Abstract
Background
Ultrasound visualization of neck vessels is the standard method used to assist with internal jugular vein (IJV) central line placement. Nevertheless, this practice has not eliminated the risk of carotid puncture and/or inadvertent arterial cannulation. Transesophageal echocardiography (TEE) effectively verifies wire placement within the heart but is invasive and not always available. We examined the feasibility and potential utility of using transthoracic echocardiography (TTE) to verify the distal wire in the right atrium (RA) before dilation and cannulation of the IJV.
Methods
Following institutional Research Ethics Board approval and signed consent, 100 patients scheduled for elective cardiac surgery were recruited. As per standard practice at our institution, all patients were to have a central line inserted under general anesthesia with TEE visualization of the guidewire. Transesophageal echocardiography (apical or subcostal four-chamber images) was performed by one of four operators while another anesthesiologist performed central line placement. Following IJV puncture, blood was rapidly aspirated and reinjected to produce microbubbles. Subsequently, a 0.035-inch j-tipped flexible guidewire was inserted and visualized with TEE. The wire was then reinserted into the RA under TTE visualization.
Results
Overall, the RA was viewed 94% (95% confidence interval [CI] 87 to 98) of the time with TTE, and both the microbubbles and guidewire were detected 91% (95% CI 84 to 96) of the time. The subjects in whom the guidewire could not be well visualized had a higher mean body mass index (33.6 vs 28.8; P = 0.01).
Conclusions
Transesophageal echocardiography is a feasible, noninvasive, and potentially useful method to confirm appropriate placement of the guidewire before dilation and cannulation of the IJV.
Résumé
Contexte
La visualisation par ultrasons des vaisseaux du cou est la méthode standard utilisée pour aider au positionnement des voies centrales au niveau de la veine jugulaire interne (VJI). Toutefois, cette pratique n’a pas éliminé le risque de ponction carotidienne et/ou la canulation artérielle faite par inadvertance. L’échocardiographie transœsophagienne (ÉTO) vérifie de façon efficace le positionnement du guide dans le cœur; toutefois, cette technique est invasive et elle n’est pas toujours disponible. Nous avons examiné la faisabilité et l’utilité potentielle de l’échocardiographie transthoracique (ÉTT) pour vérifier le positionnement du guide distal dans l’oreillette droite (OD) avant la dilatation et la canulation de la VJI.
Méthode
Après avoir obtenu l’approbation du Comité d’éthique de la recherche de notre institution et le consentement des patients, 100 patients devant subir une chirurgie cardiaque non urgente ont été recrutés. Conformément à la pratique standard de notre institution, une voie centrale devait être insérée sous anesthésie générale chez tous les patients avec une visualisation par ÉTO du guide. Une échocardiographie transœsophagienne (images apicales ou sous-costales des quatre chambres) a été réalisée par l’un de quatre opérateurs pendant qu’un autre anesthésiologiste positionnait la voie centrale. Après la ponction de la VJI, le sang a été aspiré rapidement et réinjecté afin de produire des microbulles. Par la suite, un guide flexible avec extrémité en J de 0,035 pouce a été inséré et visualisé par ÉTO. Le guide a ensuite été réinséré dans l’OD sous visualisation ÉTT.
Résultats
Globalement, l’OD a été visualisée 94 % (intervalle de confiance [CI] 95 % 87 à 98) du temps avec l’ÉTT, et les microbulles et le guide ont été détectés 91 % (IC 95 % 84 à 96) du temps. Les patients chez qui le guide n’a pas pu être bien visualisé avaient un indice de masse corporelle moyen plus élevé (33,6 vs. 28,8; P = 0,01).
Conclusion
L’échocardiographie transœsophagienne est une méthode faisable, non invasive et potentiellement utile pour confirmer le positionnement adapté du guide avant la dilatation et la canulation de la VJI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
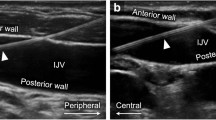
Real-time ultrasound guidance reduces complications associated with central venous cannulation and is among the accepted means of improving safety.1–3 Unfortunately, ultrasound imaging of the internal jugular vein (IJV) has not eliminated the risk of inadvertent carotid artery puncture and subsequent arterial catheter placement.4,5 Real-time ultrasound of the neck vessels alone offers visualization of the introducer needle and guidewire over a short distance, leaving room for penetration of the posterior vein wall or misdirection of the guidewire beyond the field of view. Complications, including hematoma, arterial dissection, airway compression, stroke, or death, usually occur when the guidewire is not detected in the carotid or brachiocephalic artery and the artery is subsequently damaged by the large introducer sheath.6 Visualization of the guidewire within the superior vena cava (SVC), right atrium (RA), or inferior vena cava (IVC) provides the most definitive evidence of appropriate access before dilation and catheter insertion. Sawchuk et al. (2001) showed that transesophageal echocardiography (TEE) can be used to provide this verification.7 Although other means are available to help confirm appropriate venous access, recommendations from the Society of Cardiovascular Anesthesiologists and the American Society of Echocardiography state that use of TEE or fluoroscopy to visualize the guidewire in the SVC or IVC provides “definitive confirmation of placement in the central venous system”.8 In many cardiac centres, both real-time ultrasound and TEE are used for visualization in central venous cannulation;9 however, TEE is itself invasive and its use outside cardiac operating rooms is limited by lack of equipment and expertise. With increasing use of transthoracic echocardiography (TTE) in anesthetic practice,10,11 TTE may provide a viable alternative for direct visualization of the guidewire within the right side of the heart.
We have previously shown that TTE with apical and subcostal four-chamber views can be used to visualize guidewire advancement from within the IJV to appropriate venous and cardiac structures.12 Although we showed the feasibility and potential utility of TTE in guidewire placement, we could not explore the technique`s general applicability due to an inadequate patient sample size. The purpose of this prospective observational study was to examine the feasibility and reliability of using TTE to visualize microbubbles and guidewire advancement from the IJV to the SVC, RA, or IVC as a method to confirm appropriate central line placement in a cohort of patients undergoing cardiac surgery.
Methods
Following institutional Research Ethics Board approval and signed informed consent, patients over 18 yr of age who were scheduled to undergo elective cardiac surgery with an IJV catheter and TEE were recruited. Exclusion criteria were 1) patients with severe aortic stenosis, as it was reasoned that repeated wire manipulation required by the study protocol could induce an arrhythmia that would have significant hemodynamic consequences in these patients; 2) patients with preexisting intracardiac wires or catheters, as we thought the assessors could confuse their echocardiographic signal for the guidewire, which could potentially bias the study in favour of a positive outcome; or 3) patients with contraindications to TEE. Following induction of anesthesia and intubation, a TEE probe was placed into the esophagus. The right IJV and carotid artery were identified with an ultrasound probe (5-10 MHz linear transducer; SonoSite Canada Inc, Markham, ON, Canada), and the procedure was completed under real-time ultrasound guidance. All central line catheters (9 Fr; Arrow International Inc, Reading, PA, USA) were inserted by experienced cardiac anesthesiologists or senior anesthesiology residents using the standard Seldinger technique. Transthoracic echocardiography was performed either by one of two medical students (novice) or by one of two cardiac anesthesiologists (expert) who had passed the National Board of Echocardiography Examination of Special Competence in Advanced Perioperative Transesophageal Echocardiography, but whose TTE training was limited to a two-day focused TTE course. The novices’ training consisted of observation and participation in TTE image acquisition of the apical and subcostal four-chamber views of ten patients before proceeding to independent scanning.
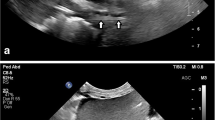
With one operator performing the TTE scanning and another placing the central line, the best-quality apical or subcostal four-chamber image that was initially obtained was used to interrogate the RA (1.5 MHz M4S GE Healthcare Canada, Mississauga, ON, Canada). The TTE operator indicated whether or not the images obtained were adequate (i.e., high quality). Following puncture of the IJV, 5 mL of blood was aspirated from the patient’s vein and immediately and rapidly reinjected. With the goal of visualizing microbubbles from the injected blood, TTE loops of the RA were obtained after injection (Fig. 1, Video 1) and used to provide ultrasound evidence that the tip of the needle was in a venous structure. Next, a standard 0.035-inch flexible j-tipped guidewire was inserted through the introducer needle/cannula, and presence of the guidewire in the SVC, RA, or IVC was confirmed with TEE in the bicaval view. If the guidewire was not visualized in the bicaval view, it was considered to be either misplaced in the arterial system or misdirected into the brachiocephalic or subclavian vein; consequently, the localization of the IJV was repeated. The wire was withdrawn 5-10 cm so that the tip was situated in the SVC. The wire was then reinserted into the RA while TTE loops were obtained to document the wire entering the RA (Fig. 2, Video 2). If the wire was not observed in the RA, the alternate view was obtained and the process of reinserting the wire was repeated. The subcostal view was used to interrogate the SVC and IVC when the wire could not be observed in the RA from either the apical or subcostal four-chamber views (Fig. 3, Video 3). Patients were initially scanned in the Trendelenburg position, but if imaging of the RA was suboptimal, the patient was changed to the supine position as this is better for acquisition of a TTE image.
Outcome measures: The primary outcome was the proportion of times that the individual performing the TTE visualized the guidewire in either the RA (apical or subcostal four-chamber views) or the IVC (subcostal view). The secondary outcome was the proportion of times that microbubbles were observed in the RA after reinjection of blood. We included this outcome as microbubbles seen in the RA would confirm initial needle placement into the venous system.
Patient characteristics collected included: age, height, weight, body mass index (BMI), and presence of chronic obstructive pulmonary disease (COPD). At the time the ultrasound images were obtained, the medical student or anesthesiologist performing the TTE documented adequacy of RA views, best TTE view, incidence of dysrhythmias, and patient position during TTE to optimize view (supine or Trendelenburg). Any complications occurring during line placement were also noted.
Statistical analysis
Patient characteristics are presented as mean (standard deviation) for continuous variables (age and BMI) and count (percentage) for categorical variables (sex and COPD). These patient characteristics were compared between patients, with and without identification of microbubbles or the guidewire, using the independent two-sided Student’s t test for continuous variables and Fisher’s two-sided exact test for categorical variables. We used the assumption/definition that TTE would have to detect the guidewire successfully at least 80% of the time to have any clinical relevance and with a binomial distribution to calculate a sample size of 97 subjects and provide 80% power to reject the null hypothesis that the detection rate is ≤ 80% at a one-sided alpha = 0.025 (same as two-sided alpha = 0.05) if the true detection rate is ≥ 90%.
Further exploratory analyses were performed. Fisher’s exact tests were used to compare detection rates among the four sonographers and between the expert and novice sonographers. The raw detection rates are presented with exact binomial 95% confidence intervals by sonographer, by expert level, and overall. The raw detection rates are compared against the null hypothesis that the true rate is ≤ 80% by a one-sided exact binomial test. All data were analyzed using SAS® version 9.2 (SAS Inc., Carey NC, USA).
Results
One hundred subjects were recruited to the study. All patients had successful guidewire placement on the first attempt, as confirmed by TEE, and there were no complications. Table 1 provides the patient characteristics and compares these characteristics between patients with and without TTE success. The sample was predominately male (72%) with an average age of 64 yr (range 42-86), average BMI 29 (range 18-47), and a 10% prevalence of COPD. In participants where the guidewire could not be visualized, the average BMI was significantly higher (33.6 vs 28.8; P = 0.01). No other patient characteristics were significantly associated with successful identification of guidewire or microbubbles.
The TTE results are summarized in Table 2. The guidewire was observed in the RA by TEE in all subjects. Since no differences were observed between the novice and expert detection rates (RA view, P = 0.42; bubble, P = 0.27; wire, P = 0.06), between the detection rates of the two novices, or between those of the two experts (P > 0.25), the data for all 100 participants were pooled. Data for the individual sonographers are still presented (Fig. 4). Overall, the RA was adequately viewed 94% (95% confidence interval [CI] 87 to 98) of the time. Microbubbles were observed in the RA in 91% of subjects (95% CI 84 to 96), and the guidewire was observed in 91% of subjects (95% CI 84 to 96). When the RA was adequately visualized, microbubbles were detected in 97% of subjects and guidewire detection was also 97%. Although both the microbubbles and guidewire were observed in the majority of subjects, in a small number of subjects, only the microbubbles or the guidewire (but not both) were seen. The RA was adequately imaged in both the subcostal and four-chamber views in 19% of subjects, while the subcostal view alone was best in 54% and the four-chamber view alone was best in 27%.
Discussion
The results of this study suggest that TTE can be a useful means of visualizing the guidewire in the SVC, RA, or IVC. Visualization of the guidewire in these locations provides definitive confirmation of correct placement before insertion of the dilator and central vein catheter. In addition, this study also indicates that TTE can be used to confirm initial needle puncture into a venous structure following aspiration and reinjection of blood, which creates microbubbles that are seen in the RA.
Techniques to confirm venous access include inspection of blood colour and flow characteristics, manometry or pressure transduction of the small-bore seeker needle/catheter, and more recently, the use of ultrasound. Real-time ultrasound-guided internal jugular cannulation has been shown to reduce complications, insertion attempts, failed catheter insertions, and time to successful insertion.13–16 Nevertheless, serious complications are still reported,4,5 and a contributing cause of this is the inability to identify the tip of the needle with certainty using ultrasound. The needle tip could pass through the posterior wall of the vein into adjacent structures.6,17 Accidental puncture of the carotid artery with the small-bore introducer needle/catheter does not appear to lead to major neurologic or vascular complications;18 however, once the large-bore dilator or catheter is inserted, major vascular injury, stroke, or death may occur, especially if the catheter is removed without surgical or interventional radiology repair.19,20 Ultrasound visualization of the guidewire in the SVC, RA, or IVC provides definitive evidence that the guidewire is appropriately placed before dilation and cannulation of the vessel.
Guidewire visualization can easily be accomplished with TEE.7,9 Previous studies have shown that appropriate wire placement can be confirmed in virtually every case using the TEE bicaval view.21,22 Nevertheless, TEE is impractical for this use as it is inherently an invasive modality that is not readily available outside an echocardiography lab or cardiac operating room. There are a few reports on the use of TTE as a technique to help confirm central line placement, but reports investigating the use of TTE for reliable visualization of a guidewire are lacking. Prekker et al. (2010) used the TTE subcostal view and injected 10 mL of saline into the distal lumen of a catheter to confirm its location with microbubbles observed in the RA.23 They speculated that injection of saline could be used before using the guidewire or dilator to avoid morbidity or mortality caused by accidental arterial injury. Their study, however, did not assess the use of TTE to visualize the guidewire. Etheridge et al. (1995) mention the use of TTE to visualize the guidewire in pediatric IJ central line insertion as part of a broader ultrasound technique, but in their study, they neither described the rate of successful TTE visualization nor used formal echocardiographic views.24 We recently published a report showing that the TTE apical and subcostal four-chamber views could be used to visualize microbubbles entering the RA when blood is withdrawn and rapidly reinjected, confirming venous access with the finder needle/catheter.10 We also showed that these views, along with the subcostal IVC view, are useful to visualize the advancement of the guidewire into the SVC, RA, or IVC. Nevertheless, in view of the results of our study, we could not make conclusions about the general applicability of the technique.
The current study extends our preliminary findings by showing the feasibility, reproducibility, and reliability of our technique to detect microbubbles or guidewire advancement during insertion/confirmation of a central venous catheter. It also delineates some limitations of TTE to identify appropriately placed guidewires or to detect RA microbubbles. Detection using TTE was unsuccessful in approximately 10% of subjects with appropriate central vein access. These failures were associated with subjects with a high BMI. The majority of cases in which TTE could not visualize microbubbles or the guidewire occurred in participants in whom the RA was not visualized at all. This suggests that improved proficiency with TTE (and ability to visualize the RA) may improve the guidewire detection rate. Nevertheless, the current investigation did show accurate visualization of microbubbles and guidewire > 90% of the time. This result was achieved using sonographers not formally trained in TTE and subjects with mechanically ventilated lungs and in suboptimal positions for imaging.
This technique has a number of limitations that preclude its use as the primary method for confirming placement of central line guidewires. First and foremost, a trained person is required to perform the TTE while another individual inserts the central line. Second, it takes time for the TTE examination to obtain adequate ultrasound windows, and in some instances, the guidewire was pulled back and re-advanced several times for adequate visualization, adding to the length of the procedure. Lastly, the scanning must be performed carefully with the ultrasound probe under sterile drapes. Current guidelines specify full-body drapes for central line insertion,3 and in our experience, scanning from apical and subcostal windows does not interfere with practices of sterile central line insertion. With the TTE operator and machine positioned on the patient’s left, the windows are accessed with the probe under the drape (Video 4). This can be performed comfortably without interfering with line insertion. In our view, the apical and subcostal windows at the xiphisternum and apex of the heart are far enough removed from the right-sided central line insertion site that no breaches in sterile technique are likely.
The current investigation has a number of limitations that may affect the generalizability of our findings. This study was conducted in a cardiac operating room where stable patients were under general anesthesia and TEE was always available. It is unclear whether the results would be similar in an intensive care or emergency room with hemodynamically unstable patients. In addition, the diameter of the guidewire used in the study was 0.035 in. It remains to be determined whether smaller diameter guidewires could be visualized as easily. Although manufacturers have produced echogenic needles for ultrasound-guided regional anesthesia, no guidewires with enhanced echogenicity are commercially available at this time. Although the medical students and cardiac anesthesiologists who performed the ultrasound examinations had no formal training in TTE, the minimal amount of education and experience required to perform TTE accurately and reliably for this purpose remains unspecified. Difficulty in discriminating the guidewire from previously placed cannulae or pacemaker wires may make this technique less useful in patients with existing indwelling lines.
Further studies would be useful to enhance the potential clinical utility of this technique. First, efforts should be made to improve the rate of guidewire detection. This may be achieved by redesigning software presets of ultrasound machines or perhaps by increasing the echogenicity of guidewires used for central line insertion. Second, training requirements need to be investigated to determine what levels of TTE proficiency are required for safe and reliable use of this technique. Finally, the utility of TTE-assisted central line insertion needs to be investigated in a wider clinical setting than the cardiac operating room setting used for this study. Such a study should have clinically relevant outcomes as it is currently unclear whether this technique can lower the rate of complications associated with central line insertion.
Results of the current investigation suggest that TTE may be a useful method to assist with confirmation of central venous access via visualizing microbubbles in the RA and the presence of a 0.035-inch guidewire in the SVC, RA, or IVC before insertion of large-bore dilators or catheters. Currently, we view this as a technique that can be used when other methods are equivocal or unavailable or when a TTE is concurrently being performed. Nevertheless, as point-of-care TTE gains wider use in anesthesia practice, this technique may become a viable alternative to the traditional methods for confirmation of central line placement.
References
Bishop L, Dougherty L, Bodenham A, et al. Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol 2007; 29: 261-78.
ACS Committee on Perioperative Care. Revised statement on recommendations for use of real-time ultrasound guidance for placement of central venous catheters. Bull Am Coll Surg 2011; 96: 36-7.
American Society of Anesthesiologists Task Force on Central Venous Access; Rupp SM, Apfelbaum JL, Blitt C, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology 2012; 116: 539-73.
Parsons AJ, Alfa J. Carotid dissection: a complication of internal jugular vein cannulation with the use of ultrasound. Anesth Analg 2009; 109: 135-6.
Stone MB, Hern HG. Inadvertent carotid artery cannulation during ultrasound guided central venous catheterization. Ann Emerg Med 2007; 49: 720.
Blaivas M. Video analysis of accidental arterial cannulation with dynamic ultrasound guidance for central venous access. J Ultrasound Med 2009; 28: 1239-44.
Sawchuk C, Fayad A. Confirmation of internal jugular guide wire position utilizing transesophageal echocardiography. Can J Anesth 2001; 48: 688-90.
Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2011; 24: 1291-318.
Mahmood F, Sundar S, Khabbaz K. Misplacement of a guide-wire diagnosed by transesophageal echocardiography. J Cardiothorac Vasc Anesth 2007; 21: 420-1.
Denault A, Fayad A, Chen R. Focused ultrasound is the next step in perioperative care. Can J Anesth 2013; 60: 741-7.
Tanzola RC, Walsh S, Hopman WM, Sydor D, Arellano R, Allard RV. Brief report: Focused transthoracic echocardiography training in a cohort of Canadian anesthesiology residents: a pilot study. Can J Anesth 2013; 60: 32-7.
Arellano R, Nurmohamed A, Rumman A, Milne B, Tanzola R. Point-of-care transthoracic echocardiography as an alternative to transesophageal echocardiography to confirm internal jugular guidewire position. Can J Anesth 2012; 59: 103-4.
Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med 1996; 24: 2053-8.
Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ 2003; 327: 361.
Leung J, Duffy M, Finckh A. Real-time ultrasonographically-guided internal jugular vein catheterization in the emergency department increases success rates and reduces complications: a randomized, prospective study. Ann Emerg Med 2006; 48: 540-7.
Milling TJ Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med 2005; 33: 1764-9.
Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med 2009; 37: 2345-9.
Oliver WC Jr, Nuttall GA, Beynen FM, Raimundo HS, Abenstein JP, Arnold JJ. The incidence of artery puncture with central venous cannulation using a modified technique for detection and prevention of arterial cannulation. J Cardiothorac Vasc Anesth 1997; 11: 851-5.
Auyong DB, Hsiung RL. Ultrasound in central venous cannulation. Adv Anesth 2010; 28: 59-79.
Jonker FH, Indes JE, Moll FL, Muhs BE. Management of iatrogenic injuries of the supra-aortic arteries. J Cardiothorac Vasc Anesth 2010; 24: 322-9.
Andropoulos DB, Stayer SA, Bent ST, et al. A controlled study of transesophageal echocardiography to guide central venous catheter placement in congenital heart surgery patients. Anesth Analg 1999; 89: 65-70.
Chaney MA, Minhaj MM, Patel K, Muzic D. Transoesophageal echocardiography and central line insertion. Ann Card Anaesth 2007; 10: 127-31.
Prekker ME, Chang R, Cole JB, Reardon R. Rapid confirmation of central venous catheter placement using an ultrasonographic “Bubble Test”. Acad Emerg Med 2010; 17: e85-6.
Etheridge SP, Berry JM, Krabill KA, Braunlin EA. Echocardiographic-guided internal jugular venous cannulation in children with heart disease. Arch Pediatr Adolesc Med 1995; 149: 77-80.
Funding
Department of Anesthesiology & Perioperative Medicine, Queen’s University and Kingston General Hospital.
Conflicts of interest
The authors have no financial interests relating to patents and/or share holdings in corporations involved in the development and/or marketing of the products evaluated in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Robert Tanzola and Ramiro Arellano participated in study conception. Robert Tanzola, Ramiro Arellano, Andrew Day, and Brian Milne participated in study design. Robert Tanzola and Ramiro Arellano participated in student supervision. Robert Tanzola, Ramiro Arellano, Aliya Nurmohamed (medical student), and Amir Rumman (medical student) participated in data collection. Andrew Day contributed to the data analysis. Robert Tanzola, Ramiro Arellano, Andrew Day, and Brian Milne participated in data interpretation. Robert Tanzola and Ramiro Arellano participated in manuscript composition. Robert Tanzola, Ramiro Arellano, Aliya Nurmohamed (medical student), Amir Rumman (medical student), Brian Milne, and Rachel Phelan participated in manuscript revision. Rachel Phelan assisted with study setup. All authors made intellectual contributions to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1: Transthoracic echocardiograms showing microbubbles (arrows) in the right atria in the apical and subcostal four-chamber views (WMV 7189 kb)
Video 2: Transthoracic echocardiograms showing the guidewires (arrows) entering the right atria in the apical and subcostal four-chamber views (WMV 7243 kb)
Video 3: Transthoracic echocardiogram showing the guidewire (arrow) in the inferior vena cava subcostal inferior vena cava view (WMV 6991 kb)
Video 4: A demonstration of technique with one operator inserting the right central line and the transthoracic echocardiography operator scanning with the probe under the sterile drapes (WMV 5355 kb)
Rights and permissions
About this article
Cite this article
Arellano, R., Nurmohamed, A., Rumman, A. et al. The utility of transthoracic echocardiography to confirm central line placement: An observational study. Can J Anesth/J Can Anesth 61, 340–346 (2014). https://doi.org/10.1007/s12630-014-0111-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0111-3