Abstract
Purpose
Aortic dissection is an infrequent but serious condition that often requires immediate operative intervention. We explore recent developments in the classification of aortic dissection and perioperative transesophageal echocardiography that assist with quantifying the severity of disease and facilitate its management.
Principal findings
We describe the pivotal role of echocardiography in relation to key surgical considerations such as cannulation, aortic root surgery, perfusion in the aortic arch vessels, stenting in hybrid arch repair, and timing of preventative surgery.
Conclusion
Developments in the classification of aortic dissection have improved our perspective and understanding of the key presenting features that affect mortality. Improvements in patient outcome may be achieved in part by appropriately timed echocardiography-guided surgery.
Résumé
Objectif
La dissection aortique est une maladie peu fréquente mais grave qui nécessite souvent une intervention opératoire immédiate. Nous explorons les développements récents survenus dans la classification de la dissection aortique et l’utilisation de l’échocardiographie transœsophagienne périopératoire pour aider à quantifier la gravité de la maladie et faciliter sa prise en charge.
Constatations principales
Nous décrivons le rôle-charnière de l’échocardiographie en relation avec diverses considérations chirurgicales clés, notamment la canulation, la chirurgie de la racine aortique, la perfusion des vaisseaux de l’arc de l’aorte, l’implantation d’endoprothèse pour la réparation hybride de l’arc, et le choix du moment opportun pour la chirurgie de prévention.
Conclusion
Les développements survenus dans la classification de la dissection aortique ont amélioré notre perspective et notre compréhension des caractéristiques de présentation clés qui affectent la mortalité. Des améliorations au niveau des pronostics des patients pourraient survenir en partie grâce à une chirurgie échoguidée planifiée au moment opportun.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aortic dissection is an infrequent but serious condition that often requires operative intervention. In this article, we discuss recent developments in classification schemes for aortic dissection in accordance with presenting factors. In addition, the role of echocardiography is considered for confirmation of correct surgical cannulation, guiding surgery involving aortic valve regurgitation, perfusion in the aortic arch vessels, hybrid arch repair, and timing of preventative surgery.
Anatomy and echocardiographic presentation
In patients with aortic dissection, there is disruption of the aortic intima and media, creating two compartments: a true lumen and a false lumen separated by an intimal flap.1 Blood in the true lumen communicates with that in the false lumen via the intimal tear (Fig. 1a). Using transesophageal echocardiography (TEE), we find that the true lumen is characterized by rapid systolic expansion, whereas the false lumen is represented by diastolic enlargement, often with evidence of spontaneous echocardiographic contrast.1,2 This feature occurs as a result of blood traversing a tear in the intimal flap and is shown by colour Doppler (Video 1a).3 In M-mode, this motion may be visualized with sufficient temporal resolution (Fig. 1b).4 Artifacts occur as a result of reverberation of ultrasound, particularly in a dilated ascending aorta and in the presence of a pulmonary artery catheter.5 An intimal flap may be distinguished from an imaging artifact when a linear structure is seen to move independently with respect to the aortic wall. Multiple two-dimensional scan views of the aorta may also be needed to confirm the presence of an intimal flap and to avoid misdiagnosis. It is likely that additional clarification may be obtained by three-dimensional TEE, which shows the dissection flap as a sheet of tissue rather than as a linear structure.6
Perioperative transesophageal echocardiography assessment of aortic dissection and associated complications. (a) Short-axis views of dissection in the descending thoracic aorta. With the use of colour flow Doppler, blood in the true lumen (TL) may be seen entering the false lumen (FL) via a tear (arrow head) of the intimal flap. There is a mobile thrombus (Tb) in the false lumen (Video 1a). (b) Colour M-mode across the long-axis view of the aortic arch showing independent mobility of the intimal flap (arrow head) with respect to the aortic wall. This image of high temporal resolution shows expansion of the true lumen (TL) in systole. (c) Mid-esophageal long-axis views of the aortic valve showing prolapse of the intimal flap into the left ventricular (LV) outflow tract and mal-coaptation of the aortic cusps during diastole (Video 1c). (d) Colour flow Doppler shows aortic regurgitation (AR) highlighted by an arrow (Video 1d). LA = left atrium; RV = right ventricle; Ao = aorta. (e) Upper esophageal long-axis (Video 1e) and (f) short-axis (Video 1f) views of the aortic arch showing an intimal flap and periaortic hematoma (H) near the origin of the left common carotid (LCC) artery. Colour Doppler shows antegrade flow in the true lumen (TL) during systole and some retrograde flow in the false lumen (FL) (Video 1e)
In experienced hands, TEE seems to be both sensitive and specific for detecting thoracic aortic dissection. In a meta-analysis of diagnostic studies evaluating the accuracy of TEE, computerized tomography, and magnetic resonance imaging, the sensitivities (95% confidence interval [CI]) were 98% (95 to 99), 100% (96 to 100), and 98% (95 to 99), respectively. The corresponding specificities (95% CI) were 95% (92 to 97), 98% (87 to 99), and 98% (95 to 100), respectively.7
Furthermore, extension of aortic dissection and hence reasons for hemodynamic instability may be identified by TEE. Detectable complications include cardiac tamponade;8 severe aortic regurgitation (Figs. 1c and 1d, Video 1c and 1d);9 proximal coronary dissection10 with myocardial ischemia;11 and severe cardiac failure.8 By identification of regional wall motion abnormalities, TEE has a particularly important role in screening for possible coronary artery disease and in determining the need for revascularization without a coronary angiogram.11
Malperfusion of other vital organs and hence adverse outcome may occur when dissection extends into the aortic arch and brachiocephalic vessels (Figs. 1e and 1f, Videos 1e and 1f).12 In addition to distinguishing malperfusion in vessels supplying the upper body, TEE has been shown to detect malperfusion in branches of the descending aorta (e.g., the celiac, mesenteric, and renal vessels) via transgastric views.13 Nevertheless, these views are difficult to obtain and care should be taken to avoid gastric injury.14
Classification and surgical approach
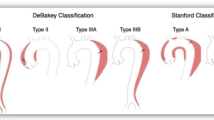
In clinical practice, the location and management of aortic dissection form the basis of its classification.15 Aortic dissection has been categorized by Stanford and DeBakey (Fig. 2).15,16 The Stanford classification is based on the location of the false lumen: Type A involving at least the ascending aorta and Type B referring to disease confined to the descending aorta beginning at the left subclavian artery.15 There are three categories in the DeBakey classification: 1) In DeBakey I, the false lumen extends from the ascending aorta to the descending aorta; 2) In DeBakey II, the false lumen was originally confined to the ascending aorta, but this category has now been modified to include disease in the aortic arch;16 and 3) In DeBakey III, as in Stanford Type B, the false lumen is confined to the descending aorta distal to the left subclavian artery. These classifications are useful for guiding requirement for surgery or conservative management: Stanford Type A or DeBakey I and II dissections require immediate surgical management, whereas Stanford Type B or DeBakey III aortic dissection may be managed conservatively or by endovascular stenting.17
Stanford, DeBakey, and Penn classification schemes for aortic dissection. In the Penn classification, Stanford Type A aortic dissection is integrated with DeBakey I (with descending aortic involvement) and DeBakey II (without descending aortic involvement). Similarly, for Stanford Type B aortic dissection, extension in the descending aortic may be in the thorax alone (DeBakey III extent A) or in both the thorax and abdomen (DeBakey III extent B). Further categorization is based on extension of clinical presentation with the following ischemic profile: a- absence of ischemia; b- branch vessel malperfusion; c- circulatory collapse; b and c- both branch vessel malperfusion and circulatory collapse. Within Stanford Type B or DeBakey III, Penn class a category may be subdivided into high risk (Type I) or low risk (Type II) of complications depending on the echo-anatomic features
There are several surgical approaches for Type A aortic dissection. Approaches for dissection in the aortic root and ascending aorta include: replacement of the ascending aorta and aortic valve, combined replacement using a valved-conduit (Bentall procedure in Fig. 3a),18,19 and valve-sparing aortic root surgery (Figs. 3b, 3c, and 3d) involving coronary arterial re-implantation.20,21 Valve-sparing aortic root repair comprises remodelling of the native aortic valve and root into neo-aortic sinuses (Yacoub procedure in Fig. 3b),22 re-suspension of the aortic valve within the tubular ascending aortic graft (David procedure in Fig. 3c),23 or integration of both techniques. Recent evidence suggests that integration of remodelling and re-suspension (Fig. 3d) may provide additional support for obtaining stable root dimensions and aortic valve competency.24-26
Aortic root surgery (a, b, c, & d) and arch procedures (e, f, & g) for Type A aortic dissection. (a) The Bentall procedure involves replacement of the ascending aorta, root, and aortic valve with a valved-conduit. (b) The Yacoub procedure involves remodelling of the aortic root and sinuses with preservation of the native aortic valve (AV). (c) In the David procedure, there is replacement of the aortic root, re-suspension of the native AV within the tube graft, and reinforcement with sub-commissural annuloplasty. (d) A new technique integrating both remodelling (Yacoub) and re-suspension with sub-commissural annuloplasty (David). (e) Hemiarch repair. (f) Total arch replacement with Carrel patch. (g) Total arch replacement with branched graft anastomosis to individual brachiocephalic vessels and elephant trunk prosthesis
Surgery to the aortic arch depends on whether there is dissection in the transverse arch and the brachiocephalic vessels.27 In the absence of involvement in the distal arch and brachiocephalic vessels, a hemiarch repair (Fig. 3e) is performed. A total arch replacement is necessary when the intimal tear occurs in the transverse arch (Carrel patch in Fig. 3f) and between the brachiocephalic vessels (branched graft in Fig. 3g).28
Recent advances in surgical technique involve use of a trifurcated graft (Fig. 4a), which is perfused intraoperatively by cannulation of the right axillary artery.29 This novel technique enables cerebral perfusion to be maintained and is known as “branch-first” aortic arch replacement.30 It involves end-to-end anastomosis of the three brachiocephalic vessels to the trifurcated graft during sequential de-branching and replacement of the diseased aortic arch (Fig. 4b). Since cerebral perfusion is almost uninterrupted, the need for deep hypothermic circulatory arrest and associated complications can be obviated.29-31
The trifurcated graft in aortic arch replacement and hybrid arch procedure. (a) The trifurcated graft. (b) The “branch-first” aortic arch replacement enables continuous perfusion of the brain and upper body using a trifurcated graft. Sequential anastomoses of the three brachiocephalic vessels to the three limbs of the graft provide almost uninterrupted cerebral perfusion as the diseased arch is debranched and replaced. This trifurcated graft receives oxygenated blood via the right axillary artery (Ax). The lower body is perfused by retrograde flow of blood from a cannula placed in the femoral artery. Since cerebral perfusion is almost uninterrupted, the need for deep hypothermic circulatory arrest and the associated complications can be obviated. IA = innominate artery; RCC = right common carotid artery; LCC = left common carotid artery; LSC = left subclavian artery. (c) Hybrid arch procedure involving an open trifurcated graft for revascularization of brachiocephalic vessels, debranching, and endovascular stenting (endostent) in the aortic arch
Hybrid arch surgery is another new development involving open revascularization of the brachiocephalic vessels and endovascular stenting.29,32 Instead of a traditional graft, an endovascular stent is deployed in the diseased aortic arch (Fig. 4c).33-37
Dissection extending from the arch into the descending thoracic aorta may require downstream repair with additional prosthetic extension, known as the elephant trunk (Figs. 3g and 4b). This tubular prosthesis may be stented (frozen) or left unstented in the proximal descending thoracic aorta to facilitate complete correction at a later stage.38,39
Additional classification
A novel framework has recently been created for grading presenting features as well as prognosis and perioperative management (Fig. 2). This Penn classification (from the University of Pennsylvania) stratifies Stanford Type A aortic dissection into the presence (DeBakey I) or absence (DeBakey II) of descending thoracic aortic involvement.40 Similarly, for Stanford Type B aortic dissection, extension in the descending aortic may be in the thorax alone (DeBakey III a) or in both the thorax and abdomen (DeBakey III b).41 Each permutation is then divided into four categories according to branch vessel disease and circulatory dysfunction: Class a–aortic dissection with absence of branch vessel malperfusion or circulatory collapse (no end-organ ischemia); Class b–aortic dissection with branch vessel malperfusion and localized ischemia in the neurologic, renal, or mesenteric organs; Class c–aortic dissection with circulatory collapse with or without cardiac involvement (generalized ischemia); Class b and c–aortic dissection with both branch vessel malperfusion and circulatory collapse (localized and generalized ischemia).40 For Type B dissection, the Penn Class a category may be subdivided into high risk (Type I) or low risk (Type II) of complications.41 Echocardiographic features suggestive of complications (Type I) include: aortic diameter > 40 mm, patent false lumen, ulcer-like projections and a primary tear in the concavity of the distal aortic arch.41
This Penn classification of aortic dissection is based on clinical manifestations superimposed on echocardiographic and other imaging modalities.40 The integration of the three classifications (Stanford, DeBakey, and Penn) with an ischemic profile provides a framework for grading the variable diagnostic features and likely prognosis.40,42 From observational data, it is evident that ischemia is associated with high mortality.43 In Type A aortic dissection, in-hospital mortality of patients presenting with local ischemia, circulatory collapse, or both were 24%, 24%, and 44%, respectively. In the absence of these features, in-hospital mortality was 14%.42 From multivariate regression, generalized ischemia or a combination of local and generalized ischemia were associated with significantly higher odds of intraoperative mortality.42 The implication of this association using the Penn classification is that patients in these categories require rapid intervention to restore perfusion and to prevent multi-organ failure. Categorization of patients in the Penn classification simplifies the heterogeneity of presentation and may allow comparison of management strategies.
Transesophageal echocardiography guidance of surgical cannulation and perfusion
Owing to the complexity of aortic disease, such as the presence of a false lumen or an intramural hematoma, there may be uncertainty regarding the optimal site of arterial cannulation for cardiopulmonary bypass.44,45 Recent evidence suggests that perioperative TEE assists correct cannulation by identifying the location of the guidewire in either the true lumen (Fig. 5a, Video 5a) or the false lumen (Fig. 5b, Video 5b).44,45 The true lumen is likely to be the chamber in which there is expansion and concavity in systole (Fig. 1b). On the other hand, the false lumen has a convex shape in systole and expands in diastole; there may be spontaneous echocardiographic contrast owing to stasis or thrombosis.1 (Fig. 5a and Video 5a).
Transesophageal echocardiography assessment of aortic cannulation, malperfusion, and elephant trunk prosthesis. (a) Mid-esophageal short-axis view of the descending thoracic aorta showing the correct placement of the guidewire (W) in the true lumen (TL). The guidewire was inserted under transesophageal echocardiography guidance. There is spontaneous echocardiographic contrast within the false lumen (FL) (Video 5a). (b) Upper-esophageal short-axis view of the aortic arch reveals incorrect placement of a guidewire (W) in the false lumen (FL). Early identification of its location prior to aortic cannulation may obviate the complications of malperfusion (Video 5b). (c) Mid-esophageal short-axis view of the descending thoracic aorta with colour flow Doppler showing blood traversing from the true lumen (TL) to the false lumen (FL) (Video 5c). Doppler spectral display confirms the direction of flow towards the transducer in systole. (d) Mid-esophageal short-axis (Video 5d) and (e) long-axis (Video 5e) views of the descending thoracic aorta showing collapse of the true lumen (TL) and pressurization of the false lumen (FL) which is enlarged. (f) After correction and redirection of proximal flow using an elephant trunk, there is re-expansion of the true lumen (TL) with appropriate flow in both the short-axis (Video 5f) and (g) long-axis (Video 5g) views. Colour Doppler shows flow via a residual intimal tear. (h) Mid-esophageal short-axis (Video 5h) and (i) long-axis (Video 5i) views of the proximal descending thoracic aorta showing a non-stented elephant trunk (ET). Colour flow Doppler shows laminar flow. (j) Upper esophageal long-axis view of the aortic arch showing partial thrombosis (Tb) within the false lumen (FL)
Flow crossing the dissection flap from true lumen to false lumen may be shown by Doppler on spectral display (Fig. 5c). By correct cannulation of the true lumen, pressurization of the false lumen and complications of malperfusion, such as paraplegia and mesenteric ischemia, are likely to be minimized.13,46 Similarly, further iatrogenic extension of dissection or aortic rupture may be obviated.47
Nevertheless, owing to the presence of multiple complex tears, pressurization of the false lumen (Figs. 5d and 5e, Videos 5d and 5e) may still occur despite initial correct cannulation and reperfusion of the true lumen.48 Immediate surgical correction may be used, e.g., an elephant trunk to redirect flow into the true lumen beyond the aortic tear (Figs. 5f and 5g, Videos 5f and 5g). Since malperfusion may still occur due to kinking of an unstented graft, TEE is needed to check adequacy of flow and possible surgical repositioning of the elephant trunk (Figs. 5h and 5i, Videos 5h and 5i) in the descending thoracic aorta.49
After surgical repair, patency of the false lumen indicates persistent leak, the possibility of late complications (e.g., aneurysm),50,51 and the need for re-intervention.52,53 Prognostically, if repair is successful, then there should be evidence of thrombosis51 (Fig. 5j) and a collapsed false lumen.50
Despite its prominent intraoperative role, TEE may not always enable monitoring of cannulation and perfusion, e.g., during cardiopulmonary bypass with an empty heart and in difficult areas such as the brachiocephalic vessels. In these circumstances, surface ultrasonography of arterial vessels, such as epiaortic scanning54 and femoral imaging,55 is likely to provide very useful additional information. Furthermore, in the case of brachiocephalic perfusion, other monitoring, such as near-infrared cerebral spectroscopy, would show end-organ receipt of perfusion.56,57
Transesophageal echocardiography assessment of aortic regurgitation
In the presence of aortic root involvement, it is necessary to assess whether aortic repair may be carried out without aortic valve replacement.58 Using TEE, it is possible to assess the functional aortic annulus, i.e., ventriculo-aortic junction and the sinotubular junction within which the aortic valve is suspended.59 Recommended echocardiographic views and measurements for patients with aortic dissection and aortic root repair include: diameter of the aortic annulus, sinuses, sinotubular junction, ascending aorta, height of aortic cusp coaptation, coaptation length, degree of aortic valve prolapse, and severity of aortic regurgitation (Fig. 6a).60-62
Transesophageal echocardiography (TEE) assessment of aortic regurgitation in aortic dissection. (a) Standard TEE measurements of the aortic root may be made at the follow points: (A) aortic annulus; (B) sinus of Valsalva; (C) sinotubular junction; (D) proximal ascending aorta. Additional measurements relating specifically to aortic regurgitation should include: (E) coaptation height, which is the perpendicular distance from the level of the aortic annulus to the point of coaptation of the aortic cusps; (F) coaptation length, which is the distance of coaptation between adjacent surfaces of the aortic cusps; (G) extent of prolapse, which is the perpendicular distance to from the aortic annulus to the lowest point of the aortic cusp; (H) jet eccentricity, which is the angle between the eccentric jet and the long axis of the aortic root. All diameters are measured perpendicular to the long axis (A, B, C, D) of the aorta and aortic annulus (E, F, G). Mid-esophageal long-axis views of the aortic valve showing different mechanisms of aortic regurgitation (AR) in aortic dissection: (b) Eccentric jet of aortic regurgitation owing to proximal extension of the intimal flap and asymmetrical prolapse of the aortic cusps (Video 6b). (c) Central aortic regurgitation due to dilatation of the aortic root and reduced coaptation of the aortic cusps (Video 6c). (d) Central aortic regurgitation secondary to prolapse of the intimal flap preventing coaptation of the aortic cusps (Video 6d)
In the presence of aortic regurgitation, it is necessary to identify its mechanisms,61 which have been shown to include: intrinsic leaflet pathology (such as thick calcified deformed cusps with restrictive mobility);62 annular dissection disrupting attachment of aortic cusps, leading to cusp prolapse (Fig. 6b, Video 6b); annular dilatation causing tethering and incomplete closure of aortic cusps (Fig. 6c, Video 6c);63 and prolapse of the dissection flap preventing closure of cusps (Fig. 6d, Video 6d).64,65
In addition to repairing the dissected aorta, the aortic valve may have to be replaced, particularly in an emergency and when leaflet calcifications and deformity with cusp prolapse are identified.66 Nevertheless, valve-sparing aortic root surgery should be possible in the absence of these abnormalities when the aortic root may be reconstructed to enable cusp coaptation.67 This situation may arise when the aortic cusps fail to coapt in the presence of aortic dilatation or a prolapsed flap which interferes with their closure. Furthermore, valve-sparing surgery is indicated when the aortic cusps coapt well during preventative aortic surgery in high-risk patients, e.g., Loeys-Dietz syndrome67-69 (Figs. 7a and 7b, Videos 7a and 7b).
Transesophageal echocardiography (TEE) assessment of valve-sparing aortic root surgery. Mid-esophageal long-axis views after valve-sparing aortic root replacement (ARR) for aortic dissection. (a) Symmetrical coaptation of aortic leaflets without cusp prolapse. The coaptation height (E) is > 8 mm and the coaptation length (F) is > 4 mm (Video 7a). (b) Transient central jet of mild aortic regurgitation during early diastole. This patient underwent the David procedure for aortopathy in association with Loeys-Dietz syndrome (Video 7b). (c) Inadequate asymmetrical coaptation due to prolapse (arrow) of the right coronary cusp beyond the aortic annulus (dotted line) (Video 7c). (d) An eccentric jet of aortic regurgitation has been detected by transesophageal echocardiography (Video 7d). LA = left atrium; LV = left ventricle; LVOT = left ventricle outflow tract
Towards the end of cardiopulmonary bypass with adequate aortic root pressure, TEE should be used to evaluate adequacy of repair and to exclude residual aortic regurgitation.62 Findings on TEE that predict recurrent aortic incompetence after a valve-sparing procedure include: an eccentric regurgitant jet, sub-annular coaptation with prolapsed cusps (Figs. 7c and 7d, Videos 7c and 7d), reduced coaptation height (E < 8 mm in Fig. 6a), and short coaptation length (F < 4 mm in Fig. 6a).61,65 Immediate re-exploration rather than conservative management would be appropriate when there is at least moderate aortic regurgitation caused by reduced coaptation or prolapsed cusps67,70 (Figs. 7c and 7d, Videos 7c and 7d). Other considerations for further aortic valve repair or replacement would depend on patient comorbidity, quality of tissue, and surgical expertise.62
Transesophageal echocardiography assessment of arch and brachiocephalic perfusion
Aortic dissection involving the arch and brachiocephalic vessels has been associated with ischemic neurological injury. Cerebral malperfusion may be further exacerbated by the requirement for deep hypothermic circulatory arrest during repair of the aortic arch. From an imaging perspective, TEE may detect malperfusion in the arch vessels and so influence the surgical plan. A dissection flap or hematoma extending into the arch and origins of the brachiocephalic vessels may be visualized (Figs. 1e and 1f, Videos 1e and 1f). These supra-aortic vessels may be assessed from the upper esophageal position showing the aortic arch (Fig. 8a at 0°, while Fig. 8b is at 90° rotation) (Videos 8a and 8b).71 The innominate artery, left common carotid artery, and subclavian artery can be seen in the short-axis view (Fig. 8c and Video 8c) by withdrawal, anteflexion, and left lateral flexion of the TEE probe.71 To obtain an orthogonal plane of the left common carotid artery in long axis (Fig. 8d), the multiplane sector is rotated to 90°. Pulse wave Doppler displays a low peak velocity waveform suggestive of a low-resistance cerebral vessel that allows continuous antegrade flow even in diastole (Fig. 8d).72,73
Perioperative transesophageal echocardiography assessment of the aortic arch and brachiocephalic vessels (a) From the upper esophageal position, the aortic arch is seen in long-axis (Video 8a) and (b) in short-axis (Video 8b) views. (c) Brachiocephalic branches of the aortic arch: innominate artery (IA), left common carotid (LCC) artery, and left subclavian (LSC) artery are seen in short axis by withdrawing the TEE probe in the upper esophageal position at 0° (Video 8c). Flow in the innominate artery (IA) is toward the transducer and appears red. Further down the aortic arch, flow in the left common carotid (LCC) artery and left subclavian (LSC) artery is away from the transducer and appears in blue. (d) Keeping the left common carotid (LCC) artery in view, the multiplane sector is rotated to 90° to visualize the LCC artery in long axis. This pulse wave Doppler image has a low peak velocity suggestive of the low resistance of the cerebral vessels, which allows continuous antegrade flow even in diastole. (e) The TEE probe is withdrawn gently with some counter-clockwise rotation to the patient’s left in order to reveal the long axis of the left subclavian (LSC) artery. Pulse wave Doppler shows a high peak velocity of a relatively high resistance of the systemic circulation. The difference between the low-velocity profile in the LCC and the high-velocity profile in the LSC artery helps to distinguish these vessels from one another. (f) The right innominate vein (IV) and innominate artery (IA) are seen from the upper esophageal position by right lateral flexion at 0°. (g) In the proximal transgastric position, it is possible to visualize an expanded aortic view of the aortic valve (AV), ascending aorta (AAo) and proximal aortic arch (blind spots) at 40°-60° (Video 8g) and at (h) 110°-130° (Video 8h). IV = innominate vein; LV = left ventricle; PT = pulmonary trunk; RV = right ventricle; RPA = right pulmonary artery; SVC = superior vena cava
To visualize the left subclavian artery in long axis (Fig. 8e), the probe is withdrawn gently with some counter-clockwise rotation towards the patient’s left. A relatively high peak Doppler velocity profile is usually detected in the left subclavian artery owing to the high resistance of the systemic circulation and rapid reflections of the pulse wave velocity (Fig. 8e).72,73 The difference between the low velocity profile in the left common carotid artery and the high velocity profile in the left subclavian artery helps to distinguish these vessels from one another.
To assess vessels on the right side of the head and neck, right lateral flexion and withdrawal of the probe from the standard upper esophageal long-axis view of the aortic arch (at 0°) may show the short-axis views of the innominate artery and vein (Fig. 8f), although visualization of these innominate vessels by TEE is not always possible.
Generally, imaging for the distal ascending aorta and proximal arch is challenging owing to the interposition of air within the trachea and the TEE probe in the esophagus. Pulsatile perfusion in the supra-aortic vessels may be identified and monitored by TEE in some (but not all) patients before and after (but not during) cardiopulmonary bypass.
Recent evidence suggests that there are three strategies for improving visualization of the aorta in the intraoperative period, particularly after inclusion of prosthetic material. First, an expanded aortic view of the entire ascending aorta and proximal arch may be obtained by placing the TEE probe in the proximal transgastric position rather than in the deep transgastric position. This novel proximal transgastric view has been obtained intraoperatively using low-frequency ultrasound for depth resolution and a probe with a good range of anteflexion (Figs. 8g and 8h).74 The probe is turned to the patient’s right and anteflexed to show the long-axis view of the aortic valve, ascending aorta, and proximal arch between 40-60° (Fig. 8g, Video 8g). An orthogonal view of the same structures and the blind spots on the proximal arch may be visualized from 110-130° (Fig. 8h, Video 8h).74
Second, an epiaortic ultrasound probe may be placed on the ascending aorta via an intermediate saline medium.75 Direct epiaortic scanning confirms the presence of aortic dissection54,76 (Fig. 9a and Video 9a) and enables the surgeon to identify dissection-free segments of the ascending aorta that may be suitable for cannulation76 (Fig. 9b and Video 9b).
Epiaortic images during surgery for repair of Type A aortic dissection. (a) In the short-axis view of the dilated proximal ascending aorta, there is a dissection flap (arrow) (Video 9a). (b) In this short-axis view close to the aortic arch, the aorta is not dilated and there is no dissection flap (Video 9b). Direct epiaortic imaging allows the surgeon to localize precisely the segment of the aorta which is suitable for cannulation
Third, an aortic view (A-view) catheter with a saline-filled balloon placed in the left main bronchus has been shown to improve TEE imaging of the blind spots. Saline allows continuous transmission of ultrasound and thus uninterrupted visualization of the distal ascending aorta and the proximal arch.77,78
In addition to visualization of the diseased aorta and the brachiocephalic vessels, TEE may assist with endovascular stenting.79,80 It has been used in identifying the true lumen via visualization and confirmation of a guidewire placed distally in the femoral artery and hence aorta81,82 (Fig. 5a, Video 5a) Moreover, TEE may have a role in sizing, determining suitable landing zones devoid of atheroma, confirming appropriate blood flow,83,84 and checking for endoleak.34,85,86
Echocardiography and timing of preventative surgery
From an anesthetic perspective, comorbidity is important to consider since it affects perioperative outcome and the timing of preventative surgery. In patients with aortic disease, several factors affecting perioperative outcome include age > 70 yr, heart failure, stroke, diabetes mellitus requiring insulin therapy, and renal dysfunction.87,88 Furthermore, the evidence suggests that specific etiological factors should be identified in aortic dissection. Besides hypertension, previous cardiac surgery,89 pregnancy,90 and cocaine abuse,91 such factors include inflammatory vascular diseases92 and congenital autosomal dominant diseases such as Marfan syndrome,93 Loeys-Dietz syndrome,68,94 Ehlers-Danlos syndrome,93 and bicuspid aortic valve95,96 (Table). In general, there is arterial medial wall degeneration, reduced distensibility, increased stiffness, and aortic dilatation.97,98
To improve survival in patients who are at risk of acute aortic dissection, it is necessary to monitor progression of disease so that optimal timing of preventative surgery may be determined.98 Surgical intervention is indicated for these patients at risk of aortic dissection because of significant mortality and morbidity under emergency situations.69 In conjunction with magnetic resonance imaging98 and computerized tomography, echocardiography69 may be used to monitor the size of the aortic root, ascending aorta, and descending thoracic aorta.2,99 From a pathological perspective, an enlarged but thin-walled aorta is an indicator of increased wall stress in accordance with the Young-Laplace equation:100
There is a general rule that operative treatment must be provided when the ascending aorta exceeds 5.5 cm.101 Nevertheless, from analysis of patients who had acute aortic dissection, it is evident that dissection occurs at smaller aortic diameters.102,103 Currently, it is likely that operative treatment should be provided when the ascending aortic diameter reaches 5.0 cm in patients with connective tissue disease104 and bicuspid aortic valve.105,106 This threshold could be reduced to 4.0-4.5 cm when there are additional considerations such as concurrent aortic valve surgery, family history, proposed pregnancy, rapid growth rate of > 0.5 cm per annum, and Loeys-Dietz syndrome.68,69
Nevertheless, adults vary in size, and thus, absolute measurements of the aorta should be adjusted accordingly. Previously, the aortic root ratio was obtained, i.e., the observed aortic root dimension divided by the maximum predicted aortic root. This latter parameter is two standard deviations above the mean predicted diameter based on age and body surface area. When the aortic root ratio reaches and exceeds 1.3, then prophylactic aortic root replacement is appropriate.107 In the recent guidelines from the American Heart Association, surgical repair in patients with Marfan syndrome is indicated when the ratio of the maximum cross-sectional area of the ascending aorta or root in square centimetres to the patient’s height in metres exceeds 10.2
In addition to absolute and relative (aortic ratio) aortic diameters, it is possible to utilize extra measurements to quantify the risk of dissection.108,109 In the presence of aortic enlargement in diameter, there is a reduction in distensibility,110 which is the relative change in diameter per unit change in pressure. In contrast to distensibility, stiffness increases, since it is the ratio of natural logarithm (systolic pressure / diastolic pressure) to relative change in diameter.111 In routine perioperative practice, distensibility and stiffness are interesting measurements that are likely to be used only as an adjunct to aortic diameters.1,2
Conclusion
Aortic dissection is an uncommon but life-threatening disease that requires urgent intervention and anesthesia.112 Recently, developments in classification have improved our perspective and understanding of the key presenting features that affect mortality.113 Improvements in patient outcome may be achieved in part by appropriately timed echocardiography-guided surgery.113,114 In future, advances in transducer technology that provide images of high resolution and in three dimensions over time may enable us to obtain incremental characterization of tear and rupture sites, aortic valve morphology, and the coronary orifice.6,115-117
References
Evangelista A, Flachskampf FA, Erbel R, et al.; European Association of Echocardiography; Document Reviewers. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr 2010; 11: 645-58.
Hiratzka LF, Bakris GL, Beckman JA, et al.; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/ AHA/ AATS/ ACR/ ASA/ SCA/ SCAI/ SIR/ STS/ SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary: A report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Anesth Analg 2010; 111: 279-315.
Rudenick PA, Bordone M, Bijnens BH, et al. Influence of tear configuration on false and true lumen haemodynamics in type B aortic dissection. Conf Proc IEEE Eng Med Biol Soc 2010; 2010: 2509-12.
Sheka K, Sadiq A, Kabalkin C, Greengart A. The use of M-mode echocardiography to identify the true lumen in aortic dissection. J Am Soc Echocardiogr 2004; 17: 1309-10.
Alter P, Herzum M, Maisch B. Echocardiographic findings mimicking type A aortic dissection. Herz 2006; 31: 153-5.
Joshi D, Bicer EI, Donmez C, et al. Incremental value of live/real time three-dimensional transesophageal echocardiography over the two-dimensional technique in the assessment of aortic aneurysm and dissection. Echocardiography 2012; 29: 620-30.
Shiga T, Wajima Z, Apfel CC, Inoue T, Ohe Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med 2006; 166: 1350-6.
Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg 2009; 37: 149-59.
Rao SL, Auerbach SR. Severe acute aortic regurgitation due to aortic dissection. Anesth Analg 2007; 104: 500-1.
Dorman SH, Barry J. Acute aortic dissection mimicking an acute coronary syndrome through occlusion of the right coronary artery. Emerg Med J 2008; 25: 462-3.
Gullu AU, Nurkalem Z, Akçar M, Eren M. Acute type A aortic dissection and left main coronary artery obstruction detected by transesophageal echocardiography. Turk Kardiyol Dern Ars 2010; 38: 211-4.
Nakamura Y, Tagusari O, Ichikawa Y, Morita A. Impact of immediate aortic repair on early and midterm neurologic status in patients with acute type A aortic dissection complicated by cerebral malperfusion. Ann Thorac Surg 2011; 92: 336-8.
Orihashi K, Sueda T, Okada K, Imai K. Perioperative diagnosis of mesenteric ischemia in acute aortic dissection by transesophageal echocardiography. Eur J Cardiothorac Surg 2005; 28: 871-6.
Orihashi K, Matsuura Y, Sueda T, et al. Abdominal aorta and visceral arteries visualized by transgastric echocardiography: technical considerations. Hiroshima J Med Sci 1997; 46: 151-7.
Kirsch ME. Editorial comment: Classification of aortic dissection: back to the future? Eur J Cardiothorac Surg 2011; 40: 1084-6.
Tsagakis K, Tossios P, Kamler M, et al. The DeBakey classification exactly reflects late outcome and re-intervention probability in acute aortic dissection with a slightly modified type II definition. Eur J Cardiothorac Surg 2011; 40: 1078-84.
White RA, Miller DC, Criado FJ, et al.; Multidisciplinary Society for Vascular Surgery Outcomes Committee. Report on the results of thoracic endovascular aortic repair for acute, complicated, Type B aortic dissection at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg 2011; 53: 1082-90.
Etz CD, Bischoff MS, Bodian C, et al. The Bentall procedure: is it the gold standard? A series of 597 consecutive cases. J Thorac Cardiovasc Surg 2010; 140: S64-70.
De Paulis R, Scaffa R, Maselli D, Weltert L, Salica A, Bellisario A. Valsalva graft in the Bentall procedure: from mechanical valve to the BioValsalva, world’s first biological aortic conduit. Surg Technol Int 2008; 17: 216-21.
Subramanian S, Leontyev S, Borger MA, Trommer C, Misfeld M, Mohr FW. Valve-sparing root reconstruction does not compromise survival in acute type A aortic dissection. Ann Thorac Surg 2012; 94: 1230-4.
Kerendi F, Guyton RA, Vega JD, Kilgo PD, Chen EP. Early results of valve-sparing aortic root replacement in high-risk clinical scenarios. Ann Thorac Surg 2010; 89: 471-6.
Maselli D, Weltert L, Scaffa R, De Paulis R. How to achieve an aortic root remodelling by performing an aortic root reimplantation. Eur J Cardiothorac Surg 2012; 42: e136-7.
Shrestha M, Baraki H, Maeding I, et al. Long-term results after aortic valve-sparing operation (David I). Eur J Cardiothorac Surg 2012; 41: 56-61.
Hanke T, Charitos EI, Stierle U, et al. Factors associated with the development of aortic valve regurgitation over time after two different techniques of valve-sparing aortic root surgery. J Thorac Cardiovasc Surg 2009; 137: 314-9.
Piccardo A, Regesta T, Pansini S, et al. Fate of the aortic valve after root reconstruction in type A aortic dissection: a 20-year follow up. J Heart Valve Dis 2009; 18: 507-13.
Kollar AC, Lick SD, Conti VR. Integrating resuspension with remodeling: early results with a new valve-sparing aortic root reconstruction technique. J Heart Valve Dis 2008; 17: 74-9.
Ince H, Nienaber CA. Management of acute aortic syndromes (Spanish). Rev Esp Cardiol 2007; 60: 526-41.
Kim JB, Chung CH, Moon DH, et al. Total arch repair versus hemiarch repair in the management of acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg 2011; 40: 881-7.
Matalanis G, Durairaj M, Brooks M. A hybrid technique of aortic arch branch transposition and antegrade stent graft deployment for complete arch repair without cardiopulmonary bypass. Eur J Cardiothorac Surg 2006; 29: 611-2.
Matalanis G, Koirala RS, Shi WY, Hayward PA, McCall PR. Branch-first aortic arch replacement with no circulatory arrest or deep hypothermia. J Thorac Cardiovasc Surg 2011; 142: 809-15.
Matalanis G, Shi WY. An Australian experience with aortic arch replacement: a novel approach without circulatory arrest or deep hypothermia. Heart Lung Circ 2011; 20: 163-9.
Szeto WY, Bavaria JE, Bowen FW, Woo EY, Fairman RM, Pochettino A. The hybrid total arch repair: brachiocephalic bypass and concomitant endovascular aortic arch stent graft placement. J Card Surg 2007; 22: 97-102.
Kolvenbach RR, Karmeli R, Pinter LS, et al. Endovascular management of ascending aortic pathology. J Vasc Surg 2011; 53: 1431-7.
Tsagakis K, Kamler M, Kuehl H, et al. Avoidance of proximal endoleak using a hybrid stent graft in arch replacement and descending aorta stenting. Ann Thorac Surg 2009; 88: 773-9.
Hughes GC, Sulzer CF, McCann RL, Swaminathan M. Endovascular approaches to complex thoracic aortic disease. Semin Cardiothorac Vasc Anesth 2008; 12: 298-319.
Roselli EE, Soltesz EG, Mastracci T, Svensson LG, Lytle BW. Antegrade delivery of stent grafts to treat complex thoracic aortic disease. Ann Thorac Surg 2010; 90: 539-46.
Milewski RK, Szeto WY, Pochettino A, Moser GW, Moeller P, Bavaria JE. Have hybrid procedures replaced open aortic arch reconstruction in high-risk patients? A comparative study of elective open arch debranching with endovascular stent graft placement and conventional elective open total and distal aortic arch reconstruction. J Thorac Cardiovasc Surg 2010; 140: 590-7.
Chen X, Huang F, Xu M, et al. The stented elephant trunk procedure combined total arch replacement for Debakey I aortic dissection: operative result and follow-up. Interact Cardiovasc Thorac Surg 2010; 11: 594-8.
Pacini D, Tsagakis K, Jakob H, et al. The frozen elephant trunk for the treatment of chronic dissection of the thoracic aorta: a multicenter experience. Ann Thorac Surg 2011; 92: 1663-70.
Augoustides JG, Szeto WY, Desai ND, et al. Classification of acute type A dissection: focus on clinical presentation and extent. Eur J Cardiothorac Surg 2011; 39: 519-22.
Augoustides JG, Szeto WY, Woo EY, Andritsos M, Fairman RM, Bavaria JE. The complications of uncomplicated acute type-B dissection: the introduction of the Penn classification. J Cardiothorac Vasc Anesth 2012; 26: 1139-44.
Olsson C, Hillebrant CG, Liska J, Lockowandt U, Eriksson P, Franco-Cereceda A. Mortality in acute type A aortic dissection: validation of the Penn classification. Ann Thorac Surg 2011; 92: 1376-82.
Augoustides JG, Geirsson A, Szeto WY, et al. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: the Penn classification. Nat Clin Pract Cardiovasc Med 2009; 6: 140-6.
Tiwari KK, Murzi M, Bevilacqua S, Glauber M. Which cannulation (ascending aortic cannulation or peripheral arterial cannulation) is better for acute type A aortic dissection surgery? Interact Cardiovasc Thorac Surg 2010; 10: 797-802.
Inoue Y, Ueda T, Taguchi S, et al. Ascending aorta cannulation in acute type A aortic dissection. Eur J Cardiothorac Surg 2007; 31: 976-9.
Shimokawa T, Takanashi S, Ozawa N, Itoh T. Management of intraoperative malperfusion syndrome using femoral artery cannulation for repair of acute type A aortic dissection. Ann Thorac Surg 2008; 85: 1619-24.
Karmonik C, Bismuth J, Shah DJ, Davies MG, Purdy D, Lumsden AB. Computational study of haemodynamic effects of entry- and exit-tear coverage in a DeBakey type III aortic dissection: technical report. Eur J Vasc Endovasc Surg 2011; 42: 172-7.
Lentini S, Savasta M, Ciuffreda F, La Monaca M, Gaeta R. Treatment of malperfusion during surgery for type A aortic dissection. J Extra Corpor Technol 2009; 41: 114-8.
Wallet F, Perbet S, Fleron MH, et al. Elephant trunk prosthesis kinking: transesophageal echocardiography diagnosis. Anesth Analg 2008; 106: 67-9.
Evangelista A, Salas A, Ribera A, et al. Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation 2012; 125: 3133-41.
Trimarchi S, Tolenaar JL, Jonker FH, et al. Importance of false lumen thrombosis in type B aortic dissection prognosis. J Thorac Cardiovasc Surg 2013; 145: S208-12.
Concistre G, Casali G, Santaniello E, et al. Reoperation after surgical correction of acute type A aortic dissection: risk factor analysis. Ann Thorac Surg 2012; 93: 450-5.
Pochettino A, Brinkman WT, Moeller P, et al. Antegrade thoracic stent grafting during repair of acute DeBakey I dissection prevents development of thoracoabdominal aortic aneurysms. Ann Thorac Surg 2009; 88: 482-9.
Inoue Y, Takahashi R, Ueda T, Yozu R. Synchronized epiaortic two-dimensional and color Doppler echocardiographic guidance enables routine ascending aortic cannulation in type A acute aortic dissection. J Thorac Cardiovasc Surg 2011; 141: 354-60.
Troianos CA, Hartman GS, Glas KE, et al.; Councils on Intraoperative Echocardiography and Vascular Ultrasound of the American Society of Echocardiography. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2011; 24: 1291-318.
Santo KC, Barrios A, Dandekar U, Riley P, Guest P, Bonser RS. Near-infrared spectroscopy: an important monitoring tool during hybrid aortic arch replacement. Anesth Analg 2008; 107: 793-6.
Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 2009; 103(Suppl 1): i3-13.
Augoustides JG, Andritsos M. Innovations in aortic disease: the ascending aorta and aortic arch. J Cardiothorac Vasc Anesth 2010; 24: 198-207.
Maselli D, De Paulis R, Scaffa R, et al. Sinotubular junction size affects aortic root geometry and aortic valve function in the aortic valve reimplantation procedure: an in vitro study using the Valsalva graft. Ann Thorac Surg 2007; 84: 1214-8.
le Polain de Waroux JB, Pouleur AC, Goffinet C, et al. Functional anatomy of aortic regurgitation: accuracy, prediction of surgical repairability, and outcome implications of transesophageal echocardiography. Circulation 2007; 116: I264-9.
le Polain de Waroux JB, Pouleur AC, Robert A, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging 2009; 2: 931-9.
Van Dyck MJ, Watremez C, Boodhwani M, Vanoverschelde JL, El Khoury G. Transesophageal echocardiographic evaluation during aortic valve repair surgery. Anesth Analg 2010; 111: 59-70.
Gallego García de Vinuesa P, Castro A, Barquero JM, et al. Functional anatomy of aortic regurgitation. Role of transesophageal echocardiography in aortic valve-sparing surgery. Rev Esp Cardiol 2010; 63: 536-43.
Whitley W, Tanaka KA, Chen EP, Glas KE. Acute aortic dissection with intimal layer prolapse into the left ventricle. Anesth Analg 2007; 104: 774-6.
Augoustides JG, Szeto WY, Bavaria JE. Advances in aortic valve repair: focus on functional approach, clinical outcomes, and central role of echocardiography. J Cardiothorac Vasc Anesth 2010; 24: 1016-20.
Guntheroth WG. A critical review of the American College of Cardiology/American Heart Association practice guidelines on bicuspid aortic valve with dilated ascending aorta. Am J Cardiol 2008; 102: 107-10.
Bonow RO, Carabello BA, Chatterjee K, et al.; 2006 Writing Committee Members;American College of Cardiology, American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 2008(118): e523-661.
Aalberts JJ, van den Berg MP, Bergman JE, et al. The many faces of aggressive aortic pathology: Loeys-Dietz syndrome. Neth Heart J 2008; 16: 299-304.
Parish LM, Gorman JH 3rd, Kahn S, et al. Aortic size in acute type A dissection: implications for preventive ascending aortic replacement. Eur J Cardiothorac Surg 2009; 35: 941-5.
Yamanaka K, Hori Y, Ikarashi J, et al. Durability of aortic valve preservation with root reconstruction for acute type A aortic dissection. Eur J Cardiothorac Surg 2012; 41: e32-6.
Orihashi K, Matsuura Y, Sueda T, et al. Aortic arch branches are no longer a blind zone for transesophageal echocardiography: a new eye for aortic surgeons. J Thorac Cardiovasc Surg 2000; 120: 466-72.
Nichols WW, Denardo SJ, Wilkinson IB, McEniery CM, Cockcroft J, O’Rourke MF. Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J Clin Hypertens 2008; 10: 295-303.
Nichols WW, O’Rourke MF. Aortic pulse wave velocity, reflection site distance, and augmentation index. Hypertension 2009; 53: e9-10.
Mahajan A, Crowley R, Ho JK, et al. Imaging the ascending aorta and aortic arch using transesophageal echocardiography: the expanded aortic view. Echocardiogr 2008; 25: 408-13.
Glas KE, Swaminathan M, Reeves ST, et al. Guidelines for the performance of a comprehensive intraoperative epiaortic ultrasonographic examination: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists; endorsed by the Society of Thoracic Surgeons. Anesth Analg 2008; 106: 1376-84.
Demertzis S, Casso G, Torre T, Siclari F. Direct epiaortic ultrasound scanning for the rapid confirmation of intraoperative aortic dissection. Interact Cardiovasc Thorac Surg 2008; 7: 725-6.
van Zaane B, Nierich AP, Buhre WF, Brandon Bravo Bruinsma GJ, Moons KG. Resolving the blind spot of transoesophageal echocardiography: a new diagnostic device for visualizing the ascending aorta in cardiac surgery. Br J Anaesth 2007; 98: 434-41.
van Zaane B, Nierich AP, Brandon Bravo Bruinsma GJ, et al. Diagnostic accuracy of modified transoesophageal echocardiography for pre-incision assessment of aortic atherosclerosis in cardiac surgery patients. Br J Anaesth 2010; 105: 131-8.
Tsagakis K, Pacini D, Di Bartolomeo R, et al. Arch replacement and downstream stent grafting in complex aortic dissection: first results of an international registry. Eur J Cardiothorac Surg 2011; 39: 87-93.
Uchida N, Katayama A, Tamura K, Sutoh M, Kuraoka M, Ishihara H. Frozen elephant trunk technique and partial remodeling for acute type A aortic dissection. Eur J Cardiothorac Surg 2011; 40: 1066-71.
Orihashi K, Sueda T, Okada K, Imai K. Detection and monitoring of complications associated with femoral or axillary arterial cannulation for surgical repair of aortic dissection. J Cardiothorac Vasc Anesth 2006; 20: 20-5.
Ayyash B, Tranquilli M, Elefteriades JA. Femoral artery cannulation for thoracic aortic surgery: safe under transesophageal echocardiographic control. J Thorac Cardiovasc Surg 2011; 142: 1478-81.
Koschyk DH, Nienaber CA, Knap M, et al. How to guide stent-graft implantation in type B aortic dissection? Comparison of angiography, transesophageal echocardiography, and intravascular ultrasound. Circulation 2005; 112: I260-4.
Rocchi G, Lofiego C, Biagini E, et al. Transesophageal echocardiography-guided algorithm for stent-graft implantation in aortic dissection. J Vasc Surg 2004; 40: 880-5.
Koullias GJ, Wheatley GH 3rd. State-of-the-art of hybrid procedures for the aortic arch: a meta-analysis. Ann Thorac Surg 2010; 90: 689-97.
Antoniou GA, El Sakka K, Hamady M, Wolfe JH. Hybrid treatment of complex aortic arch disease with supra-aortic debranching and endovascular stent graft repair. Eur J Vasc Endovasc Surg 2010; 39: 683-90.
American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography: American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesth Analg 2008; 106: 685-712.
Bauer SM, Cayne NS, Veith FJ. New developments in the preoperative evaluation and perioperative management of coronary artery disease in patients undergoing vascular surgery. J Vasc Surg 2010; 51: 242-51.
Collins JS, Evangelista A, Nienaber CA, Bossone E, et al.; International Registry of Acute Aortic Dissection (IRAD). Differences in clinical presentation, management, and outcomes of acute type A aortic dissection in patients with and without previous cardiac surgery. Circulation 2004; 110: II237-42.
European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM); Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al.; ESC Committee for Practice Guidelines. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 3147-97.
Marella GL, Furnari C, Perfetti E, Arcudi G. Aortic dissection and cocaine use. J Forensic Leg Med 2011; 18: 329-31.
He R, Guo DC, Sun W, et al. Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J Thorac Cardiovasc Surg 2008; 136: 922-9.
Callewaert B, Malfait F, Loeys B, De Paepe A. Ehlers-Danlos syndromes and Marfan syndrome. Best Pract Res Clin Rheumatol 2008; 22: 165-89.
Van Hemelrijk C, Renard M, Loeys B. The Loeys-Dietz syndrome: an update for the clinician. Curr Opin Cardiol 2010; 25: 546-51.
Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009; 53: 2288-95.
Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55: 2789-800.
Nesi G, Anichini C, Tozzini S, Boddi V, Calamai G, Gori F. Pathology of the thoracic aorta: a morphologic review of 338 surgical specimens over a 7-year period. Cardiovasc Pathol 2009; 18: 134-9.
Nollen GJ, Groenink M, Tijssen JG, Van Der Wall EE, Mulder BJ. Aortic stiffness and diameter predict progressive aortic dilatation in patients with Marfan syndrome. Eur Heart J 2004; 25: 1146-52.
Erbel R, Eggebrecht H. Aortic dimensions and the risk of dissection. Heart 2006; 92: 137-42.
Beller CJ, Gebhard MM, Karck M, Labrosse MR. Usefulness and limitations of computational models in aortic disease risk stratification. J Vasc Surg 2010; 52: 1572-9.
Cozijnsen L, Braam RL, Waalewijn RA, et al. What is new in dilatation of the ascending aorta? Review of current literature and practical advice for the cardiologist. Circulation 2011; 123: 924-8.
Pape LA, Tsai TT, Isselbacher EM, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116: 1120-7.
Neri E, Barabesi L, Buklas D, et al. Limited role of aortic size in the genesis of acute type A aortic dissection. Eur J Cardiothorac Surg 2005; 28: 857-63.
Vahanian A, Alfieri O, Andreotti F, et al.; Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33: 2451-96.
Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation 2009; 119: 880-90.
Etz CD, Zoli S, Brenner R, et al. When to operate on the bicuspid valve patient with a modestly dilated ascending aorta. Ann Thorac Surg 2010; 90: 1884-90.
Aalberts JJ, Waterbolk TW, van Tintelen JP, Hillege HL, Boonstra PW, van den Berg MP. Prophylactic aortic root surgery in patients with Marfan syndrome: 10 years’ experience with a protocol based on body surface area. Eur J Cardiothorac Surg 2008; 34: 589-94.
Vitarelli A, Conde Y, Cimino E, et al. Aortic wall mechanics in the Marfan syndrome assessed by transesophageal tissue Doppler echocardiography. Am J Cardiol 2006; 97: 571-7.
Baumgartner D, Baumgartner C, Schermer E, et al. Different patterns of aortic wall elasticity in patients with Marfan syndrome: a noninvasive follow-up study. J Thorac Cardiovasc Surg 2006; 132: 811-9.
Boudoulas KD, Vlachopoulos C, Raman SV, et al. Aortic function: from the research laboratory to the clinic. Cardiology 2012; 121: 31-42.
Antonini-Canterin F, Carerj S, Di Bello V, et al.; Research Group of the Italian Society of Cardiovascular Echography (SIEC). Arterial stiffness and ventricular stiffness: a couple of diseases or a coupling disease? A review from the cardiologist’s point of view. Eur J Echocardiogr 2009; 10: 36-43.
Cheung AT. An Evolving role of anesthesiologists in the management of thoracic aortic diseases. Anesth Analg 2010; 111: 259-60.
Augoustides JG, Patel PA, Savino JS, Bavaria JE. The heart team approach to acute type A dissection: a new paradigm in the era of the integrated Penn classification and the Essen concept. Eur J Cardiothorac Surg 2013; 43: 404-5.
Jakob H, Tsagakis K, Dohle DS, et al. Hybrid room technology as a prerequisite for the modern therapy of aortic dissection (German). Herz 2011; 36: 525-30.
Scohy TV, Geniets B, McGhie J, Bogers AJ. Feasibility of real-time three-dimensional transesophageal echocardiography in type A aortic dissection. Interact Cardiovasc Thorac Surg 2010; 11: 112-3.
Evangelista A, Aguilar R, Cuellar H, et al. Usefulness of real-time three-dimensional transoesophageal echocardiography in the assessment of chronic aortic dissection. Eur J Echocardiogr 2011; 12: 272-7.
Sasaki S, Watanabe H, Shibayama K, et al. Three dimensional transesophageal echocardiographic evaluation of coronary involvement in patients with acute type A aortic dissection. J Am Soc Echocardiogr 2013; 26: 837-45.
Conflicts of interest
None declared.
Funding
No financial support was given, but the time needed to write this article was provided by the Department of Anaesthesia, University Hospital of Wales, Cardiff. This work is attributed to the Department of Anaesthesia, University Hospital of Wales and the Wales Heart Research Institute, Cardiff, United Kingdom. The work has not been presented at any meetings and no individual acknowledgement is necessary.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Christine N.H. Tan and Alan G. Fraser were responsible for the conception and design of the manuscript. They both contributed to the literature search and screening of the resulting papers for the original draft. Alan G. Fraser further revised the article critically for important intellectual content.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (WMV 4449 kb)
Supplementary material 2 (WMV 3041 kb)
Supplementary material 3 (WMV 2433 kb)
Supplementary material 4 (WMV 4833 kb)
Supplementary material 5 (WMV 3873 kb)
Supplementary material 6 (WMV 1921 kb)
Supplementary material 7 (WMV 3937 kb)
Supplementary material 8 (WMV 4161 kb)
Supplementary material 9 (WMV 2561 kb)
Supplementary material 10 (WMV 18912 kb)
Supplementary material 11 (WMV 3968 kb)
Supplementary material 12 (WMV 2240 kb)
Supplementary material 13 (WMV 5185 kb)
Supplementary material 14 (WMV 1537 kb)
Supplementary material 15 (WMV 3809 kb)
Supplementary material 16 (WMV 1248 kb)
Rights and permissions
About this article
Cite this article
Tan, C.N.H., Fraser, A.G. Perioperative transesophageal echocardiography for aortic dissection. Can J Anesth/J Can Anesth 61, 362–378 (2014). https://doi.org/10.1007/s12630-014-0113-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0113-1