Abstract
Purpose
Estimated continuous cardiac output (esCCO) is a new and noninvasive cardiac output (CO) monitoring device using pulse wave transit time. The aim of this study was to assess rapid changes in CO using esCCO (ΔCOesCCO) without invasive calibration and to compare the results with those using transthoracic Doppler echocardiography (ΔCOTTE).
Methods
Fifty-four consecutive patients were enrolled in this study following elective cardiac surgery. The COesCCO and COTTE were collected during four consecutive steps: 1) at baseline, 2) during passive leg raising (PLR), 3) at return to baseline, and 4) after a fluid challenge. The relationship between ΔCOesCCO and ΔCOTTE induced by PLR and a fluid challenge was assessed and a polar plot analysis was performed. Relationship, Bland-Altman analysis, and percentage error for absolute values of COesCCO and COTTE were also performed.
Results
Twenty-four patients were excluded from the analysis. No correlation was found between ΔCOesCCO and ΔCOTTE during PLR (r = 0.07; P = 0.732; n = 30) and after a fluid challenge (r = 0.24; P = 0.394; n = 14). The polar plot analysis showed that 21 data points (87%) of significant changes in CO were above the 30° radial sector lines and confirmed that esCCO was unable to track changes in CO. A weak positive relationship was found between absolute values of COesCCO and COTTE (r = 0.28; P = 0.004). Bias, precision, and limits of agreement were 0.25 L·min−1, 2.4 L·min−1, and −4.4 to 4.9 L·min−1, respectively. The percentage error was 80%.
Conclusions
Estimated continuous cardiac output without external calibration seems unable to assess rapid changes in CO following cardiac surgery and was not interchangeable with transthoracic Doppler echocardiography.
Résumé
Objectif
L’estimation continue du débit cardiaque (esCCO) est une nouvelle technique de monitorage continu et non invasif du débit cardiaque utilisant la vitesse de propagation de l’onde de pouls. L’objectif de l’étude était de comparer les variations rapides de mesure de débit cardiaque entre l’esCCO (ΔCOesCCO) sans calibration invasive et l’échocardiographie Doppler transthoracique (ΔCOETT).
Méthodes
Cinquante-quatre patients consécutifs ont participé à l’étude après avoir subi une chirurgie cardiaque non urgente. Les données de COesCCO et COETT ont été récoltées au cours de quatre étapes consécutives: 1) état de base, 2) épreuve de lever de jambes passif (ELJ), 3) retour à l’état de base, et 4) après remplissage vasculaire. La relation entre la ΔCOesCCO et la ΔCOETT induites par l’ELJ et le remplissage vasculaire a été évaluée et une analyse par diagramme polaire a été réalisée. La corrélation, l’analyse de Bland-Altman et le pourcentage d’erreur pour les valeurs absolues de COesCCO et COETT ont également été réalisés.
Résultats
Vingt-quatre patients ont été exclus de l’analyse. Aucune corrélation n’a été observée entre la ΔCOesCCO et la ΔCOETT pendant l’ELJ (r = 0,07, P = 0,732, n = 30) ni après remplissage vasculaire (r = 0,24, P = 0,394, n = 14). L’analyse en diagramme polaire a démontré que 21 points (87 %) parmi les variations de débit cardiaque significatives se trouvaient au dessus de la zone radiale de 30°, confirmant que l’esCCO n’était pas assez précise pour détecter les changements de débit cardiaque. Une faible relation positive a été observée entre les valeurs absolues de COesCCO et COETT (r = 0,28, P = 0,004). Le biais, la précision et les limites d'agrémentétaient 0,25 L·min−1, 2,4 L·min−1, et −4,4 à 4,9 L·min−1, respectivement. Le pourcentage d’erreur était de 80 %.
Conclusion
L’esCCO sans calibration externe semble non utilisable pour évaluer les variations rapides du débit cardiaque après une chirurgie cardiaque et l’esCCO n’était pas interchangeable avec l’échocardiographie Doppler transthoracique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Advanced hemodynamic monitoring, including measurement of cardiac output (CO), has been shown to decrease morbidity and mortality in moderate- to high-risk patients during the perioperative period.1 Nevertheless, intermittent bolus pulmonary artery thermodilution, considered to be the reference approach for CO monitoring, is a highly invasive method and not feasible for regular use in routine practice.2 Among noninvasive CO monitoring devices, Doppler transthoracic echocardiography (TTE) has been validated as a reference method in critical care;3-5 however, TTE is time-consuming and requires expensive equipment and operator training. Moreover, the poor echogenicity of numerous critically ill patients decreases its efficiency at the bedside.3 New noninvasive convenient technologies could significantly improve the use of CO monitoring in high-risk patients and adherence of practitioners to the international guidelines.2,6 Recently, the Japanese company, Nihon Kohden, marketed a device (i.e., estimated continuous cardiac output [esCCO], Tokyo, Japan) that estimates CO by measuring pulse wave transit time (PWTT) derived from an electrocardiogram, digital pulse oximeter, and noninvasive brachial pressure. Two proof-of-concept studies using an external calibration with bolus pulmonary artery thermodilution and invasive arterial pressure showed encouraging results.7,8 Another study conducted in the intensive care unit (ICU) to compare noninvasive esCCO without external CO calibration with TTE was less straightforward.9 Further validation studies using different comparators and conducted in various subgroups of high-risk patients are highly desirable before recommending a wider use of esCCO at the bedside, in the ICU, or in the operating room.
Accordingly, the aim of this study conducted in cardiac surgical ICU patients was to compare noninvasive esCCO without external CO calibration with TTE during rapid changes in CO. We tested the hypothesis that esCCO could be an acceptable and convenient noninvasive alternative to TTE in that setting.
Methods
Patient population
The study was conducted in accordance with the Statement for Reporting Studies of Diagnostic Accuracy (the STARD initiative).10 Fifty-four consecutive adult patients admitted to the ICU following elective cardiac surgery with cardiopulmonary bypass were prospectively investigated at the University Hospital of Caen (Caen, France) from November 2012 to March 2013. Institutional approval was obtained from the local ethics committee (Comité de Protection des Personnes Nord-Ouest III, CHU de Caen, avenue de la Côte de Nacre, BP 95182, 14033 Caen Cedex 9, France, Number A12-D22-VOL.13, June 9, 2012). Authorization was granted to waive written informed consent because data were collected during routine care that conformed to standard procedures currently used in our institution. Nevertheless, verbal consent was obtained from all study participants. All patients scheduled for elective cardiac surgery with cardiopulmonary bypass (coronary artery bypass grafting, aortic and/or mitral valve replacement or repair, or combined cardiac surgery) were eligible for the study. The patients were enrolled if, according to the judgment of the attending anesthesiologist, they presented an indication of fluid loading during the postoperative period in the ICU. Patients undergoing emergent surgery (less than 24 hr), redo surgery, off-pump coronary artery bypass grafting, and complex unusual procedures were not included in the study. Additional exclusion criteria included patients with irregular heart rhythm when study hemodynamic data were gathered, patients with poor echogenicity, and/or patients with a poor-quality esCCO signal.
Perioperative management
General anesthesia and postoperative management followed the institutional standard. In the operating theatre, a Leadercath 20G (Vygon) radial artery catheter (Ecouen, France) and a jugular central venous catheter were inserted after induction of general anesthesia. Intra- and postoperative fluid management were left to the discretion of the attending anesthesiologist who was not involved in the study protocol and was guided by routine clinical and hemodynamic parameters. At the time of the study, patients either breathed spontaneously or received tracheal intubation and lung ventilation (volume-controlled regimen or assisted ventilation).
Transthoracic echocardiography cardiac output (COTTE) calculation
A single trained operator (X.B.) blinded to the esCCO measurements performed transthoracic echocardiography using a standard transthoracic probe (S5-1, Philips Healthcare, Bothell, WA, USA) and a dedicated unit (CX Cart 50, Philips Healthcare, Bothell, WA, USA). The COTTE was calculated using the sub-aortic velocity-time integral (VTISA), the diameter of the left ventricular outflow tract (LVOT), and heart rate (HR) using the following formula: COTTE = (VTISA × (diameter of LVOT)2 × Π/4) × HR. The diameter of the LVOT was measured in the parasternal long axis view, and the VTISA was measured on the five-chamber apical view using pulsed Doppler and averaged over three consecutive measurements. Two mandatory quality criteria were essential for patient enrollment in the study: the Doppler click of aortic valve closure and a dual spectrum VTISA (Fig. 1), confirming the right position of the pulsed-wave Doppler cursor and its repeatability over the study period.11
Example of a systolic still-frame image of the heart in the apical five-chamber view illustrating the Doppler recording sub-aortic velocity-time integral and the presence of the two quality criteria necessary to include the patient: the Doppler click of the aortic valve closure (large arrow) and a dual spectrum of VTISA (fine arrow). These criteria helped confirm the right position of the pulsed-wave Doppler cursor and its reproducibility over the study period
esCCO cardiac output (COesCCO) calculation
In all patients, an electrocardiograph, a noninvasive brachial pressure device, and a digital oximeter were connected to the Vismo monitor (Nihon Kohden, Tokyo, Japan), and a second investigator (C.L.M.D.K.) carried out a noninvasive calibration. After recording patient characteristics, including age, sex, height, weight, and noninvasive brachial pressure, the esCCO device determined a reference value for calibration. The signal quality index was displayed continuously on the monitor. The esCCO calculated the stroke volume (SV) continuously using the negative correlation between SV and PWTT. The PWTT was calculated by averaging 64 consecutive heart beats. The COesCCO was performed with the formulas previously described7: 1) CO = SV × HR; 2) SV = K × PP where K was the experimental constant and PP the pulse pressure; and 3) PP = (α × PWTT) + β where α and β were experimental constants. The PWTT represented the sum of the pre-ejection period and the delay in pulse wave arrival time from the ascending aorta to the site of the peripheral pulse oximeter sensor. The PWTT was calculated as the interval between the R wave on the electrocardiogram and the arrival of the peripheral SpO2 pulse wave.
Study protocol
Patients were enrolled postoperatively following the attending anesthesiologist’s decision to administer a fluid challenge (6% hydroxyethyl starch 500 mL 130/0.4) over 15 min. For each patient, measurements were recorded: 1) at baseline in the 45° semi-recumbent position; 2) during passive leg raising (PLR), which consisted of simply pivoting the entire bed using an automatic pivotal motion, as previously described;12 3) at return to baseline in the 45° semi-recumbent position; and 4) ten minutes after a fluid challenge in PLR responders only. The positive response to PLR was defined by an increase in COTTE ≥ 10%.13 The highest value of COTTE was observed within the first 90 sec following the PLR maneuver.14 The highest value of COesCCO was recorded during the period ranging from the beginning of the PLR to two minutes after the highest value of COTTE, in accordance with the fact that PWTT was calculated by averaging 64 consecutive heart beats.7 The same method was applied after the fluid challenge. Finally, a positive response to the fluid challenge was defined by an increase in COTTE of at least 15%.15 At each step, all hemodynamic parameters (including COTTE, COesCCO, and PWTT) were recorded. During the recording of study data, ventilator settings, sedation, and vasoactive drugs remained unchanged. The noninvasive brachial pressure was measured only at the time of the initial calibration, as recommended by the manufacturer.
Endpoints
The primary endpoint was the relationship between rapid changes in CO (ΔCOTTE and ΔCOesCCO) following PLR. Secondary endpoints were 1) the relationship and agreement between absolute values of COTTE and COesCCO and 2) the ability of ΔCOesCCO to track rapid changes in ΔCOTTE.
Statistical analysis
On the basis of a previous report9 and according to our primary endpoint, we calculated that 29 patients were necessary to show a correlation coefficient r = 0.50 between ΔCOTTE and ΔCOesCCO with a 5% type I error rate and a 20% type II error rate. Data are expressed as mean (SD) or median [25th-75th percentile] for non-normally distributed variables (Kolmogorov-Smirnov test) or numbers and percentages, as appropriate. Continuous variables were analyzed with the unpaired Mann-Whitney U test. Categorical variables were analyzed with the Fisher’s exact test or the Khi-2 test, as appropriate. Changes in hemodynamic parameters after PLR and the fluid challenge were compared using the paired Wilcoxon test. The relationships between ΔCOTTE and ΔCOesCCO and the absolute values of COTTE and COesCCO were determined by linear regression. A modified Bland-Altman analysis for repeated measurements was used to compare bias, precision (SD of bias), and limits of agreement (LOA) (bias ± 1.96 SD) between COTTE and COesCCO.16 The percentage error to determine acceptable limits of agreement between both devices was calculated using the formula given by Critchley and Critchley.17 Trends were analyzed using polar plotting of CO changes among consecutive time points, as previously described.18,19 A P value of < 0.05 was considered to be statistically significant and all P values were two-tailed. Statistical analyses were performed using MedCalc® Software bvba version 12.5.0 (Mariakerke, Belgium) and Deltagraph® version 5.6 (RockWare Inc., Golden, CO, USA).
Results
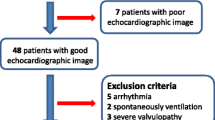
Twenty-four of the 54 consecutive adult patients enrolled in the study were excluded because of poor echogenicity (n = 8), the absence of the two predefined TTE criteria of quality (n = 14), and the absence of an esCCO signal (n = 2) (Fig. 2). The intra-observer variability for CO measurement assessed from five consecutive TTE exams was 4%. When present, the esCCO signal quality index was excellent at each time in all patients, indicating that COesCCO was valid for analysis. No complication related to the use of esCCO was observed over the study period. Baseline characteristics for the remaining 30 patients are indicated in Table 1. Patients were included on postoperative day 0 [0-1]. At the time of the study, 16 (53%) patients breathed spontaneously and 14 (47%) patients received tracheal intubation and lung ventilation (volume-controlled regimen = 9; assisted ventilation = 5).
The ΔCOTTE and ΔCOesCCO were compared during PLR (n = 30) and after the fluid challenge (n = 14). Responders to PLR (n = 14) increased COTTE by 25 (14)% on average while COesCCO varied by −1 (4)% (P < 0.001). Responders to the fluid challenge (n = 11) increased COTTE by 38 (21)% on average, while COesCCO varied by 3 (12)% (P = 0.09). The COTTE increased significantly after both PLR and the fluid challenge, whereas no significant difference was observed in COesCCO (Table 2). No statistical correlation was found between ΔCOTTE and ΔCOesCCO during PLR (n = 30; Y = −1.49 + 0.03X; R2 = 0.00; r = 0.07; P = 0.732), and after the fluid challenge (n = 14; Y = 7.64 − 0.13X; R2 = 0.06; r = 0.24; P = 0.394) (Figs. 3A and 3B, respectively).
The relationship between cardiac output changes (%) measured by transthoracic Doppler echocardiography (ΔCOTTE) and by estimated continuous cardiac output (ΔCOesCCO): (A) during passive leg raising (n = 30) and (B) after fluid challenge (n = 14). Dotted line is the 95% confidence interval of linear fit
The range of COTTE absolutes values was 2.9-13.6 L·min−1 [6.1 (2.3) L·min−1], while the range of COesCCO absolute values was 2.7-8.8 L·min−1 [5.9 (1.5) L·min−1]; P = 0.730. A weak positive relationship was found between the absolute values of COTTE and COesCCO (n = 104; Y = 9.55 + 0.44x; R2 = 0.079; r = 0.28; P = 0.004). The bias, precision, and LOA between COTTE and COesCCO were 0.25 L·min−1, 2.4 L·min−1, and −4.4 to 4.9 L·min−1, respectively (Fig. 4). The percentage error between COTTE and COesCCO was 80%, meaning that both methods were not interchangeable.
After the 0.5 L·min−1 central exclusion zone, polar plot analysis showed that 21 data points (87%) were above the 30° radial sector lines, confirming the low trending of esCCO to track rapid changes in CO (Fig. 5).
Polar plot comparing changes in cardiac output during each step of the study: baseline to PLR (n = 30), PLR to return to baseline (n = 30), and baseline to after fluid challenge (n = 14) using transthoracic Doppler echocardiography and estimated continuous cardiac output (esCCO). The first circle of 0.5 L·min−1 represents the central exclusion zone (n = 50), while remaining points showed the significant variations of cardiac output (n = 24). Twenty-one points (87%) were above the 30° radial sector lines
Discussion
The main finding of the study was the inability of esCCO to detect rapid changes of CO in adult patients undergoing elective cardiac surgery with cardiopulmonary bypass. The changes of CO determined by the noninvasive esCCO device without external CO calibration were not correlated with ΔCOTTE during PLR and after the fluid challenge. The values of precision and limits of agreement significantly exceeded the boundaries allowing interchangeable use of both devices.
In the present study, we used TTE as the reference method for CO assessment. An excellent correlation with use of a bolus pulmonary artery catheter has been previously reported in ICU patients.4,5 Our intra-observer variability for COTTE measurement was low (much lower than the changes in CO we observed during PLR and after fluid loading) and similar to previous reports.20 Moreover, we required high-quality TTE criteria, which explains the high rate of exclusion and strengthens the internal validity of our results. It is noteworthy that TTE offered a continuous beat-to-beat and noninvasive CO measurement while esCCO averaged 64 consecutive heart beats. Subsequently, we recorded the highest values of COesCCO within the two minutes following the highest values of COTTE, as previously described. In accordance with the manufacturer guidelines, a single calibration was performed at the start of the study period using measurement of noninvasive brachial pressure. Thus, the esCCO device was used in an entirely noninvasive manner in full accordance with a modern concept of CO monitoring, i.e., to encourage a noninvasive, beat-to-beat, plug and play, easy to use device.6 As no CO monitoring tool can currently replace thermodilution,21 the ability of new mini- and noninvasive CO monitors to assess trends could be more important at the bedside than their accuracy in measuring absolute values of CO under stable hemodynamic conditions.18 The esCCO device fulfills these specifications and thereby justifies the main objective of the present study.
We found that esCCO was convenient and easy to use in almost all cardiac surgical patients when compared with TTE, which is both operator- and patient-dependent and of limited clinical usefulness. This result highlights the need for simple plug-and-play CO monitors, such as esCCO, for use in critically ill patients. Nevertheless, the esCCO device was unable to track the direction of rapid changes in CO induced by PLR and the fluid challenge, as shown by both the absence of correlation between ΔCOTTE and ΔCOesCCO and the polar plot analysis. A previous study reported a significant correlation between spontaneous changes in COTTE and COesCCO over time in critically ill patients.9 The same study also showed a poor concordance rate and finally concluded that esCCO failed to trend CO reliably. Recently, Kim et al. suggested that the variations of PWTT were correlated with invasive systolic blood pressure during induction of anesthesia and could represent a valuable alternative to invasive systolic blood pressure in high-risk hypertensive patients.22 The indirect arterial pressure approach derived from CO is misleading, however, and could be notoriously insufficient at the bedside.23 In our study, neither systolic arterial pressure nor pulse pressure were correlated with PWTT (data not shown). To date, the esCCO device uses an undisclosed built-in proprietary algorithm that incorporates patient characteristics and PWTT to estimate arterial compliance. The CO calculation is based on the assumption that the SV is proportional to pulse pressure and arterial compliance. Unfortunately, acute changes in arterial compliance are not taken into account, even when serial noninvasive brachial pressure measurements are performed.9 The use of vasopressors could strongly modify the CO measurement taken with the esCCO device. Nevertheless, our results were similar when patients receiving norepinephrine were excluded from the analysis (data not shown). Passive leg raising and the fluid challenge were expected to modify blood volume and density and subsequently impact the velocity of arterial blood flow. Surprisingly, PWTT remained unchanged throughout the current study. Cardiac failure could also influence SV and PWTT. Postoperative inotropic support was used in 20% of our patients, leading to a quite normal left ventricular ejection fraction. In accordance with manufacturer recommendations, we measured noninvasive brachial pressure only at the beginning of the study. A systematic calibration of noninvasive brachial pressure before each measurement of COesCCO could improve the reliability of the device; however, a recent study found that this approach failed to improve agreement between TTE and esCCO.9 These last results emphasize the lack of interchangeability between esCCO and TTE. In contrast, studies using both a pulmonary artery catheter and invasive continuous arterial pressure for external calibration showed a good correlation between absolute values of CO measurements.7,8 Even so, the need for external invasive calibration significantly limits the applicability of esCCO as a complete noninvasive monitoring tool. Unfortunately, implementing an automated exclusion algorithm with the esCCO device seems unable to improve its reliability.24
Some comments are necessary concerning the limitations of the present study. First, the assessment of CO with TTE was unreliable in nearly 40% of our study population, mainly because of the absence of both predefined quality criteria required to reach intra-observer variability below 5%. This limitation clearly highlights the need for a simple plug-and-play CO monitor. Second, despite many attempts to reposition the pulse oximeter, two patients were excluded from esCCO analysis due to the absence of a signal. This could be another limitation of the device in routine practice. Third, the PWTT was calculated by averaging data of 64 consecutive heart beats, while TTE provided a beat-to-beat CO measurement. We tried to solve this problem by recording the highest value of COesCCO within the two minutes following the highest value of COTTE. Fourth, this phase II validation study was conducted in a subgroup of postoperative cardiac surgical patients. Further studies are required to assess the usefulness of esCCO as a trend CO monitor in other patient populations and to evaluate the influence of external invasive calibration at the bedside on the accuracy and reliability of esCCO.
In conclusion, the use of the noninvasive esCCO device without external CO calibration seemed unable to assess rapid changes in CO induced by PLR and a fluid challenge, and the device was not interchangeable with TTE following elective cardiac surgery.
References
Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg 2011; 112: 1392-402.
Maguire S, Rinehart J, Vakharia S, Cannesson M. Technical communication: respiratory variation in pulse pressure and plethysmographic waveforms: intraoperative applicability in a North American academic center. Anesth Analg 2011; 112: 94-6.
Huang SJ, McLean AS. Appreciating the strengths and weaknesses of transthoracic echocardiography in hemodynamic assessments. Cardiol Res Pract 2012; DOI:10.1155/2012/894308.
Evangelista A, Garcia-Dorado D, Garcia Del Castillo H, Gonzalez-Alujas T, Soler-Soler J. Cardiac index quantification by Doppler ultrasound in patients without left ventricular outflow tract abnormalities. J Am Coll Cardiol 1995; 25: 710-6.
McLean AS, Needham A, Stewart D, Parkin R. Estimation of cardiac output by noninvasive echocardiographic techniques in the critically ill subject. Anaesth Intensive Care 1997; 25: 250-4.
de Waal EE, Wappler F, Buhre WF. Cardiac output monitoring. Curr Opin Anaesthesiol 2009; 22: 71-7.
Ishihara H, Okawa H, Tanabe K, et al. A new non-invasive continuous cardiac output trend solely utilizing routine cardiovascular monitors. J Clin Monit Comput 2004; 18: 313-20.
Yamada T, Tsutsui M, Sugo Y, et al. Multicenter study verifying a method of noninvasive continuous cardiac output measurement using pulse wave transit time: a comparison with intermittent bolus thermodilution cardiac output. Anesth Analg 2012; 115: 82-7.
Bataille B, Bertuit M, Mora M, et al. Comparison of esCCO and transthoracic echocardiography for non-invasive measurement of cardiac output intensive care. Br J Anaesth 2012; 109: 879-86.
Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003; 138: W1-12.
Lewis JF, Kuo LC, Nelson JG, Limacher MC, Quinones MA. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation 1984; 70: 425-31.
Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 2006; 34: 1402-7.
Preau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med 2010; 38: 819-25.
Cavallaro F, Sandroni C, Marano C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med 2010; 36: 1475-83.
Monnet X, Teboul JL. Volume responsiveness. Current Opin Crit Care 2007; 13: 549-53.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 2007; 17: 571-82.
Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 1999; 15: 85-91.
Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg 2010; 111: 1180-92.
Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth 2011; 25: 536-46.
Bergenzaun L, Gudmundsson P, Ohlin H, et al. Assessing left ventricular systolic function in shock: evaluation of echocardiographic parameters in intensive care. Crit Care 2011; 15: R200.
Peyton PJ, Chong SW. Minimally invasive measurement of cardiac output during surgery and critical care: a meta-analysis of accuracy and precision. Anesthesiology 2010; 113: 1220-35.
Kim SH, Song JG, Park JH, Kim JW, Park YS, Hwang GS. Beat-to-beat tracking of systolic blood pressure using noninvasive pulse transit time during anesthesia induction in hypertensive patients. Anesth Analg 2013; 116: 94-100.
Le Manach Y, Hofer CK, Lehot JJ, et al. Can changes in arterial pressure be used to detect changes in cardiac output during volume expansion in the perioperative period? Anesthesiology 2012; 117: 1165-74.
Ishihara H, Sugo Y, Tsutsui M, et al. The ability of a new continuous cardiac output monitor to measure trends in cardiac output following implementation of a patient information calibration and an automated exclusion algorithm. J Clin Monit Comput 2012; 26: 465-71.
Acknowledgements
The authors thank Olivier Montembault (Nihon Kohden, Tokyo, Japan) for kindly providing all facilities for the esCCO measurements. The manufacturer had no input into the design or conduct of the study or the decision to submit the manuscript for publication.
Funding
The study was supported entirely by the University Hospital, Caen, France.
Competing interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
All authors contributed to the concept and design of the study, reviewed the analysis, and participated in the preparation of the submitted manuscript. Xavier Balaire performed the transthoracic echocardiography. Marc-Olivier Fischer and Charles Le Mauff de Kergal performed data acquisition. Clément Boisselier requested ethical and administrative authorization. Marc-Olivier Fischer, Jean-Luc Hanouz, and Jean-Luc Fellahi designed the study, performed statistical analysis, and wrote the manuscript.
Rights and permissions
About this article
Cite this article
Fischer, MO., Balaire, X., Le Mauff de Kergal, C. et al. The diagnostic accuracy of estimated continuous cardiac output compared with transthoracic echocardiography. Can J Anesth/J Can Anesth 61, 19–26 (2014). https://doi.org/10.1007/s12630-013-0055-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-0055-z