Abstract
Purpose
The use of peripheral tramadol to block pain has been advocated. However, since its actions in the periphery have not been elucidated fully, we tested the hypothesis that peripheral tramadol blocks peripheral glutamate-induced nociceptive behaviour in mice.
Methods
First, we compared the duration of paw licking after intraplantar (ipl.) glutamate administration, with and without tramadol, using a randomized blinded controlled design. Next, we established the half maximal effective concentrations (EC50s) for local tramadol and reference compound lidocaine in the hot water tail-flick latency test and the glutamate-induced paw allodynia assay.
Results
Tramadol reduced glutamate-induced paw licking from 33 ± 12 sec to 4 ± 4 sec (mean ± SD; t test, P < 0.05; n = 6 per group). The tramadol and lidocaine EC50 nerve conduction blocks in the tail did not differ significantly (84 ± 24 mM vs 69 ± 5 mM, respectively). Although tramadol reduced glutamate-induced allodynia (EC50, 46 ± 13 mM), lidocaine was more potent (EC50, 13 ± 5 mM; Dixon’s up-and-down method; P < 0.05). Tramadol was 2.5 times as effective at blocking nerve conduction in the tail compared with allodynia in the paw.
Conclusions
Local tramadol administration blocked nociceptive behaviour in mice induced by peripheral glutamate. Compared with lidocaine, the relative potency of tramadol was lower for blocking glutamate-induced allodynia than for sensory nerve conduction blockade, suggesting the activation of a pronociceptive receptor system in the periphery.
Résumé
Objectif
L’utilisation de tramadol périphérique a été proposée pour bloquer la douleur. Cependant, comme nous ne connaissons pas pleinement ses actions sur les nerfs périphériques, nous avons émis l’hypothèse que le tramadol périphérique bloquait les comportements nociceptifs provoqués par le glutamate périphérique chez la souris.
Méthode
Nous avons d’abord comparé la durée du léchage de la patte après une administration intraplantaire (ipl.) de glutamate, avec ou sans tramadol, en utilisant une méthode contrôlée randomisée en aveugle. Ensuite, nous avons déterminé les concentrations efficaces moyennes (CE50) pour le tramadol administré localement et un composé de lidocaïne comme référence à l’aide du test de latence du retrait de la queue et du test d’allodynie de la patte provoquée par le glutamate.
Résultats
Le tramadol a réduit le léchage de patte provoqué par le glutamate de 33 ± 12 sec à 4 ± 4 sec (moyenne ± ET; test t, P < 0,05; n = 6 par groupe). Les CE50 pour le bloc de conduction nerveuse réalisé avec le tramadol et la lidocaïne au niveau de la queue n’ont pas montré de différence significative (84 ± 24 mM vs 69 ± 5 mM, respectivement). Bien que le tramadol ait réduit l’allodynie induite par le glutamate (CE50, 46 ± 13 mM), la lidocaïne était plus puissante (CE50, 13 ± 5 mM; méthode de l’escalier de Dixon; P < 0,05). Le tramadol était 2,5 fois plus efficace pour le blocage de la conduction nerveuse dans la queue que pour le blocage de l’allodynie dans la queue.
Conclusion
L’administration locale de tramadol a bloqué les comportements nociceptifs induits par du glutamate périphérique chez les souris. Par rapport à la lidocaïne, la puissance relative du tramadol était plus faible pour bloquer l’allodynie induite par le glutamate que pour le blocage de la conduction nerveuse sensitive, ce qui suggère qu’il y a activation d’un système de récepteurs pronociceptifs périphériques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently, tramadol has been approved in Canada as an oral sustained-release formulation for treatment of chronic pain. Tramadol is an atypical analgesic known to have local anesthetic actions as well as central opioidergic, noradrenergic, and serotonergic actions.1 , 2 Tramadol has been advocated increasingly for a local effect in blocking postoperative pain after tendon repair, tendon repair of the hand, and arthroscopic knee surgery.1 , 3 Intra-articular administration has been particularly effective; for instance, an intra-articular tramadol-bupivacaine combination prolongs the duration of postoperative analgesia after outpatient arthroscopic knee surgery.4 Furthermore, after outpatient arthroscopic partial meniscectomy, intra-articular tramadol plus pericapsular incisional bupivacaine provides better analgesia than intra-articular plus pericapsular incisional bupivacaine.5 , 6
Evidence in the literature suggests that tramadol antagonizes glutamate N-methyl-D-aspartic acid (NMDA) receptors7 , 8 that are known to be involved in the pathophysiology of chronic pain.9 Tramadol, fentanyl, sufentanil, but not morphine, block voltage-gated sodium channels.9
Tramadol’s effects following systemic intravenous and oral administration are well described, but there are few studies examining the pharmacological effects of local subcutaneous administration of tramadol. Local anesthesia by tramadol in amphibians and mammals is presumably produced by blockade of sodium channels.1 , 2 , 10 Glutamate, the primary excitatory amino acid neurotransmitter, is released along with other pain mediators in response to tissue damage.9 Formalin-induced pain behaviour is linked to local glutamate release and is blocked by tramadol.11
Considering previous findings implicating both glutamate receptor antagonism and nerve conduction block in tramadol’s mechanism of action, we hypothesized that tramadol would have conjunctive analgesic effects in nociceptive systems involving glutamate receptor-mediated transmission.12 In pharmacological terms, this would be reflected by a higher relative potency in systems involving glutamate receptor activation via nociceptive systems nearly exclusively mediated by nerve conduction.13
Methods
Animals
All animal experiments were performed according to protocols approved by the Animal Care Committee at The University of British Columbia. Female CD-1 mice weighing 20-30 g (Charles River Laboratories Inc., Wilmington, MA, USA) were used in the experiments. They were housed in groups of 12 per cage with food and water freely available in an environmentally controlled room kept at an ambient temperature of 25 ± 1°C (mean ± SD), a humidity of 55 ± 5%, and a reversed 12 hr/12 hr dark-light cycle.
Drugs
We obtained RS-Tramadol from Spectrum Chemicals & Laboratory Products (Gardena, CA, USA), lidocaine from AstraZeneca (Wilmington, DE, USA), and glutamate from Sigma (St. Louis, MO, USA). Glutamate solutions were prepared by dissolving L-glutamic acid in 0.3 mM KCl at a pH of 8. Other solutions were dissolved in normal saline (pH = 5.5). All solutions were freshly prepared on each day of the experiment.
Glutamate-induced nociception in the paw
To determine the initial effect of a high concentration of tramadol on glutamate-induced paw licking, 20 μL of glutamate (220 mg·mL−1 = 30 μmol) was co-injected with or without 20 μL of tramadol (19.5 mg·mL−1 = 1.3 μmol total injected) under the ventral surface of the right hindpaw (ipl.; n = 6 per group). After ipl. injection of glutamate, the animals were placed alone in an enclosed transparent plexiglass container (30 x 15 x 15 cm), and then the subsequent time spent licking and biting the injected paw was recorded as a behavioural endpoint indicative of nociception.9 , 10 A video camera mounted over the observation chamber recorded all experiments.
Effects of tramadol and lidocaine on glutamate-induced allodynia in the paw
We determined the drug’s half maximal effective concentration (EC50) on glutamate-induced allodynia, a more sensitive assay than glutamate-induced licking.14 We used a random double-staircase method to determine the EC50 for glutamate-induced allodynia when injected ipl. under the ventral surface of the right hindpaw of the mouse at a volume of 20 μL (total n = 11).15 The random double-staircase method is a variant of the staircase method (also known as the up-and-down method), which is an efficient method to determine the EC50 while reducing animal suffering in research by using the least number of animals.16 The staircase method is an adaptive trial. It involves a series of trials in which the dose at a given trial is determined by the response result of the previous trial. If there is no response in a trial, the dose of the subsequent trial is doubled. If there is a response, the dose for the subsequent trial is halved. The random double-staircase method resolves the problem of blinding by concurrently operating two staircases with the order of presentation randomized.
The von Frey filament thickness required to produce three consecutive withdrawals when pressed against the injected hindpaw was determined before and after injection. Allodynia was defined as a decrease of 50% in threshold of von Frey filament thickness required for three consecutive withdrawals. Preliminary testing to determine an appropriate dose of glutamate to produce allodynia yielded an EC50 of 550 ± 170 mM.
The dose of glutamate selected was the EC95 for the production of robust and consistent allodynia (20 μL of 110 mg·mL−1 or 750 mM). The EC50 for tramadol and lidocaine in reducing glutamate-induced allodynia was determined according to the same method (total n = 11 per drug). Observations were conducted five minutes after glutamate or after co-injection of glutamate with drug injection.
Effect of tramadol on hot water tail-flick test
We conducted a randomized blinded experiment to determine the EC50 for tramadol and for lidocaine on the tail-flick latency after immersion in hot water (n = 6 per group). We used a modification of the method of Grant et al. 17 In brief, mice were placed in restraining tubes and the 2 cm distal tips of their tails were submerged into a 55°C water bath. Initially, the latencies to tail flick were screened in the mice, and only those with latencies less than four seconds were selected for experiments. Bilateral 20 μL injections of tramadol (22.5 mg·mL−1; 1.5 μmol) or lidocaine (16.4 mg·mL−1; 1.4 μmol) were performed 7 cm proximal to the tip of the tail using 29 gauge hypodermic needles. The needles were inserted with the bevel facing medially until their tips contacted the caudal vertebrae. The needles were then withdrawn by 1 mm before injection. The distance between the application of heat at the distal tip of the tail and the site of injection was at least 5 cm. Tail-flick latency was tested at one, three, five, ten, and 15 min after injection. The time to recover to control latency (< four seconds) was determined in a group of five animals administered with tramadol at five, 30, and 60 min.
Subsequently, the random double staircase method (total n = 11) was used to determine the EC50s for tramadol and lidocaine on the tail-flick test (Figure 1).15 , 16 The four-second cut-off latency for tail withdrawal from hot water was also considered a positive response, i.e., the deciding criteria for a dose increment. To avoid injury to the animals, the test was terminated when the latency exceeded 15 sec. With at least three animals exceeding and three animals not exceeding the criterion for local anesthesia in mice, nine to twelve animals were required to determine an EC50 value.18
Statistical analysis
Values are presented as means ± standard deviation and n represents the sample size. Statistical analyses for the random double staircase method were conducted according to Dixon.15 Two-tailed Student’s t tests were used to assess statistical significance between groups, and outliers were excluded according to Grubb’s criterion (GraphPad Prism 5.0, GraphPad Software, San Diego, CA, USA); P < 0.05. Intention-to-treat analysis was not undertaken, as all animals in our experiments of this type continue through the entire experiment.
Results
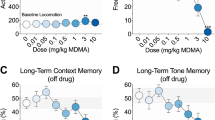
A total of 149 animals were used. There were no protocol violations. Tramadol reduced glutamate-induced paw licking time by > 90% (4 ± 4 sec vs 33 ± 12 sec; mean ± SD; n = 6; P < 0.05) (Figure 2A). Intraplantar injection of glutamate lowered the nociceptive threshold as measured by responses to von Frey filaments. The EC50 for tramadol blockade of allodynia (a decrease of 50% of von Frey filament thickness) was 46 ± 13 mM, and the EC50 for lidocaine was 13 ± 5 mM (Figure 2A).
A Graph comparing half maximal effective concentrations (EC50s) of tramadol and lidocaine on the glutamate-induced allodynia test. Mean ± standard deviation; total n = 66 mice with three staircases per group, two groups, and 11 mice per staircase; *P < 0.05. B Graph comparing half maximal effective concentrations (EC50s) of tramadol and lidocaine on the tail-flick test. Mean ± standard deviation, total n = 66 mice with three staircases per group, two groups, and 11 mice per staircase
Time courses for tramadol and lidocaine in the hot water tail-flick test were similar. The pre-treatment latency of the tramadol and lidocaine groups were not significantly different (n = 6; P > 0.05). While the onset of action of tramadol was less than one minute, the duration of action was approximately one hour (0.95 ± 0.11 hr; n = 5). The EC50 to increase tail latency to 50% of the maximum was 84 ± 24 mM for tramadol and 69 ± 5 mM for lidocaine (Figure 2B).
Tramadol was 30% as potent as lidocaine at blocking allodynia, while it was 82% as potent as lidocaine at blocking the peripheral nerve. Compared with the local anesthetic, lidocaine, tramadol was 2.5 times as effective at blocking nerve conduction in the tail compared with blocking allodynia in the paw.
Discussion
Our study has confirmed that peripheral tramadol administration inhibits nociceptive behaviour induced by peripheral glutamate application in the paw. Furthermore, we have shown that tramadol produces peripheral sensory nerve blockade in the tail-flick test.
As mentioned earlier, the antinociceptive actions of tramadol include blocking glutamate NMDA receptors.8 Indeed, systemic administration of tramadol also produces anticonvulsant effects that are known to be mediated through NMDA receptors.8 Moreover, it has been demonstrated conclusively that tramadol inhibits the activity of NMDA receptors expressed in Xenopus oocytes in vitro. 8 , 19 Furthermore, peripheral tramadol administered intraplantarily in rats reduces formalin-induced nociception in rats,20 which is partially mediated by glutamate release.11
Tramadol applied around the nerves in the tail reversibly inhibited the tail-flick response elicited by immersion of the tail in hot water, indicative of a local anesthetic action. There is evidence that tramadol produces local anesthesia in amphibians and mammals1 , 10 via blockade of sodium channels.2 Regarding the possible activity of glutamate receptor antagonists in local anesthetic assays, preliminary studies in our laboratory have shown that a glutamate receptor antagonist did not produce nerve conduction block when administered in the tail at a concentration of 1 mM (data not shown).
Tramadol acted primarily at the site of injection, not centrally, since the antinociception in one paw following tramadol with formalin was not found in the contralateral paw that had received formalin only.20
Other possible mechanisms of local anesthetic action distinct from sodium channel blockade are unlikely, since tramadol has no effects on inhibitory glycine or gamma-aminobutyric acid (GABA) receptors at clinically relevant concentrations.7 Primary activity through opioid action is unlikely, since direct application of peripheral intraplantar naloxone does not reverse tramadol’s effect on reducing heat-induced nociception,10 and naloxone does not reverse tramadol’s ability to block the licking response to formalin in the paw.20 Thus, it is likely that tramadol produces its analgesic effects in the tail-flick test by blockade of sodium channels.
The surprising observation that tramadol has a lower relative potency for glutamate-induced nociceptive blockade than for nerve conduction blockade raises the possibility that tramadol has some pronociceptive properties. This is supported by a recent publication showing that tramadol is an agonist at transient receptor potential vanilloid 1 receptors (TRPV1).3 An alternative explanation of the lower potency of tramadol in the paw compared with lidocaine exposed to glutamate would be a decreased efficacy of tramadol relative to lidocaine on damaged peripheral nerves rather than undamaged nerves. Indeed, tramadol has been demonstrated to be ineffective in injured peripheral nerves.7 However, in the aforementioned study, nerves were damaged by constriction for a long period—there was no evidence of chronic nerve damage in our experiment, as the allodynia reversed with time.
It has been noted that tramadol produces skin erythema, flare, and urticaria, and initiates a burning skin sensation, presumably due to the known TRPV1 activation.10 This raises the likelihood of tissue damage and calls for further investigation. In addition, this possibility has implications for the clinical use of local tramadol injection.
While further studies are required to elucidate the pronociceptive actions of tramadol, our findings indicate that these actions decrease tramadol’s analgesic potency in the periphery.
References
Kargi E, Babuccu O, Altunkaya H, et al. Tramadol as a local anaesthetic in tendon repair surgery of the hand. J Int Med Res 2008; 36: 971-8.
Haeseler G, Foadi N, Ahrens J, Dengler R, Hecker H, Leuwer M. Tramadol, fentanyl and sufentanil but not morphine block voltage-operated sodium channels. Pain 2006; 126: 234-44.
Marincsak R, Toth B, Czifra G, Szabo T, Kovacs L, Biro T. The analgesic drug, tramadol, acts as an agonist of the transient receptor potential vanilloid-1. Anesth Analg 2008; 106: 1890-6.
Beyzadeoglu T, Yilmaz C, Bekler H, Gokce A, Sayin M. Intraarticular tramadol plus pericapsular incisional bupivacaine provides better analgesia than intraarticular plus pericapsular incisional bupivacaine after outpatient arthroscopic partial meniscectomy. Knee Surg Sports Traumatol Arthrosc 2007; 15: 564-8.
Zeidan A,Kassem R,Nahleh N,Maaliki H,El-Khatib M,Struys MM,Baraka A. Intraarticular tramadol-bupivacaine combination prolongs the duration of postoperative analgesia after outpatient arthroscopic knee surgery. Anesth Analg 2008; 107: 292-9.
Zeidan A, Struys MM. Intraarticular Tramadol or “Hot Chili Peppers”? (Reply). Anesth Analg 2008; 107: 2093.
Hara K, Sata T. The effects of the local anesthetics lidocaine and procaine on glycine and gamma-aminobutyric acid receptors expressed in xenopus oocytes. Anesth Analg 2007; 104: 1434-9.
Manocha A, Sharma KK, Mediratta PK. On the mechanism of anticonvulsant effect of tramadol in mice. Pharmacol Biochem Behav 2005; 82: 74-81.
Beirith A, Santos AR, Calixto JB. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res 2002; 924: 219-28.
Mert T, Gunes Y, Gunay I. Local analgesic efficacy of tramadol following intraplantar injection. Eur J Pharmacol 2007; 558: 68-72.
Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res 1998; 787: 161-4.
Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 1992; 260: 275-85.
Zhang Y, Laster MJ, Eger EI II, Sharma M, Sonner JM. Lidocaine, MK-801, and MAC. Anesth Analg 2007; 104: 1098-102.
Zhou S, Bonasera L, Carlton SM. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport 1996; 7: 895-900.
Dixon WJ. Quantal response variable experimentation: the up and down method. In: McArthur C (Ed.). Statistics in Endocrinology. Cambridge: MIT Press; 1970: 251-67.
Cornsweet TN. The staircase-method in psychophysics. Am J Psychol 1962; 75: 485-91.
Grant GJ, Zakowski MI, Vermeulen K, Langerman L, Ramanathan S, Turndorf H. Assessing local anesthetic effect using the mouse tail flick test. J Pharmacol Toxicol Methods 1993; 29: 223-6.
Lichtman AH. The up-and-down method substantially reduces the number of animals required to determine antinociceptive ED50 values. J Pharmacol Toxicol Methods 1998; 40: 81-5.
Hara K, Minami K, Sata T. The effects of tramadol and its metabolite on glycine, gamma-aminobutyric acidA, and N-methyl-D-aspartate receptors expressed in xenopus oocytes. Anesth Analg 2005; 100: 1400-5.
Pozos-Guillen AJ, Aguirre-Banuelos P, Arellano-Guerrero A, Castaneda-Hernandez G, Hoyo-Vadillo C, Perez-Urizar J. Isobolographic analysis of the dual-site synergism in the antinociceptive response of tramadol in the formalin test in rats. Life Sci 2006; 79: 2275-82.
Acknowledgements
We thank Professor Richard Wall, Department of Anesthesiology, Pharmacology & Therapeutics, for his advice and molecular modelling.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J.T.C., Chung, C.C.W., Whitehead, R.A. et al. Effects of local tramadol administration on peripheral glutamate-induced nociceptive behaviour in mice. Can J Anesth/J Can Anesth 57, 659–663 (2010). https://doi.org/10.1007/s12630-010-9301-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-010-9301-9