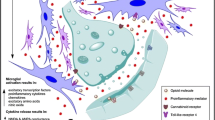
Abstract
Opioids are very potent and efficacious drugs, traditionally used for both acute and chronic pain conditions. However, the use of opioids is frequently associated with the occurrence of adverse effects or clinical problems. Other than adverse effects and dependence, the development of tolerance is a significant problem, as it requires increased opioid drug doses to achieve the same effect. Mechanisms of opioid tolerance include drug-induced adaptations or allostatic changes at the cellular, circuitry, and system levels. Dose escalation in long-term opioid therapy might cause opioid-induced hyperalgesia (OIH), which is a state of hypersensitivity to painful stimuli associated with opioid therapy, resulting in exacerbation of pain sensation rather than relief of pain. Various strategies may provide extra-opioid analgesia. There are drugs that may produce independent analgesic effects. A tailored treatment provided by skilled personnel, in accordance with the individual condition, is mandatory. Any treatment aimed at reducing opioid consumption may be indicated in these circumstances. Interventional techniques able to decrease the pain input may allow a decrease in the opioid dose, thus reverting the mechanisms producing tolerance of OIH. Intrathecal therapy with local anesthetics and a sympathetic block are the most common techniques utilized in these circumstances.



Similar content being viewed by others
References
Breivik H, Eisenberg E, O’Brien T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013;13:1229.
Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11:1230–9.
Mercadante S, Portenoy RK. Opioid poorly-responsive cancer pain: part 2: basic mechanisms that could shift dose response for analgesia. J Pain Symptom Manag. 2001;21:255–64.
Cahill CM, Walwyn W, Taylor AMW, Pradhan AAA, Evans CJ. Allostatic mechanisms of opioid tolerance beyond desensitization and downregulation. Trends Pharmacol Sci. 2016;37:963–76.
Gulur P, Williams L, Chaudhary S, Koury K, Jaff M. Opioid tolerance—a predictor of increased length of stay and higher readmission rates. Pain Physician. 2014;17:E503–7.
Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–63.
Siegel S, Hinson RE, Krank MD, McCully J. Heroin ‘overdose’ death: contribution of drug-associated environmental cues. Science. 1982;216:436–7.
Voon P, Hayashi K, Milloy MJ, Nguyen P, Wood E, Montaner J, et al. Pain among high-risk patients on methadone maintenance treatment. J Pain. 2015;16:887–94.
Gupta S. Hyperalgesia induced by opioid drugs. J Ration Pharmacother Res. 2018;4:22–30.
Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Physician. 2009;12:679–84.
Compton P, Canamar CP, Hillhouse M, Ling W. Hyperalgesia in heroin dependent patients and the effects of opioid substitution therapy. J Pain. 2012;13:401–9.
Rieb LM, Norman WV, Martin RE, Berkowitz J, Wood E, McNeil R, et al. Withdrawal-associated injury site pain (WISP): a descriptive case series of an opioid cessation phenomenon. Pain. 2016;157:2865–74.
Arout C, Edens E, Ismene L, Petrakis IL, Sofuoglu M. Targeting opioid-induced hyperalgesia in clinical treatment: neurobiological considerations. CNS Drugs. 2015;29:465–86.
Wilson-Poe AR, Lau BK, Vaughan CW. Repeated morphine treatment alters cannabinoid modulation of GABAergic synaptic transmission within the rat periaqueductal grey. Br J Pharmacol. 2015;172:681–90.
Wilson-Poe AR, Jeong HI, Vaughan CW. Chronic morphine reduces the readily releasable pool of GABA, a presynaptic mechanism of opioid tolerance. J Physiol. 2017;595:6541–55.
Lau BK, Vaughan CW. Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol. 2014;29:159–64.
Chieng B, Christie MJ. Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994;113:303–9.
Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol. 1997;498:463–72.
Connor M, Bagley EE, Chieng BC, Christie MJ. β-Arrestin-2 knockout prevents development of cellular mu-opioid receptor tolerance but does not affect opioid-withdrawal-related adaptations in single PAG neurons. Brit J Pharmacol. 2015;172:492–500.
Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–86.
Meng ID, Harasawa I. Chronic morphine exposure increases the proportion of on-cells in the rostral ventromedial medulla in rats. Life Sci. 2007;80:1915–20.
Ippolito DL, Temkin PA, Rogalski SL, Chavkin C. N-terminal tyrosine residues within the potassium channel Kir3 modulate GTPase activity of Galphai. J Biol Chem. 2002;277:32692–6.
Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci. 2002;22:4328–34.
Zhao YL, Chen SR, Chen H, Pan HL. Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-d-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. J Biol Chem. 2012;287:25073–85.
Price DD, Mayer DJ, Mao J, Caruso FS. NMDA-receptor antagonists and opioid receptor interactions as related to analgesia and tolerance. J Pain Symptom Manag. 2000;19(1 Suppl.):S7–11.
Gintzler AR, Chakrabarti S. Chronic morphine-induced plasticity among signalling molecules. Novartis Found Symptom. 2004;261:191–3.
Hull LC, Llorente J, Gabra BH, et al. The effect of protein kinase C and G protein-coupled receptor kinase inhibition on tolerance induced by mu-opioid agonists of different efficacy. J Pharmacol Exp Ther. 2010;332:1127–35.
Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA. 2010;107:11608–13.
Bobeck EN, Ingram SL, Hermes SM, Aicher SA, Morgan MM. Ligand-biased activation of extracellular signal-regulated kinase 1/2 leads to differences in opioid induced antinociception and tolerance. Behav Brain Res. 2016;298:17–24.
Morgan MM, Bobeck EN, Ingram SL. Glutamate modulation of antinociception, but not tolerance, produced by morphine microinjection into the periaqueductal gray of the rat. Brain Res. 2009;1295:59–66.
Xie JY, Herman DS, Stiller C-O, Gardell LR, Ossipov MH, Lai J, et al. Cholecystokinin in the rostral ventromedial medulla mediates opioid- induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25:409–16.
Thomas J, Mustafa S, Johnson J, Nicotra L, Hutchinson M. The relationship between opioids and immune signaling in the spinal cord. Handb Exp Pharmacol. 2015;227:207–38.
Xu J-TT, Yaster M, Tao Y-XX, et al. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Investig 2014;124:592–603.
Harada S, Nakamoto K, Tokuyama S. The involvement of midbrain astrocyte in the development of morphine tolerance. Life Sci. 2013;93:573–8.
Eidson LN, Murphy AZ. Blockade of toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci. 2013;33:15952–63.
Beutler B, Du X, Poltorak A. Identification of Toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: genetic and evolutionary studies. J Endotoxin Res. 2001;7:277–80.
Thomas J, Mustafa S, Johnson J, Nicotra L, Hutchinson M. The relationship between opioids and immune signaling in the spinal cord. Handb Exp Pharmacol. 2015;227:207–38.
Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407.
Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–61.
Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165:569–83.
Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–73.
Wang X, Loram LC, Ramos K, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci USA. 2012;109:6325–30.
Little JW, Cuzzocrea S, Bryant L, Esposito E, Doyle T, Rausaria S, et al. Spinal mitochondrial-derived peroxynitrite enhances neuroimmune activation during morphine hyperalgesia and antinociceptive tolerance. Pain. 2013;154:978–86.
Salvemini D, Doyle T, Kress M, Nicol G. Therapeutic targeting of the ceramide-to-sphingosine 1-phosphate pathway in pain. Trends Pharmacol Sci. 2013;34:110–8.
You MH, Kim BM, Chen CH, Begley MJ, Cantley LC, Lee TH. Death-associated protein kinase 1 phosphorylates NDRG2 and induces neuronal cell death. Cell Death Differ. 2017;24:238–50.
Muscoli C, Doyle T, Dagostino C, Bryant L, Chen Z, Watkins LR, et al. Counterregulation of opioid analgesia by glial-derived bioactive sphingolipids. J Neurosci. 2010;30:15400–8.
Grace PM, Gaudet AD, Staikopoulos V, Maier SF, Hutchinson MR, Salvemini D, et al. Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci. 2016;39:862–79.
Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol. Sci. 2009;30:581–91.
Nakamoto K, Kawasaki S, Kobori T, et al. Involvement of matrix metalloproteinase-9 in the development of morphine tolerance. Eur J Pharmacol. 2012;683:86–92.
Hutchinson MR, Zhang Y, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun. 2010;24:83–95.
Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–9.
Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ. Toll-like receptor 4 mediates morphine-induced neuroinflammation and tolerance via soluble tumor necrosis factor signaling. Neuropsychopharmacology. 2017;42:661–70.
Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, et al. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun. 2009;23:240–50.
Johnston IN, Milligan ED, Wieseler-Frank J, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–65.
Johnson JL, Rolan PE, Johnson ME, Bobrovskaya L, Williams DB, Johnson K, et al. Codeine-induced hyperalgesia and allodynia: investigating the role of glial activation. Transl Psychiatry. 2014;4:e482.
Gruber-Schoffnegger D, Drdla-Schutting R, Honigsperger C, Wunderbaldinger G, Gassner M, Sandkuhler J. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-alpha and IL-1beta is mediated by glial cells. J Neurosci. 2013;33:6540–51.
Melik Parsadaniantz S, Rivat C, Rostene W, Reaux-Le Goazigo A. Opioid and chemokine receptor crosstalk: a promising target for pain therapy? Nat Rev Neurosci. 2015;16:69–78.
Zhang N, Rogers TJ, Caterina M, Oppenheim JJ. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. J Immunol. 2004;173:594–9.
Rivat C, Sebaihi S, Van Steenwinckel J, et al. Src family kinases involved in CXCL12-induced loss of acute morphine analgesia. Brain Behav Immun. 2014;38:38–52.
Lin CP, Kang KH, Lin TH, et al. Role of spinal CXCL1 (GROalpha) in opioid tolerance: a human-to-rodent translational study. Anesthesiology. 2015;122:666–76.
Luo X, Wang X, Xia Z, Chung SK, Cheung CW. CXCL12/ CXCR61 axis: an emerging neuromodulator in pathological pain. Rev Neurosci. 2016;27:83–92.
Takayama N, Ueda H. Morphine-induced chemotaxis and brain-derived neurotrophic factor expression in microglia. J Neurosci. 2005;25:430–5.
Horvath RJ, Romero-Sandoval EA, De Leo JA. Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and mu opioid receptor protein expression while enhancing perivascular microglial ED2. Pain. 2010;150:401–13.
Zhou D, Chen ML, Zhang YQ, Zhao ZQ. Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J Neurosci. 2010;30:8042–7.
Chen ML, Cao H, Chu YX, et al. Role of P2X7 receptor-mediated IL-18/IL-18R signaling in morphine tolerance: Multiple glial-neuronal dialogues in the rat spinal cord. J Pain. 2012;13:945–58.
Ferrini F, Trang T, Mattioli TA. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl– homeostasis. Nat Neurosci. 2013;16:183–92.
Mattioli TA, Leduc-Pessah H, Skelhorne-Gross G, et al. Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PLoS One. 2014;9:e97361.
Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–31.
Ossipov MH, Lai J, Vanderah TW, Porreca F. Induction of pain facilitation by sustained opioid exposure: relationship to opioid antinociceptive tolerance. Life Sci. 2003;73:783–800.
Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT. Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev. 2016;68:419–57.
Faris PL, Komisaruk BR, Watkins LR, Mayer DJ. Evidence for the neuropeptide cholecystokinin as an antagonist of opiate analgesia. Science. 1983;219:310–2.
Rothman RB. A review of the role of anti-opioid peptides in morphine tolerance and dependence. Synapse. 1992;12:129–38.
McNally GP. Pain facilitatory circuits in the mammalian central nervous system: their behavioral significance and role in morphine analgesic tolerance. Neurosci Biobehav Rev. 1999;23:1059–78.
Dourish CT, O’Neill MF, Schaffer LW, Siegl PK, Iversen SD. The cholecystokinin receptor antagonist devazepide enhances morphine-induced analgesia but not morphine-induced respiratory depression in the squirrel monkey. J Pharmacol Exp Ther. 1990;255:1158–65.
Hoffmann O, Wiesenfeld-Hallin Z. The CCK-B receptor antagonist Cl 988 reverses tolerance to morphine in rats. NeuroReport. 1994;5:2565–8.
Simonin F, Schmitt M, Laulin J-P, et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc Natl Acad Sci USA. 2006;103:466–71.
Elhabazi K, Trigo JM, Mollereau C, et al. Involvement of neuropeptide FF receptors in neuroadaptive responses to acute and chronic opiate treatments. Br J Pharmacol. 2012;165:424–35.
Depner UB, Reinscheid RK, Takeshima H, Brune K, Zeilhofer HU. Normal sensitivity to acute pain, but increased inflammatory hyperalgesia in mice lacking the nociception precursor polypeptide or the nociceptin receptor. Eur J Neurosci. 2003;17:2381–7.
Rizzi A, Nazzaro C, Marzola GG, et al. Endogenous nociceptin/orphanin FQ signalling produces opposite spinal antinociceptive and supraspinal pronociceptive effects in the mouse formalin test: pharmacological and genetic evidences. Pain. 2006;124:100–8.
Garcia-Recio S, Gascón P. Biological and pharmacological aspects of the NK1-receptor. Biomed Res Int. 2015;2015:495704.
Tumati S, Largent-Milnes TM, Keresztes AI, et al. Tachykinin NK(1) receptor antagonist co-administration attenuates opioid withdrawal-mediated spinal microglia and astrocyte activation. Eur J Pharmacol. 2012;684:64–70.
Liang DY, Li X, Clark JD. Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J Pain. 2013;14:36–47.
Doehring A, Geisslinger G, Lotsch J. Epigenetics in pain and analgesia: an imminent research field. Eur J Pain. 2011;15:11–6.
Doehring A, Oertel BG, Sittl R, Lotsch J. Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. Pain. 2013;154:15–23.
Viet CT, Dang D, Aouizerat BE, Miaskowski C, Ye Y, Viet DT, et al. OPRM1 methylation contributes to opioid tolerance in cancer patients. J Pain. 2017;18:1046–59.
Chao YC, Xie F, Li X, Guo R, Yang N, Zhang C, et al. Demethylation regulation of BDNF gene expression in dorsal root ganglion neurons is implicated in opioid-induced pain hypersensitivity in rats. Neurochem Int. 2016;97:91–8.
He Y, Wang ZJ. Let-7 microRNAs and opioid tolerance. Front Genet. 2012;3:110.
He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci. 2010;30:10251–8.
Wu Q, Zhang L, Law PY, Wei LN, Loh HH. Long-term morphine treatment decreases the association of mu-opioid receptor (MOR1) mRNA with polysomes through miRNA23b. Mol Pharmacol. 2009;75:744–50.
Mercadante S. Portenoy RK Opioid poorly-responsive cancer pain. Part 3. Clinical strategies to improve opioid responsiveness. J Pain Symptom Manag. 2001;21:338–54.
Edwards HL, Mulvey MR, Bennett MI. Cancer-related neuropathic pain. Cancers (Basel). 2019;11(3):373.
Lee JT, Sanderson CR, Xuan W, Agar M. Lidocaine for cancer pain in adults: a systematic review and meta-analysis. J Palliat Med. 2019;22:326–34.
Mercadante S, Fulfaro F, Casuccio A. A randomized controlled study on the use of anti-inflammatory drugs in patients with cancer pain on morphine therapy: effects on dose escalation and a pharmacoeconomic analysis. Eur J Cancer. 2002;38:1358–66.
Chabot-Doré AJ, Schuster DJ, Stone LS, Wilcox GL. Analgesic synergy between opioid and α2-adrenoceptors. Br J Pharmacol. 2015;172:388–402.
Belgrade M, Hall S. Dexmedetomidine infusion for the management of opioid-induced hyperalgesia. Pain Med. 2010;11:1819–26.
Mercadante S. The patient with difficult cancer pain. Cancers (Basel). 2019;11(4):565.
Mercadante S, Caruselli A, Casuccio A. The use of ketamine in a palliative-supportive care unit: a retrospective analysis. Ann Palliat Med. 2018;7:205–10.
Pasternak GW. Molecular biology of opioid analgesia. J Pain Symptom Manag. 2005;19:S2.
Connor M, Osborne PB, Christies MJ. Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol. 2004;143:685–96.
Hashimoto T, Saito Y, Yamada K, et al. Enhancement of morphine analgesic effect with induction of mu-opioid receptor endocytosis in rats. Anesthesiology. 2006;105:574–80.
Kieffer BL, Evans CJ. Opioid tolerance—in search of the holy grail. Cell. 2002;108:587–90.
Mercadante S, Bruera E. Opioid switching in cancer pain: from the beginning to nowadays. Crit Rev Oncol Hematol. 2016;99:241–8.
Mercadante S. Managing difficult pain conditions in the cancer patient. Curr Pain Headache Rep. 2014;18:395.
Mercadante S. Switching methadone: a 10-year experience of 345 patients in an acute palliative care unit. Pain Med. 2012;13:399–404.
Reddy A, Yennurajalingam S, Pulivarthi K, Palla SL, Wang X, Kwon JH, et al. Frequency, outcome, and predictors of success within 6 weeks of an opioid rotation among outpatients with cancer receiving strong opioids. Oncologist. 2013;18:212–20.
Mercadante S, Arcuri E. Hyperalgesia and opioid switching. Am J Hosp Palliat Care. 2005;22:291–4.
Prommer E. Rotating methadone to other opioids: a lesson in the mechanisms of opioid tolerance and opioid-induced pain. J Palliat Med. 2006;9:488–93.
Mercadante S, Caraceni A. Conversion ratios for opioid switching in the treatment of cancer pain: a systematic review. Palliat Med. 2011;25:494–503.
Dale O, Moksnes K, Kaasa S. European Palliative Care Research Collaborative pain guidelines: opioid switching to improve analgesia or reduce side effects. A systematic review. Palliat Med. 2011;25:494–503.
Bruera E, Pereira J, Watanabe S, Belzile M, Kuehn N, Hanson J. Opioid rotation in patients with cancer pain. A retrospective comparison of dose ratios between methadone, hydromorphone, and morphine. Cancer. 1996;78:852–7.
Mercadante S, Casuccio A, Calderone L. Rapid switching from morphine to methadone in cancer patients with poor response to morphine. J Clin Oncol. 1999;17:3307–12.
Mercadante S, Bianchi M, Villari P, Ferrera P, Casuccio A, Fulfaro F, et al. Opioid plasma concentration during switching from morphine to methadone: preliminary data. Support Care Cancer. 2003;11:326–31.
Kurita GP, Sjøgren P, Klepstad P, Mercadante S. Interventional techniques to management of cancer-related pain: clinical and critical aspects. Cancers (Basel). 2019;11(4):443.
Mercadante S, Klepstad P, Kurita GP, Sjøgren P, Giarratano A. Sympathetic blocks for visceral cancer pain management: a systematic review and EAPC recommendations. Crit Rev Oncol Hematol. 2015;96:577–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this article.
Conflicts of interest
Sebastiano Mercadante, Edoardo Arcuri, and Angela Santoni have no conflicts of interest to declare related to this article.
Rights and permissions
About this article
Cite this article
Mercadante, S., Arcuri, E. & Santoni, A. Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs 33, 943–955 (2019). https://doi.org/10.1007/s40263-019-00660-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00660-0