Abstract
Purpose
Lung deflation during one-lung ventilation (OLV) is thought to be faster using a double-lumen endotracheal tube (DL-ETT) than with a bronchial blocker, especially when the non-ventilated lumen is opened to allow egress of air from the operative lung. Nevertheless, ambient air can also be entrained into the non-ventilated lumen before pleural opening and subsequently delay deflation. We therefore hypothesized that occluding the non-ventilated DL-ETT lumen during OLV before pleural opening would prevent air entrainment and consequently enhance operative lung deflation during video-assisted thoracoscopic surgery (VATS).
Methods
Thirty patients undergoing VATS using DL-ETT to allow OLV were randomized to having the lumen of the operative lung either open (control group) or occluded (intervention group) to ambient air. The primary outcome was the time to lung collapse evaluated intraoperatively by the surgeons. The T50, an index of rate of deflation, was also determined from a probabilistic model derived from intraoperative video clips presented in random order to three observers.
Results
The median [interquartile range] time to lung deflation occurred faster in the intervention group than in the control group (24 [20–37] min vs 54 [48–68] min, respectively; median difference, 30 min; 95% confidence interval [CI], 14 to 46; P < 0.001). The estimated T50 was 32.6 min in the intervention group compared with 62.3 min in the control group (difference, − 29.7 min; 95% CI, − 51.1 to − 8.4; P = 0.008).
Conclusion
Operative lung deflation during OLV with a DL-ETT is faster when the operative lumen remains closed before pleural opening thus preventing it from entraining ambient air during the closed chest phase of OLV.
Trial registration
www.clinicaltrials.gov (NCT03508050); registered 27 September 2017.
Résumé
Objectif
On pense que la déflation pulmonaire pendant la ventilation unipulmonaire (VUP) est plus rapide à l’aide d’un tube endotrachéal à double lumière (TET-DL) qu’avec un bloqueur bronchique, surtout lorsque la lumière non ventilée est ouverte pour permettre l’évacuation de l’air du poumon opéré. Néanmoins, l’air ambiant peut également être entraîné dans la lumière non ventilée avant l’ouverture pleurale et ainsi retarder la déflation. Nous avons donc émis l’hypothèse que l’occlusion de la lumière non ventilée du TET-DL pendant la VUP avant l’ouverture de la plèvre empêcherait l’entraînement d’air et accélérerait par conséquent la déflation du poumon opéré pendant une chirurgie thoracoscopique vidéo-assistée (VATS).
Méthode
Trente patients subissant une VATS avec un TET-DL pour permettre une VUP ont été randomisés à une ouverture (groupe témoin) ou à une occlusion (groupe intervention) de la lumière du poumon opéré à l’air ambiant. Le critère d’évaluation principal était le temps jusqu’au collapsus du poumon tel qu’évalué pendant l’opération par les chirurgiens. Le T50, un indice du taux de déflation, a également été déterminé à partir d’un modèle probabiliste dérivé de clips vidéo peropératoires présentés de façon randomisée à trois observateurs.
Résultats
Le temps médian [écart interquartile] jusqu’à la déflation du poumon était plus court dans le groupe d’intervention que dans le groupe témoin (24 [20-37] min vs 54 [48-68] min, respectivement; différence médiane, 30 min; intervalle de confiance [IC] à 95 %, 14 à 46; P < 0,001). Le T50 estimé était de 32,6 min dans le groupe d’intervention comparativement à 62,3 min dans le groupe témoin (différence, -29,7 min; IC 95 %, -51,1 à -8,4; P = 0,008).
Conclusion
La déflation du poumon opéré pendant une VUP avec un TET-DL est plus rapide quand la lumière opératoire reste fermée avant l’ouverture pleurale, l’empêchant ainsi d’entraîner l’air ambiant pendant la phase pré ouverture pleurale de la VUP.
Enregistrement de l’étude
www.clinicaltrials.gov (NCT03508050); enregistrée le 27 septembre 2017.
Similar content being viewed by others
One-lung ventilation (OLV) is an integral part of thoracic anesthesia, particularly for video-assisted thoracoscopic surgery (VATS). Lung isolation with either a double-lumen endotracheal tube (DL-ETT) or a bronchial blocker (BB) enables operative lung collapse to facilitate surgical exposure of intra-thoracic structures. Ventilation of the operative lung is interrupted by clamping the corresponding DL-ETT lumen (Fig. 1) or by inflating the endobronchial balloon of the BB. It is widespread practice amongst anesthesiologists to open the internal lumen of the BB or the bronchoscopy port of the operative lumen of the DL-ETT during OLV in the belief that it will facilitate lung collapse through venting of the lung and subsequent egress of air.
Studies comparing the quality of lung collapse between DL-ETTs and BBs have been inconclusive with respect to optimal management of the operative lung.1,2,3,4 Contrary to most reports,5,6 our research group has obtained faster lung collapse using BBs then DL-ETTs, notably when the internal channel of the BB remains occluded.7 Based on those findings, Moreault et al.8 showed that a negative pressure develops in the non-dependent lung before pleural opening when using either lung isolation device, resulting in entrainment of ambient air via the internal channel of the BB or the bronchoscopy port of the DL-ETT if left open to air.
Herein, we hypothesized that the time to collapse of the operative lung would be improved if the lung isolation device is prevented from communicating with ambient air before the pleura is opened. Thus, we compared the conventional management of having the operative lung lumen of the DL-ETT left open to air (control group) with an intervention group where the operative lumen is clamped until after pleural opening. The primary endpoint was the time to lung collapse during OLV in patients undergoing VATS (as assessed by the surgeon during the procedure).
Methods
We conducted a prospective single-blinded randomized-controlled trial on patients requiring OLV for lobectomy or segmentectomy using a VATS approach. We excluded patients with contraindications to the insertion of a DL-ETT (difficult intubation, tracheostomy, tracheobronchial abnormalities on bronchoscopy or computed tomography-scan findings), severe chronic obstructive pulmonary disease (forced expiratory volume in the first second < 50%), prior thoracic surgery or radiotherapy, known pleural or interstitial pathology, active pulmonary infection, or an endobronchial mass. It was also decided a priori to exclude patients post-randomization if significant desaturation (SaO2 < 90%) occurred and/or if there was a need for re-inflation of the collapsed lung during surgery. Every patient excluded post-randomization was to be replaced by an additional randomized patient. The study protocol was approved on 28 July 2017 by the local Research Ethics Board (Centre de Recherche de l’Institut Universitaire de Cardiologie et de Pneumologie de Québec-Université Laval CER#21436) and registered at ClinicalTrials.gov (#NCT03508050). All participants provided written informed consent.
Lung isolation management
Research staff enrolled consecutive patients meeting the eligibility criteria. Sealed envelopes containing the computer-generated group assignment were opened immediately prior to anesthesia induction. After application of routine monitoring,9 with invasive blood pressure monitoring where appropriate, anesthesia was induced with intravenous midazolam, propofol, sufentanil, and rocuronium, and maintained with sevoflurane or desflurane. Following induction, patients were intubated with a left-sided DL-ETT (Mallinckrodt Medical, Cornamaddy, Athlone, Westmeath, Ireland). The DL-ETT size was based on Brodsky’s recommendations10 and its position was confirmed by flexible bronchoscopy.11 Intubation and positioning of the DL-ETT was either performed or directly supervised by the attending anesthesiologist.
During two-lung ventilation, the tidal volume was set at 6–8 mL·kg−1 using ideal body weight, with a respiratory rate of 10 breaths·min−1, positive end-expiratory pressure (PEEP) of 5 cmH2O, and a fraction of inspired oxygen (FiO2) of 1.0. The fresh gas flow was set at 2 L·min−1. After lateral decubitus patient positioning, the DL-ETT placement position was re-confirmed with flexible bronchoscopy. Continuous spirometry was used to monitor for bronchial cuff leakage and/or misplacement.12 During OLV, we used tidal volumes of 4–6 mL·kg−1 at a respiratory rate of 14–18 breaths·min−1, with a PEEP of 5 cmH2O. The FiO2 was initially set at 1.0 and gradually titrated down while maintaining a SaO2 > 92%.
Lung collapse endpoints
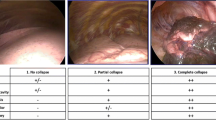
During the operative procedure, the surgeons were blinded to the group assignment by maintaining a cover over the DL-ETT connector during surgery. The time of complete lung collapse (primary objective) was recorded by the research assistant as soon as the surgeon deemed that it had occurred. The clinical quality of lung collapse was assessed and scored by the surgeon according to a three-point visual grading scale (Fig. 2).7
The secondary endpoints were the clinical quality of lung collapse scored by the surgeon upon entering the thoracic cavity, and at ten and 20 min following pleural opening; the incidence of completed lung collapse at the end of surgery; expired O2 concentration at initiation of OLV and at pleural opening; expiratory volume at pleural opening; quality of oxygenation at 25 min; intervention to optimize lung collapse; surgery duration; and reported postoperative chest radiographic anomalies. The thoracoscopic portion of each operation was also continuously video recorded for separate postoperative assessment of lung collapse by three evaluators blinded to group assignments.
Before initiation of OLV, a custom-made device was connected to the operative lung connector of the DL-ETT (Figs 3 and 4). The apparatus consisted of an oxygen analyzer (MaxO2 ME; Maxtec, Salt Lake City, UT, USA), a 2-L Douglas bag (Roxon, Etobicoke, ON, Canada), and a three-way stopcock (Hans Rudolph Inc., Shawnee, KS, USA). This device allowed us to measure the expiratory volume and expiratory concentration of O2 during two one-minute periods, the first one at the initiation of OLV and the second one just following the pleural opening.
Set up for measurements of O2 concentration and expiratory volume. A: double-lumen tube (DL-ETT). B: clamp directed to the non-ventilated DL-ETT lumen on the patient side of the bronchoscopy port. C: in-line oxygen analyzer (D). E: three-way stopcock connected to a two-litre Douglas bag (F). G: clamp on the non-ventilated DL-ETT lumen on the ventilator-side of the bronchoscope port. H: ventilator circuit. Z: clamp on the non-ventilated DL-ETT lumen on the patient side
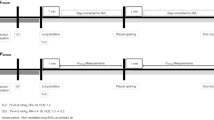
Study timeline. Panel A The patient is placed in lateral decubitus position during two-lung ventilation. Panel B One-lung ventilation is initiated by clamping the DL-ETT distal limb of the operative lung and attaching the custom-made device (Fig. 3) to the proximal limb. The stopcock was opened to room air (Fig. 3) and oxygen measurements were obtained from the operative lung lumen of the DL-ETT in both study groups. Three oxygen concentration measurements were obtained at 15, 35, and 55 sec. Immediately after obtaining the oxygen measurements (one minute after the initiation of OLV), patients were entered into their respective study group. Panel C Patients are entered into their respective study group (one minute after initiation of OLV). In the control group, the bronchoscopy port was left open to ambient air for the duration of surgery, while in the intervention group, the non-ventilated DL-ETT lumen was clamped on the patient side of the bronchoscopy port until pleural opening (Clamp Z in Fig. 3 and panel C in Fig. 4). A study period of closed chest OLV was maintained for a period of ten to 15 min before starting the surgical procedure. Panel D At the moment of pleural opening, the stopcock was opened to the Douglas bag in both study groups and the clamp (clamp Z) occluding the operative lumen of the DL-ETT in the intervention group was removed. Oxygen measurements were again obtained at 15, 35, and 55 sec, and the residual gas escaping from the operative lung was collected in the Douglas bag for 60 sec in both study groups. Thereafter, the bronchoscopy port was left open to ambient air in both groups until the end of the operation. DL-ETT = double-lumen endotracheal tube; OLV = one-lung ventilation
The gas volume collected in the Douglas bag was measured with a calibrated 0.5-L syringe (Rudolph Volume Calibration Seringue/Series 5550; Hans Rudolph Inc, Shawnee, KS, USA) and a pneumotachometer (Rudolph Linear Pneumotachs/series 4700; Hans Rudolph Inc.). The pneumotachometer signal was amplified with Pneumotach Amplifier 1 (Series 1110; Hans Rudolph Inc.) then digitized at 200 Hz using an MP100 analogic/numeric system. The volume was measured by the integration of the flow using the ACQKnowledge software package (BIOPAC Systems, Goleta, CA, USA). The timeline of the experimental period is detailed in Fig. 4.
Twenty-five minutes after pleural opening, the FiO2 and SaO2 were recorded, and an arterial blood gas was performed. The duration of surgery was defined as time from pleural opening to insertion of the lung specimen bag into the thoracic cavity. Interventions required to reconfirm or correct lung isolation were recorded. Postoperative chest radiographs were obtained in the postanesthetic recovery room and reviewed by a radiologist for the presence of atelectasis or lung contusion.
Video analysis of lung collapse
Video recordings were edited by one of the investigators (S.P.) into clips of 10–15 sec duration at three-minute intervals for the first 30 min of surgery, five minutes intervals for the next 30 min of surgery, and ten-minute intervals for the remainder of the procedure unless complete lung collapse had been reached (as assessed by the surgeon). If complete lung collapse did occur, two video clips were created at five and ten minutes after complete lung collapse, followed by video clips every 20 min until the end of the surgery. A total of 423 clips were created (241 in the control group and 182 in the intervention group). Video clips were duplicated and rearranged in two playlists based on two different randomization sequences (A and B) generated via Excel. Three blinded evaluators (one thoracic surgeon, one staff cardiothoracic anesthesiologist, and one respirologist) were asked to score lung collapse on each individual clip of the two random playlists using the same visual grading scale used by the surgeons (Fig. 2).
Statistical analysis
The required sample size was calculated based on an estimated mean (standard deviation [SD]) time to reach complete lung collapse of 10 (4) min vs 28 (25) min based on previously published data.7 Based on an alpha of 0.05 and a power of 0.80, 11 patients were required per group. We chose to include 15 patients per group for a total of 30 patients.
Continuous and nominal variables were expressed using mean (SD), median [interquartile range (IQR)], or percentage (%). Characteristic variables from perioperative data expressed in percentage were analyzed using Fisher’s exact test.
Continuous variables were analyzed using one-way analysis of variance (ANOVA). For some variables, separate residual variances in each group were used because the effect that specifies heterogeneity in the covariance structure was significant (heteroscedasticity) compared with the same variance between groups. The Satterthwaite’s degree of freedom statement was added for variables analyzed using unequal variance structures. Expired O2 data measured at initiation of OLV were analyzed using a two-way ANOVA. Two experimental factors were defined: one associated with the comparison between two groups (intervention vs control), factor fixed and one associated with the comparison among results from the time periods (15, 35, and 55 sec), factor fixed with interaction terms between the fixed factors. The data were analyzed using a repeated mixed model. An autoregressive covariance structure was used to consider the dependency among repeated measurements. A group effect was defined for variance estimations as the covariance structure was heterogeneous. The same statistical approach was used to analyze expired O2 at pleural opening. The normality assumptions were verified with the Shapiro–Wilk tests after a Cholesky factorization on residuals. The results were considered significant with a P < 0.05. All analyses were conducted using the statistical package SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA) and R (R Core Team 2016, Foundation for Statistical Computing, Vienna, Austria).
Probability model for the video analysis of lung collapse
A probability model was used to derive the T50, an index of the rate of lung collapse. The T50, the time at which the probability of observing a total lung collapse is 50%, is based on a general model previously published7,13:
where P (total LC) is the probability of observing total lung collapse (LC) at a given time, T50 is the time when the probability is 50%, and N is the hill coefficient representing the steepness of this sigmoidal relationship.
The model was fitted to the data (scoring of the post-surgical video clips by three blinded observers). The maximum likelihood estimation was used to estimate the parameters of the model. The first-order normal approximation bootstrap method14 was used to estimate the standard error (SE) of the parameters. A two-tailed t-test was performed. A P < 0.05 was considered statistically significant. For every parameter of the model, the SE and difference between groups, the 95% confidence interval (CI) of the difference between groups, and the associated P values are reported.
We assessed goodness of fit using the percentage of data points predicted correctly, the performance probability Pk described by Smith,15 and the visual assessment of the “measured” probability described by Somma.13
A more detailed discussion of our statistical analysis can be found in eAppendix 1 (available as Electronic Supplementary Material [ESM]).
Results
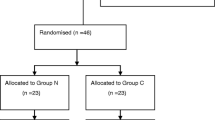
Of the 45 screened patients over a three-month period, 15 patients per group were randomized and assessed (Fig. 5). One video recording was unreadable, reducing the total number of video studies to fourteen in the intervention arm. Demographic variables (Table 1) and perioperative data (Table 2) were similar between both groups.
The median [IQR] time to lung deflation occurred faster in the intervention group than the control group (24 [20–37] min vs 54 [48–68] min, respectively; median difference, 30 min; 95% CI, 14 to 46; P = 0.001).
Primary and secondary outcomes are displayed in Table 3. Complete lung collapse occurred earlier in the intervention group and the quality of lung collapse was better in the intervention group from the ten-minute time point onward than in the control group. At the 20-min time point, complete lung collapse had occurred in only one patient in the control group (7%) vs seven patients in the intervention group (47%; P = 0.04). At the conclusion of surgery, there were fewer patients (46.7%) in the control group reaching complete lung collapse than in the intervention group (86.7%) (P = 0.05).
Fifty-five seconds after pleural opening, the expired oxygen concentration in the control group was significantly lower than in the intervention group (11.9 vs 41.9%; P < 0.001) (Table 3). The residual gas volume after pleural opening was significantly greater in the control group compared with the intervention group (421 vs 178 mL; P < 0.001).
Finally, there was no difference in the quality of oxygenation at 25 min, need for interventions to optimize lung collapse, surgical duration, or chest radiography between the groups.
The results of the video clip analysis are found in Table 4. When the results of the video clip randomization review sequences (A and B) were pooled, the T50 was 32.6 (3.8) min in the intervention group vs 62.3 (9.4) min in the control group (mean difference, − 29.7 min; 95% CI, − 51.1 to − 8.4; P = 0.008).
Figure 6 shows the predicted and “measured” probabilities of observing lung deflation scores ≥ 2 (and only =3) as a function of time. The model offers a nearly unbiased prediction of the probability of observing scores ≥ 2 and only = 3. Additional detailed discussion on our results can be found in eAppendix 2 (see ESM).
Discussion
Our study shows that not allowing the non-ventilated DL-ETT lumen to communicate with ambient air during the closed chest time of OLV significantly accelerates lung collapse compared with the traditional practice of opening the bronchoscopy port to “vent” the collapsing lung. This was shown both by the primary endpoint of blinded clinical evaluation (24 vs 54 min; P < 0.001) and the secondary endpoint of the blinded video analysis determining lung deflation rate (T50 = 32.6 min vs 62.3 min; P = 0.008). Furthermore, we showed that allowing the operative lung to communicate with ambient air during closed chest OLV significantly increased the residual volume of gas in the operative lung at pleural opening (421 vs 178 mL; P < 0.001) and markedly reduced the oxygen concentration of the residual gas volume (11.9 vs 41.9%; P < 0.001) compared with excluding the operative lung from contact with the surrounding air during the same period.
Our group previously reported a faster complete lung collapse during OLV with the use of BB when compared with a DL-ETT control group.7 In that study, the internal channel of the BB was kept occluded throughout the procedure, and the cuff of the BB was deflated for a one-minute period upon opening of the pleural space.7 This study protocol was therefore comparable to what we describe for the intervention group in the present trial and illustrates the superiority of clamping or occluding the lumen directed to the operative lung.
We attempted to fill the void in knowledge about the physiology of lung collapse during OLV. In a separate study, we showed that, irrespective of lung isolation device (BB or DL-ETT), negative pressure develops in the operative lung during the closed chest stage of OLV resulting in entrainment of ambient air through the bronchoscopy port of the DL-ETT connector if left open upon lung isolation.6 This finding may explain why we previously observed faster lung collapse with an occluded BB compared with a DL-ETT open to ambient air.7
Previous studies on the physiology of lung collapse during OLV partly explain this result of better lung deflation when the operative lung DL-ETT lumen is kept closed before pleural opening. Pfitzner et al.16 showed that, before pleural opening, the positive pressure ventilation of the dependent lung generates paradoxical ambient air movement in and out of the operative lung through mediastinal displacement. The ensuing alveolar gas exchange resulted in an influx of ambient air into the lung via the open DL-ETT lumen. They hypothesized that air entrainment would increase nitrogen in the alveoli and lower partial pressure of oxygen in arterial blood and that the presence of nitrogen in the lung could delay the subsequent lung deflation upon pleural opening. In a different study, they proposed an ambient pressure oxygen reservoir apparatus connected to the operative lung’s bronchoscopy port to prevent the re-entry of nitrogen into the operative lung and hasten its subsequent collapse.17 In 2016, Pfitzner argued that while there was no published clinical study showing the efficacy of the ambient pressure oxygen reservoir apparatus, there was sufficient indirect and rationale evidence to support its routine use.18 Nevertheless, their three theories were never tested until now.
Interestingly, there is evidence supporting Pfitzner work16 from the 1952 study of Dale and Rahn on closed chest OLV of dogs.19 They showed an entrainment of gas to the non-ventilated lung, and that it was six to eight times faster if the operative lung was connected to a spirometer filled with 100% O2 instead of room air.
In 2009, Ko et al., compared the rate of lung collapse during OLV when different gas mixtures (air, O2 with FiO2 = 1.0 or N2O/O2 with FiO2 = 0.4) were used during two-lung ventilation prior to lung isolation. They found that the use of air prior to OLV delayed the speed of lung collapse and advocated de-nitrogenation prior to OLV as a means of improving the rate of lung collapse.20
Recently, Zhang et al., showed the role of pre-emptive OLV to improve the speed of lung collapse during VATS.21 The pre-emptive OLV they described was defined as a period of OLV prior to pleural opening during which time the operative lumen of the DL-ETT was clamped instead of being left open to ambient air. Their experimental pre-emptive OLV group is virtually identical to our intervention group, but their control group is different. While the bronchoscopy port of the operative lung was left open to ambient air in their control group as was done in ours, OLV of the control group was initiated immediately prior to the pleural opening. This limited the possibility of air entrainment in the lung in their control group. Conversely, in our control group, we initiated OLV and kept the bronchoscopy port of the operative lung open for a mean duration of 12 min prior to pleural opening, allowing significant time for ambient air to enter the lung and presumably slowing the speed of lung collapse compared with Zhang’s control group. They observed an improvement of time to satisfactory lung collapse of 35% in their intervention group compared with their control group while the improvement of time to total lung collapse was improved by 56% in our intervention group compared with our control group. The greater difference observed in our study could be explained by a slower lung collapse in our control group.
We added the T50 as a secondary endpoint similar to our previous study evaluating BBs vs DL-ETTs.7 This endpoint differs from the majority of publications20,21,22,23,24,25,26,27 on this topic, which chose surgical exposure or qualitative lung collapse as judged by the surgeon. We chose blinded assessments of randomized video clips based on a visual grading scale by three observers to ensure objectivity. Nevertheless, use of the same grading scale by surgeons at predefined times during the procedure resulted in similar results.
There are limitations in our study. While our approach aimed to minimize the subjective nature of evaluating lung collapse, our method relies on the quality of the video clips collected. Even if efforts were made to record a scanning view of the entire lung, there is always a possibility that some of the video clips were suboptimal.
The lung deflation optimization technique used in this study is described in Fig. 7 to allow for improved understanding and an easier clinical application. This technique needs to be carefully applied to obtain the expected results.
Lung deflation optimization technique. 1- Clamp the distal limb of the NVL to initiate OLV. 2- Open the bronchoscopy port of the NVL for one minute maximum. 3- Monitor the spirometry loop to detect inadequate lung isolation during this first minute. 4- Close the bronchoscopy port * after this first minute and keep it close until the pleural opening. 5- Open the bronchoscopy port at pleural opening and until the end of OLV. * From a clinical perspective, closing the bronchoscopy port is equivalent to clamping the patient side DLT lumen of the NVL that we used for the study purposes. According to conventional technique, the fourth step is omitted. Consequently, the bronchoscopy port of the NVL is kept open throughout the OLV (see Fig. 1). DLT = double-lumen tube; NVL = non-ventilating lung; OLV = one-lung ventilation
Conclusions
Our study used the time to complete lung collapse to show that the conventional management of closed chest OLV that “vents” the operative lung by opening the bronchoscopy port may not be optimal. Rather than facilitating lung collapse, opening the bronchoscopy port of the operative lung at the time of closed chest OLV allows for ambient air entrainment resulting in re-nitrogenation of the lung, increasing the residual gas volume at the time of pleural opening, decreasing the gas absorption (pre- and post-pleural opening), and reducing the speed of lung collapse. Preventing the operative lung from communicating with ambient air prior to pleural opening during OLV results in lower residual gas volumes with higher residual oxygen concentrations and, most importantly, markedly faster speed of lung collapse.
References
Huitema AN, Koers L, Preckel B. Data interpretation on the use of double-lumen tube (DLT) versus bronchial blocker (BB) for one-lung ventilation. J Cardiothorac Vasc Anesth 2017. DOI: https://doi.org/10.1053/j.jvca.2015.12.018.
Neustein SM. Pro: bronchial blockers should be used routinely for providing one-lung ventilation. J Cardiothorac Vasc Anesth 2015; 29: 234-6.
Clayton-Smith A, Bennett K, Alston RP, et al. A comparison of the efficacy and adverse effects of double-lumen endobronchial tubes and bronchial blockers in thoracic surgery: a systematic review and meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth 2015; 29: 955-66.
Brodsky JB. Con: a bronchial blocker is not a substitute for a double-lumen endobronchial tube. J Cardiothorac Vasc Anesth 2015; 29: 237-9.
Campos JH, Kernstine KH. A comparison of a Left-Sided Broncho-Cath® with the torque control blocker univent and the wire-guided blocker. Anesth Analg 2003; 96: 283-9.
Lu Y, Dai W, Zong Z, et al. Bronchial blocker versus left double-lumen endotracheal tube for one-lung ventilation in right video-assisted thoracoscopic surgery. J Cardiothorac Vasc Anesth 2018; 32: 297-301.
Bussières JS, Somma J, del Castillo JL, et al. Bronchial blocker versus left double-lumen endotracheal tube in video-assisted thoracoscopic surgery: a randomized-controlled trial examining time and quality of lung deflation. Can J Anesth 2016; 63: 818-27.
Moreault O, Couture EJ, Provencher S, et al. Double-lumen tubes and bronchial blockers exhibit similar lung collapse physiology during lung isolation. Can J Anesth 2021. DOI: https://doi.org/10.1007/s12630-021-01938-y.
Dobson G, Chong M, Chow L, et al. Guidelines to the practice of anesthesia - revised edition 2018. Can J Anesth 2018; 65: 76-104.
Brodsky JB, Lemmens HJ. Tracheal width and left double-lumen tube size: a formula to estimate left-bronchial width. J Clin Anesth 2005; 17: 267-70.
Bussières JS, Slinger P. Correct positioning of double-lumen tubes. Can J Anesth 2012; 59: 431-6.
Bardoczky GI, Levarlet M, Engelman E, deFrancquen P. Continuous spirometry for detection of double-lumen endobronchial tube displacement. Br J Anaesth 1993; 70: 499-502.
Somma J, Donner A, Zomorodi K, et al. Population pharmacodynamics of midazolam administered by target controlled infusion in SICU patients after CABG surgery. Anesthesiology 1998; 89: 1430-43.
Efron B, Tibshirani R. Statistical data analysis in the computer age. Science 1991; 253: 390-5.
Smith WD, Dutton RC, Smith NT. Measuring the performance of anesthetic depth indicators. Anesthesiology 1996; 84: 38-51.
Pfitzner J, Peacock MJ, McAleer PT. Gas movement in the nonventilated lung at the onset of single-lung ventilation for video-assisted thoracoscopy. Anaesthesia 1999; 54: 437-43.
Pfitzner J, Peacock MJ, Daniels BW. Ambient pressure oxygen reservoir apparatus for use during one-lung anaesthesia. Anaesthesia 1999; 54: 454-8.
Pfitzner J. The role of an ambient pressure oxygen source during one-lung ventilation for thoracoscopic surgery. Anaesth Intensive Care 2016; 44: 20-7.
Dale WA, Rahn H. Rate of gas absorption during atelectasis. Am J Physiol 1952; 170: 606-13.
Ko R, McRae K, Darling G, et al. The use of air in the inspired gas mixture during two-lung ventilation delays lung collapse during one-lung ventilation. Anesth Analg 2009; 108: 1092-6.
Zhang Y, Yan W, Fan Z, et al. Preemptive one lung ventilation enhances lung collapse during thoracoscopic surgery: a randomized controlled trial. Thorac Cancer 2019; 10: 1448-52.
Campos JH, Massa FC. Is there a better right-sided tube for one-lung ventilation? A comparison of the right-sided double-lumen tube with the single-lumen tube with right-sided enclosed bronchial blocker. Anesth Analg 1998; 86: 696-700.
Campos JH, Massa FC, Kernstine KH. The incidence of right upper-lobe collapse when comparing a right-sided double-lumen tube versus a modified left double-lumen tube for left-sided thoracic surgery. Anesth Analg 2000; 90: 535-40.
Campos JH, Kernstine KH. A comparison of a left-sided broncho-cath with the torque control blocker univent and the wire-guided blocker. Anesth Analg 2003; 96: 283-9.
Yoo JY, Kim DH, Choi H, Kim K, Chae YJ, Park SY. Disconnection technique with a bronchial blocker for improving lung deflation: a comparison with a double-lumen tube and bronchial blocker without disconnection. J Cardiothorac Vasc Anesth 2014; 28: 904-7.
Li Q, Zhang X, Wu J, Xu M. Two-minute disconnection technique with a double-lumen tube to speed the collapse of the non-ventilated lung for one-lung ventilation in thoracoscopic surgery. BMC Anesthesiol 2017. DOI: https://doi.org/10.1186/s12871-017-0371-x.
Quan X, Yi J, Huang Y, Zhang X, Shen L, Li S. Bronchial suction does not facilitate lung collapse when using a double-lumen tube during video-assisted thoracoscopic surgery: a randomized controlled trial. J Thorac Dis 2017; 9: 5244-8.
Author contributions
Jacques Somma and Jean S. Bussières made substantial contributions to study conception and design, interpretation of data, drafting the article, revising the article critically for important intellectual content, and final approval of the version to be published. Étienne J. Couture and Steeve Provencher made substantial contributions to study conception and design, interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published. Sabrina Pelletier, Antoine Somma, and Sarah-Elizabeth Guay were involved in acquisition of data, revising the article critically for important intellectual content, and final approval of the version to be published. Olivier Moreault, Jens Lohser, Paula A. Ugalde, Louise Vigneault, and Jérome Lemieux made substantial contributions to study conception and design, revising the article critically for important intellectual content, and final approval of the version to be published.
Acknowledgements
We express our gratitude to Mr. Serge Simard, M.Sc., for his help in statistical analysis.
Disclosures
None.
Funding statement
Fondation de l’Institut Universitaire de Cardiologie et de Pneumologie de Québec
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Former Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Somma, J., Couture, É.J., Pelletier, S. et al. Non-ventilated lung deflation during one-lung ventilation with a double-lumen endotracheal tube: a randomized-controlled trial of occluding the non-ventilated endobronchial lumen before pleural opening. Can J Anesth/J Can Anesth 68, 801–811 (2021). https://doi.org/10.1007/s12630-021-01957-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-021-01957-9