli, since airflow is prevented by occluding the non-ventilated lumen. Conversely, when the non-ventilated lung remains in contact with ambient air, O2 absorption by the alveoli results in a continuous entrainment of air (FiO2 0.21) into the non-ventilated lung until pleural opening. Ultimately, this air (i.e., nitrogen) entrainment probably reduces PAO2 and eventually reduces the speed of subsequent absorption atelectasis since it is proportional to the solubility of the gas in the alveoli.23 This is consistent with earlier observation by Pfitzner et al. who documented the entry of atmospheric air into the non-ventilated lung a few minutes before opening the pleura with the DL-ETT.11 In their discussion, they proposed the air influx could increase nitrogen in the alveoli and lower PAO2, which would slow the lung collapse. Our results confirmed that movement of air occurs with the DL-ETT during OLV before pleural opening, and that the same phenomenon happens with the BB when its internal channel is open. We also showed that this inflow was considerable before pleural opening, whereas no clinically significant movement of air occurred when the pleural space was open to ambient air. These phenomena likely impacts the speed of lung collapse during OLV.
Interestingly, previously published papers could not convincingly confirm whether DL-ETT or BB were superior in terms of quality and speed of lung collapse during VATS.3,6,7,24 It has been hypothesized that the smaller internal channel of the BB may be responsible for the slower lung collapse as it may restrict the egress of gas during the first phase of lung collapse. This hypothesis was supported by trials showing a faster collapse using the disconnection technique and bronchial suction with the BB.9,25 In our study, while the diameter of the lumen of the DL-ETT and BB differed significantly, a similar volume of air inflow was observed with both devices. We thus speculate that differences in occlusion methods rather than the isolation device could also have influenced these results.26 For example, in our previous study,8 a significantly faster collapse was observed with the BB compared with the DL-ETT. In that study, we had chosen to occlude the internal channel of the BB while the DL-ETT lumen was open. We believe that the occlusion precluded the entrainment of nitrogen, likely resulting in a higher PAO2 and a shorter time to complete lung collapse in the BB group compared with the DL-ETT. Based on that hypothesis, the occlusion of the DL-ETT lumen should reproduce the same results, although this statement remains to be confirmed by future studies.
This study has a number of limitations. First, ten patients were removed from the analysis as they met post-randomization exclusion criteria. These exclusion criteria were defined a priori and reflect real-life limitations and challenges of lung isolation and OLV with specific devices. Additionally, given that the primary goal of this study was to investigate the changes in Vresorp and Pairway during OLV with DL-ETT and BB, analyzing the data as intention-to-treat would have led to misleading results. Second, because of the need for specialized airway adaptors and measuring tools, we were unable to blind the main investigator and attending anesthesiologist to the assigned study group. Also, the clinical impact of occluding the non-ventilated lung on the speed of lung collapse remains unanswered. Finally, future studies will aim to reveal the clinical impact on per-operative outcomes, such as the quality of lung collapse, its impact on the length of surgery and the incidence of postoperative respiratory complications.27
Conclusion
The use of OLV before pleural opening results in the entrainment of a significant volume of air into the non-ventilated lung when the lumen of the isolation device is kept open. This phenomenon is prevented by occluding the central lumen before pleural opening, resulting in a progressive build-up of negative pressure in the non-ventilated lung. Future clinical studies are needed to confirm these physiologic results and their impact on lung collapse and operative outcomes.
References
Shah RD, D’Amico TA. Modern impact of video assisted thoracic surgery. J Thorac Dis 2014; 6(Suppl 6): S631-6.
Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008; 135: 247-54.
Campos JH, Reasoner DK, Moyers JR. Comparison of a modified double-lumen endotracheal tube with a single-lumen tube with enclosed bronchial blocker. Anesth Analg 1996; 83: 1268-72.
Bauer C, Winter C, Hentz JG, Ducrocq X, Steib A, Dupeyron JP. Bronchial blocker compared to double-lumen tube for one-lung ventilation during thoracoscopy. Acta Anaesthesiol Scand 2001; 45: 250-4.
Campos JH, Kernstine KH. A comparison of a left-sided Broncho-Cath with the torque control blocker univent and the wire-guided blocker. Anesth Analg 2003; 96: 283-9.
Clayton-Smith A, Bennett K, Alston RP, et al. A comparison of the efficacy and adverse effects of double-lumen endobronchial tubes and bronchial blockers in thoracic surgery: a systematic review and meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth 2015; 29: 955-66.
Dumans-Nizard V, Liu N, Laloë PA, Fischler M. A comparison of the deflecting-tip bronchial blocker with a wire-guided blocker or left-sided double-lumen tube. J Cardiothorac Vasc Anesth 2009; 23: 501-5.
Bussières JS, Somma J, Del Castillo JL, et al. Bronchial blocker versus left double-lumen endotracheal tube in video-assisted thoracoscopic surgery: a randomized-controlled trial examining time and quality of lung deflation. Can J Anesth 2016; 63: 818-27.
Yoo JY, Kim DH, Choi H, Kim K, Chae YJ, Park SY. Disconnection technique with a bronchial blocker for improving lung deflation: a comparison with a double-lumen tube and bronchial blocker without disconnection. J Cardiothorac Vasc Anesth 2014; 28: 904-7.
Grocott HP. Optimizing lung collapse with a bronchial blocker: it’s not what you use, but how you use it. J Cardiothorac Vasc Anesth 2018; 32: e93-4.
Pfitzner J, Peacock MJ, McAleer PT. Gas movement in the nonventilated lung at the onset of single-lung ventilation for video-assisted thoracoscopy. Anaesthesia 1999; 54: 437-43.
Altman DG, Bland JM. Treatment allocation by minimisation. BMJ 2005; . https://doi.org/10.1136/bmj.330.7495.843.
Merchant R, Chartrand D, Dain S, et al. Guidelines to the practice of anesthesia - revised edition 2016. Can J Anesth 2016; 63: 86-112.
Brodsky JB, Lemmens HJ. Tracheal width and left double-lumen tube size: a formula to estimate left-bronchial width. J Clin Anesth 2005; 17: 267-70.
Fortier G, Coté D, Bergeron C, Bussières JS. New landmarks improve the positioning of the left Broncho-Cath double-lumen tube-comparison with the classic technique. Can J Anesth 2001; 48: 790-4.
Moreault O, Lacasse Y, Bussières JS. Calculating ideal body weight: keep it simple. Anesthesiology 2017; 127: 203-4.
Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301-8.
Pfitzner J, Peacock MJ, Harris RJ. Speed of collapse of the non-ventilated lung during single-lung ventilation for thoracoscopic surgery: the effect of transient increases in pleural pressure on the venting of gas from the non-ventilated lung. Anaesthesia 2001; 56: 940-6.
Ko R, McRae K, Darling G, et al. The use of air in the inspired gas mixture during two-lung ventilation delays lung collapse during one-lung ventilation. Anesth Analg 2009; 108: 1092-6.
Yoshimura T, Ueda K, Kakinuma A, Sawai J, Nakata Y. Bronchial blocker lung collapse technique: nitrous oxide for facilitating lung collapse during one-lung ventilation with a bronchial blocker. Anesth Analg 2014; 118: 666-70.
Joyce CJ, Baker AB, Kennedy RR. Gas uptake from an unventilated area of lung: computer model of absorption atelectasis. J Appl Physiol 1985; 1993(74): 1107-16.
Joyce CJ, Baker AB, Parkinson R, Zacharias M. Nitrous oxide and the rate of gas uptake from an unventilated lung in dogs. Br J Anaesth 1996; 76: 292-6.
Vooren PH. A nomographic ruler for body temperature, pressure, saturated with water (BTPS) correction. Am Rev Respir Dis 1967; 96: 324-5.
Lu Y, Dai W, Zong Z, et al. Bronchial blocker versus left double-lumen endotracheal tube for one-lung ventilation in right video-assisted thoracoscopic surgery. J Cardiothorac Vasc Anesth 2018; 32: 297-301.
El-Tahan MR. A comparison of the disconnection technique with continuous bronchial suction for lung deflation when using the Arndt endobronchial blocker during video-assisted thoracoscopy: a randomised trial. Eur J Anaesthesiol 2015; 32: 411-7.
Bussières JS, Moreault O, Couture EJ, Provencher S. Optimizing lung collapse with a bronchial blocker: it’s not what you use, but how you use it, part II. J Cardiothorac Vasc Anesth 2019; . https://doi.org/10.1053/j.jvca.2018.09.025.
Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth 2017; 118: 317-34.
Author contributions
Olivier Moreault contributed to study conception and design, acquisition of data, interpretation of data, drafting the article, revising the article critically for important intellectual content, and final approval of the version to be published. Etienne J. Couture contributed to study conception and design, interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published. Steeve Provencher contributed to study conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published. Jacques Somma, Jens Lohser, Paula A. Ugalde, Jérôme Lemieux, and François Lellouche contributed to study conception and design, revising the article critically for important intellectual content, and final approval of the version to be published. Jean S. Bussières contributed to study conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, and final approval of the version to be published.
Acknowledgement
We express our gratitude to Mrs. Catherine Tremblay, B. eng., M. Sc., for help with the physics considerations and Mr. Serge Simard, M. Sc., for help with the statistics.
Disclosures
None.
Funding statement
Bourse Rosario-Denis, Fondation Anesthésie-Réanimation du Québec; Fondation Institut Universitaire de Cardiologie et de Pneumologie de Québec.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, former Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moreault, O., Couture, E.J., Provencher, S. et al. Double-lumen endotracheal tubes and bronchial blockers exhibit similar lung collapse physiology during lung isolation. Can J Anesth/J Can Anesth 68, 791–800 (2021). https://doi.org/10.1007/s12630-021-01938-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-021-01938-y