Abstract
Purpose
Ischemia/reperfusion injury (IRI) remains a clinical challenge. We tested the hypothesis that fluid therapy using hydroxyethyl starch (HES) 130/0.4 during the early phase of IRI in rat liver decreases markers of hepatic injury.
Methods
We induced liver IRI in three groups of rats anesthetized with ketamine and chlorpromazine by means of 60 min of segmental hepatic ischemia followed by 120 min of reperfusion. At the onset of reperfusion, Group 1 (IRI + HES; n = 12) was given 13 mL·kg−1 of HES; Group 2 (IRI + HS; n = 12) received the same volume of 7.5% saline (HS), and Group 3 (IRI-only; n = 12) received no fluid. Three other groups of 12 animals each were sham-operated and received the same fluid as the test groups. We euthanized the animals after three hours, drew blood for alanine aminotransferase (ALT) quantification, and took ischemic liver samples for histomorphological study.
Results
Serum ALT activity was greater in all of the IRI groups than in the sham-operated animals. The ALT activity was 1,081 ± 575 IU·L−1 in IRI + HES Group 1; 2,363 ± 1,839 IU·L−1 in IRI + HS Group 2; and 2,866 ± 2,491 IU·L−1 in IRI-only Group 3. There was a statistically significant difference between the IRI + HES and the IRI-only groups (P = 0.001), but not between the IRI + HS and the IRI-only groups (P > 0.05). Likewise, histological scores were greater in all IRI groups compared with the sham-operated animals. Scores were higher in the IRI-only group (median 3.5) than in the groups receiving fluid (IRI + HES median 2; IRI + HS median 3). The difference between IRI + HES and IRI-only was statistically significant (P = 0.008) but not so between IRI + HS and IRI-only (P > 0.05).
Conclusions
Giving HES 130/0.4 attenuates rat liver IRI compared with no fluid, while giving HS does not. This suggests a role for HES in hepatoprotection associated with liver IRI.
Résumé
Objectif
La lésion d’ischémie/reperfusion (IR) reste un défi clinique. Nous avons émis l’hypothèse qu’une thérapie liquidienne à base d’hydroxyéthylamidon (HES) 130/0,4 administrée pendant la phase précoce d’IR dans un modèle hépatique de rat, diminuerait les marqueurs de la lésion hépatique.
Méthode
Nous avons provoqué une lésion d’IR au niveau du foie dans trois groupes de rats anesthésiés à la kétamine et à la chlorpromazine en créant une ischémie hépatique segmentaire de 60 min suivie de 120 min de reperfusion. Au début de la reperfusion, le groupe 1 (IR + HES; n = 12) a reçu 13 mL·kg−1 de HES; le groupe 2 (IR + HS; n = 12) a reçu le même volume de sérum physiologique 7,5 % (HS), et le groupe 3 (IR seule; n = 12) n’a pas reçu de liquide. Trois autres groupes composés de 12 animaux chacun ont été opérés de manière fictive et ont reçu les mêmes liquides que les groupes à l’étude. Nous avons euthanasié les animaux après trois heures, extrait du sang pour procéder à la quantification de l’alanine aminotransférase (ALT) et avons pris des échantillons de foie ischémique pour réaliser une étude histomorphologique.
Résultats
L’activité sérique de l’ALT était plus élevée dans tous les groupes IR que dans les animaux opérés de manière fictive. L’activité de l’ALT était de 1 081 ± 575 IU·L−1 dans le groupe 1 (IR + HES); de 2 363 ± 1 839 IU·L−1 dans le groupe 2 (IR + HS); et de 2 866 ± 2 491 IU·L−1 dans le groupe 3 (IR seule). Il y a eu une différence statistiquement significative entre les groupes IR + HES et IR seule (P = 0,001), mais pas entre les groupes IR + HS et IR seule (P > 0,05). De la même façon, les résultats histologiques étaient plus élevés dans tous les groupes IR par rapport aux animaux ayant subi une opération fictive. Les scores étaient plus élevés dans le groupe IR seule (valeur médiane 3,5) que dans les groupes recevant des liquides (IR + HES valeur médiane 2; IR + HS valeur médiane 3). La différence entre les groupes IR + HES et IR seule était statistiquement significative (P = 0,008), ce qui n’était pas le cas pour la différence entre les groupes IR + HS et IR seule (P > 0,05).
Conclusion
Le HES 130/0,4, mais pas le sérum physiologique, atténue la lésion d’ischémie/reperfusion dans un modèle hépatique de rat sans liquide. Cela suggère que le HES pourrait jouer un rôle de protection hépatique dans les cas d’IR au niveau du foie.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Liver ischemia/reperfusion injury (IRI) occurs during restoration of hepatic blood flow after a period of disrupted circulation. Following the hypoxemic injury in the ischemic period, the subsequent reintroduction of oxygen into the liver leads to an aggressive inflammatory response divided into early and delayed phases. In the early phase, which corresponds to the first six hours of reperfusion in the rat model, the injury is mediated mainly by reactive oxygen intermediates and cytokine production, essentially for leukocyte recruitment. The involvement of activated leukocytes and microcirculatory changes predominantly characterizes the delayed phase, which extends for up to 24 hr in the rat model.1
Liver IRI is a relevant clinical problem that can occur during the perioperative period in a variety of clinical situations, such as extensive hepatectomy, liver transplantation, and shock.2 - 5 Despite improvements in surgical techniques and pharmacological strategies over the past several decades, liver IRI remains a cause of liver failure, especially if surgery is conducted in patients with severe liver disease.2 , 6
In contrast to the earlier hydroxyethyl starch (HES) solutions, which had a large molecular weight and a high degree of substitution, the new lower molecular weight HES solutions with a lower degree of substitution, such as HES 130/0.4, have shown a favourable pharmacological profile with fewer side effects.7 - 13 Best known for their use as intravascular volume expanders, HES solutions are also associated with microcirculatory improvement and anti-inflammatory properties.14 - 18
Despite their adverse effects in other settings, namely, on renal function, coagulation, and tissue storage, it was suggested previously that the older high molecular weight HES can reduce the deleterious effect of IRI.14 , 19 Distinct types of HES show clear differences in pharmacokinetic, clinical efficacy, and adverse effects.9 , 10 Therefore, the conclusions of studies performed with a specific type of HES may be valid only for that product. Any extrapolation from a certain type of hydroxyethyl starch to another with different molecular weight, molar substitution ratio, C2/C6 ratio, or even source of the starch must be made with considerable caution.19
We conducted this study to test the hypothesis that third generation HES 130/0.4 attenuates liver IRI and to explore the potential role of its molecule, in comparison with another volume expander (7.5% saline), as one of the effectors of liver protection.
Methods
Animals and experimental design
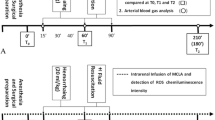
Adult male Wistar rats (Charles River Laboratories, L’Arbresle Cedex, France) weighing 307 ± 28 g were housed in a temperature-controlled room on a 12 hr light/dark cycle with access to normal rat chow and drinking water ad libitum. The Ethics Committee of the University of Coimbra, Faculty of Medicine approved the study, which meets applicable European legislation and the standards of the National Institutes of Health as set forth in the Guide for the Care and Use of Laboratory Animals (National Research Council, Washington: National Academy Press, 1996).
To explore the role of HES on hepatic IRI, we randomized the rats into six groups (n = 12, each). Three groups had IRI induced; two of these groups received either HES (IRI + HES) or 7.5% saline solution (IRI + HS), and the third group (IRI-only) was untreated and served as control. Three other groups (Sham-only, Sham + HES and Sham + HS) were sham-operated and served as normal controls. Group Sham-only had surgical manipulation only and the other two sham-operated groups received HES or 7.5% saline solution.
Staff delivered the HES or 7.5% saline solutions blindly in similar unidentified syringes. They injected the solutions intravenously at the onset of reperfusion at a volume of 13 mL·kg−1 (1.3 mL per 100 g body weight). To preserve masking, staff not involved in the study prepared the study solutions.
We euthanized the rats three hours after the beginning of the surgical procedure.
Throughout the study, investigators were unaware of the fluid administered, and staff used codes to mask the groups in alanine aminotransferase (ALT) serum determinations and histopathological samples.
Rat model of hepatic ischemia/reperfusion injury
We selected a non-lethal model of segmental (70%) hepatic warm ischemia to allow prevention of mesenteric congestion20 and performed all surgical procedures in intramuscular ketamine (115 mg·kg−1) and chlorpromazine (3.5 mg·kg−1) anesthetized animals with body temperatures maintained at 37°C. We performed a midline abdominal incision to enter the peritoneal cavity and placed a microvascular clamp (TKL-2) (Biover AG, Hergiswil, Switzerland) for 60 min across the vascular structures common to the left and median hepatic lobes in the groups that included ischemia/reperfusion. We inspected ischemic color change to confirm the clamp’s correct placement and removed the clip after the 60-min period. Immediately before reperfusion in the IRI + HES and IRI + HS groups, staff blindly administered 1 mL of the respective test solution into the left femoral vein in slow bolus and slowly infused the remaining volume (approximately 2 mL·min−1) after removing the clip. The animals in the IRI-only group did not receive intravascular solution.
The Sham-only animals underwent the same surgical procedure with the exception of vascular clamping. They did not receive a fluid injection. Animals in the Sham + HES or Sham + HS groups underwent a similar sham procedure (without IRI), and a volume of either 1.3 mL·kg−1 of HES or 7.5% saline solution, respectively, was injected into the femoral vein.
At 120 min after reperfusion, we drew blood from the aorta for ALT quantification (primary outcome) and took ischemic liver tissue samples for subsequent histomorphological study (secondary outcome). We also noted perioperative mortality (secondary outcome). The surviving animals were euthanized by exsanguination.
Serum alanine aminotransferase levels
Serum ALT levels were evaluated based on the following protocol: blood samples were centrifuged to separate the plasma, and the plasma samples were stored at -20°C until use for ALT assay. The ALT levels, expressed in international units per litre (IU·L−1), were determined using a standard dry chemistry system (Johnson & Johnson Vitros 250) (Ortho-Clinical Diagnostic, Inc., Rochester, NY, USA).
Histological analysis of hepatocellular damage
Liver specimens were fixed in 10% buffered formalin and embedded in paraffin. Sections were cut at a thickness of 5 μm and stained with hematoxylin and eosin, and the stained sections were used to assess liver damage. Two independent blind observers graded the histological injury using a scoring system from 0 (none) to 4 (severe) previously described,21 , 22 with modifications. Histopathological changes observed in representative hematoxylin and eosin stained liver sections were given histological scores based on the extent of hepatocellular injury as follows: 0: normal liver architecture; 1: minimal injury (swelling, congestion, single cell necrosis); 2: mild injury, with one or more minute foci of necrosis, the largest involving < 1% of the examined sectional area of the lobule; 3: moderate injury, as in 2, but the necrotic foci occupying 1-5% of the lobule; and 4: severe injury, as above, but with necrotic foci covering > 5% of the lobule.
Necrosis was defined when one or more of the following characteristics were seen: nuclear pyknosis, cytoplasmic hypereosinophilia, loss of distinct cellular borders, hemorrhage, and sinusoidal congestion. The percent area of necrosis was estimated using digital photomicrographs that were taken from five randomly selected fields of each tissue section (Nikon Coolscope, Digital Microscope, Emílio de Azevedo Campos, S.A. Porto, Portugal), and the average percent area of necrosis resulted from observing at least ten slides per group.
Statistical analysis
We determined the required sample size based on the mean value of 2,218.8 ± 825.3 IU·L−1 for the IRI-only group from a previous in vivo study.23 Twelve rats per group were required to detect a 45% reduction in ALT value, with a type I error of 0.05 and a power of 80%.
Statistical analysis was performed using SPSS statistical software package version 11.5 (SPSS, Inc., Chicago, IL, USA), and normality of distribution was determined using Kolmogorov-Smirnov and Shapiro-Wilk tests. Changes in ALT values (normally distributed data) were statistically analyzed by one-way analysis of variance (ANOVA) followed by least significant difference post hoc test for multiple comparisons. Histological scores were statistically analyzed by non-parametric tests (Kruskal-Wallis test), and, where differences were identified, pairwise comparisons were performed using the Mann Whitney test with appropriate correction with the Holm-Bonferroni method. Chi square test with Fisher’s exact test was used for categorical data (mortality). All differences were considered significant at P < 0.05. Values are expressed as means ± standard deviation (SD) and as median and interquartile range (IQ), as appropriate, and n is the number of rats in each group.
Results
Mortality
All of the animals reached the defined end-point, with the exception of two in the IRI + HS group that died during intravenous administration of 7.5% saline solution (P > 0.05). These two were excluded from the analysis of markers of liver injury due to the absence of results.
Effect of ischemia/reperfusion injury
The IRI-only group showed a greater ALT activity than the Sham-only group. Liver histology in the IRI-only group, but not in the Sham-only group, showed the early typical changes characteristic of the non-lethal warm ischemia model, such as sinusoidal congestion and areas of necrosis.
Effect of fluid therapy with HES or HS in sham animals
Intravenous HES 130/0.4 or 7.5% saline solution to the liver of the sham-operated rats did not cause significant detrimental effects on hepatic cell architecture (histological severity grades ranging from 0 [none] to 1 [minimal injury]). Similarly, fluid treatment in these groups did not alter ALT levels when compared with sham-operated animals that received no intravenous fluids (Sham + HES: 214 ± 157 IU·L−1 and Sham + HS: 218 ± 230 IU·L−1 vs Sham-only: 149 ± 142 IU·L−1; both P > 0.05).
Effect of fluids in ischemia/reperfusion injury
The ALT activity was less in the hydroxyethyl starch (IRI + HES) group 1,081 ± 575 IU·L−1 than in the no fluid (IRI-only) group (2,866 ± 2,491 IU·L−1) (P = 0.001). There was no statistically significant difference between the group receiving HS (2,363 ± 1,839 IU.L−1) and the group given no fluid (P > 0.05) (Figure 1).
Alanine aminotransferase (ALT) levels in animals that were subjected to one hour of 70% warm ischemia and two hours of reperfusion and in sham-operated controls. After one hour of ischemia and two hours of reperfusion, the maximal reduction of liver ischemia/reperfusion injury (IRI) was observed in the hydroxyethyl starch (HES)-treated rats (IRI + HES) vs the hypertonic saline (HS)-treated rats (IRI + HS) and the no fluid IRI control rats (IRI-only); *P = 0.022 and †P = 0.001, respectively. Sham-operated control groups all showed similar ALT values
Similarly, histological injury scores were greater in the IRI-only group (median 3.5, IQ 1.75) than in groups receiving HES (IRI + HES: median 2, IQ 1.5) (P = 0.008) (Figure 2). Severe injury was seen in 60% and 10% of the animals, respectively (Figure 3). There was no statistically significant difference in histology scores between saline-treated animals (IRI + HS median 3, IQ 1.25) and IRI-only rats (P > 0.05).
Histomorphological results. Photomicrographs of liver sections after three hours in sham-operated rats and in rats subjected to one hour of 70% warm ischemia and two hours of reperfusion (IRI). Hydroxyethyl starch (HES)-treated rats (IRI + HES) (picture histological score = 1) demonstrate a notable improvement in liver structure resembling more the normal histological appearance observed in the Sham-only group (picture score = 0). The hypertonic saline (IRI + HS) group (picture score = 2) shows a more generalized sinusoidal congestion than IRI + HES and signs of focal derangement of hepatocellular organization (white arrows). Black arrows in the IRI-only group (picture score = 4) show extensive signs of apoptosis and necrosis and derangement of hepatocellular organization. Images are representative liver sections of at least ten rats per group. Hematoxylin and eosin stain; magnification × 10. The horizontal bar in the pictures represents 100 μm
Liver injury histological scores graded from 0 (none) to 4 (severe). The majority (75%) of hydroxyethyl starch (HES)-treated rats (IRI + HES) showed minimal to mild injury, in contrast to the hypertonic saline (HS)-treated (IRI + HS) and the untreated IRI (IRI-only) rats, where 50% of the cases had moderate (grade 3) and severe injuries (grade 4), respectively
Discussion
Using an established model of hepatic IRI,20 this study shows that intravascular administration of HES 130/0.4 before induced reperfusion lesion reduces the markers of severity of the early phase of liver ischemia/reperfusion injury, suggesting a protective effect. If supported by further clinical studies, this investigation provides a base for the rational use of this product for improving outcome in surgeries involving risk of liver ischemia/reperfusion.
The third generation hydroxyethyl starches, which include HES 130/0.4, may have advantages over other replacement fluids. Several clinical studies investigated the effects of HES 130/0.4 for perioperative volume replacement. There is evidence of a better safety profile with HES 130/04 compared with the older colloids, and it is often preferred over pure crystalloid regimens because of its prolonged intravascular half-life and positive impact on blood rheology and tissue oxygenation.24 - 29 Moreover, Lang et al. 18 showed a significant attenuation of inflammatory markers, such as interleukins 6 and 8 and soluble intercellular adhesion molecule-1 (sICAM-1), when this solution was used in patients submitted to major abdominal surgery instead of a crystalloid-based volume therapy.
Recent studies in stroke and kidney rat models30 , 31 demonstrated that an HES 130/0.4 solution could attenuate the severity of IRI, although the efficacy in the liver IRI was not determined. We explored this subject further, showing that HES 130/0.4 protects liver against IRI and providing new insights into the mechanisms of its action.
Previous studies demonstrated the benefits of HES solutions, supporting the idea that there are several potential mechanisms through which HES may contribute to organ preservation in the context of IRI.14 - 18 , 24 - 29 We shared the commonly held perception that both volume effect and molecule characteristics are among the most important factors in organ protection; however, the current study demonstrated that the HES molecule plays a role in hepatoprotection against reperfusion injury. The infused volume of HES solution (13 mL·kg−1) adjusted to the weight of the rats was within the doses reported for volume repletion in clinical studies.25 , 26 , 32 , 33 An equivalent volume of 7.5% saline solution was used as an appropriate control of volume effect. First, although we are far from finding an ideal volume control for HES, theoretically, the osmotic and volume-expanding properties of the 7.5% saline solution make it the best alternative.34 Furthermore, it has been studied widely in the same experimental contexts as starches24 and has been in clinical use for many decades.35 , 36
Normal saline, one of the most commonly used solutions, was not chosen as a control because its volume effect is not comparable with that of colloids, given that it is easily lost from the intravascular space and, therefore, does not act effectively as a volume expander. The distribution among the three compartments (intravascular, extracellular, and intracellular) depends highly on the concentration of sodium. Thus, in order for normal saline to act as a valid volume control in this study, three to five times the amount of normal saline than that of the HES would be necessary. Under the conditions of our model, i.e., using rats, this approach was not possible without substantial mortality. We overcame this limitation by using 7.5% saline solution as volume control.
In this study, we analyzed separately the protective roles of two interrelated concepts in fluid therapy against liver IRI, namely, volume and molecule effects. Our data support the HES molecule as a major candidate for exerting this protective effect. Indeed, two types of fluid delivered in equivalent amounts showed different results. While the rats that received saline solution demonstrated no statistically significant improvement in the parameters used for analysis of liver injury, the HES-treated group demonstrated significant improvement of these parameters. These data strongly suggest that the observed improvement occurred beyond the volume-effect. Consequently, we acknowledge the possibility for the HES molecule to play a contributing role in liver IRI attenuation.
The present study, which is supported in a vast sample with appropriate control groups, provides further insight into the role that the novel HES generation plays in liver IRI through its molecule effect. Given its profound impact on the markers of early phase IRI, this novel HES molecule presents as an attractive preventive solution. However, its clinical impact warrants further studies. The effect of HES on clinical IRI is not easy to access given the large variability of hepatic surgery from centre to centre and the limited availability of homogeneous patients.
The only two deaths observed in this study occurred in the IRI + HS group during 7.5% sodium chloride administration. Since the volume per weight was similar in IRI + HES and IRI + HS groups, this suggests that mortality was not attributable to fluid overload but probably to the potential ionic disturbances occasionally observed with hypertonic fluids.
In summary, within the limitations of an animal study, we report here that HES 130/0.4 attenuates pathologic and biologic markers of liver IRI, likely through its molecule. Whether this finding will have clinical implication deserves further studies.
References
Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J 1990; 4: 3355-9.
Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury - a fresh look. Exp Mol Pathol 2003; 74: 86-93.
Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg 1994; 178: 454-8.
Hilmi I, Horton CN, Planinsic RM, et al. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl 2008; 14: 504-8.
Dart RC, Liebler DC, Sipes IG. Hepatic injury and lipid peroxidation during hemorrhagic shock and resuscitation. Life Sci 1993; 53: 1685-9.
Ke B, Lipshutz GS, Kupiec-Weglinski JW. Gene therapy in liver ischemia and reperfusion injury. Curr Pharm Des 2006; 12: 2969-75.
Gallandat Huet RC, Siemons AW, Baus D, et al. A novel hydroxyethyl starch (Voluven) for effective perioperative plasma volume substitution in cardiac surgery. Can J Anesth 2000; 47: 1207-15.
Langeron O, Doelberg M, Ang ET, Bonnet F, Capdevila X, Coriat P. Voluven, a lower substituted novel hydroxyethyl starch (HES 130/0.4), causes fewer effects on coagulation in major orthopedic surgery than HES 200/0.5. Anesth Analg 2001; 92: 855-62.
Jungheinrich C, Scharpf R, Wargenau M, Bepperling F, Baron JF. The pharmacokinetics and tolerability of an intravenous infusion of the new hydroxyethyl starch 130/0.4 (6%, 500 mL) in mild-to-severe renal impairment. Anesth Analg 2002; 95: 544-51.
Jungheinrich C, Neff TA. Pharmacokinetics of hydroxyethyl starch. Clin Pharmacokinet 2005; 44: 681-99.
Kozek-Langenecker SA. Effects of hydroxyethyl starch solutions on hemostasis. Anesthesiology 2005; 103: 654-60.
Gandhi SD, Weiskopf RB, Jungheinrich C, et al. Volume replacement therapy during major orthopedic surgery using Voluven (hydroxyethyl starch 130/0.4) or hetastarch. Anesthesiology 2007; 106: 1120-7.
Godet G, Lehot JJ, Janvier G, Steib A, De Castro V, Coriat P. Safety of HES 130/0.4 (Voluven(R)) in patients with preoperative renal dysfunction undergoing abdominal aortic surgery: a prospective, randomized, controlled, parallel-group multicentre trial. Eur J Anaesthesiol 2008; 25: 986-94.
Zikria BA, Subbarao C, Oz MC, et al. Macromolecules reduce abnormal microvascular permeability in rat limb ischemia/reperfusion injury. Crit Care Med 1989; 17: 1306-9.
Handrigan MT, Burns AR, Donnachie EM, Bowden RA. Hydroxyethyl starch inhibits neutrophil adhesion and transendothelial migration. Shock 2005; 24: 434-9.
Rittoo D, Gosling P, Simms MH, Smith SR, Vohra RK. The effects of hydroxyethyl starch compared with gelofusine on activated endothelium and the systemic inflammatory response following aortic aneurysm repair. Eur J Vasc Endovasc Surg 2005; 30: 520-4.
Dieterich HJ, Weissmuller T, Rosenberger P, Eltzschig HK. Effect of hydroxyethyl starch on vascular leak syndrome and neutrophil accumulation during hypoxia. Crit Care Med 2006; 34: 1775-82.
Lang K, Suttner S, Boldt J, Kumle B, Nagel D. Volume replacement with HES 130/0.4 may reduce the inflammatory response in patients undergoing major abdominal surgery. Can J Anesth 2003; 50: 1009-16.
Westphal M, James MF, Kozek-Langenecker S, Stocker R, Guidet B, Van Aken H. Hydroxyethyl starches: different products–different effects. Anesthesiology 2009; 111: 187-202.
Spiegel HU, Bahde R. Experimental models of temporary normothermic liver ischemia. J Invest Surg 2006; 19: 113-23.
Kaplan N, Yagmurdur H, Kilinc K, Baltaci B, Tezel S. The protective effects of intravenous anesthetics and verapamil in gut ischemia/reperfusion-induced liver injury. Anesth Analg 2007; 105: 1371-8.
Omar R, Nomikos I, Piccorelli G, Savino J, Agarwal N. Prevention of postischaemic lipid peroxidation and liver cell injury by iron chelation. Gut 1989; 30(4): 510–4.
Cheng XD, Jiang XC, Liu YB, Peng CH, Xu B, Peng SY. Effect of ischemic preconditioning on P-selectin expression in hepatocytes of rats with cirrhotic ischemia-reperfusion injury. World J Gastroenterol 2003; 9: 2289-92.
Nascimento P Jr, de Paiva Filho O, de Carvalho LR, Braz JR. Early hemodynamic and renal effects of hemorrhagic shock resuscitation with lactated Ringer’s solution, hydroxyethyl starch, and hypertonic saline with or without 6% dextran-70. J Surg Res 2006; 136: 98-105.
Standl T, Burmeister MA, Schroeder F, et al. Hydroxyethylstarch (HES) 130/0.4 provides larger and faster increases in tissue oxygen tension in comparison with prehemodilution values than HES 70/0.5 or HES 200/0.5 in volunteers undergoing acute normovolemic hemodilution. Anesth Analg 2003; 96: 936-43.
Paul M, Dueck M, Joachim Herrmann H, Holzki J. A randomized controlled study of fluid management in infants and toddlers during surgery: hydroxyethyl starch 6% (HES 70/0.5) vs lactated Ringer’s solution. Paediatr Anaesth 2003; 13: 603-8.
Woessner R, Grauer MT, Dieterich HJ, et al. Influence of a long-term, high-dose volume therapy with 6% hydroxyethyl starch 130/0.4 or crystalloid solution on hemodynamics, rheology and hemostasis in patients with acute ischemic stroke. Results of a randomized, placebo-controlled, double-blind study. Pathophysiol Haemost Thromb 2003; 33: 121-6.
Ando Y, Terao Y, Fukusaki M, et al. Influence of low-molecular-weight hydroxyethyl starch on microvascular permeability in patients undergoing abdominal surgery: comparison with crystalloid. J Anesth 2008; 22: 391-6.
Neff TA, Fischler L, Mark M, Stocker R, Reinhart WH. The influence of two different hydroxyethyl starch solutions (6% HES 130/0.4 and 200/0.5) on blood viscosity. Anesth Analg 2005; 100: 1773-80.
Xiong L, Lei C, Wang Q, Li W. Acute normovolaemic haemodilution with a novel hydroxyethyl starch (130/0.4) reduces focal cerebral ischaemic injury in rats. Eur J Anaesthesiol 2008; 25: 581-8.
Zhou DC, Ma XX, Xie JR, Luo F, Jin M. Protective effects of hydroxyethyl starch (130/0.4) against ischemia reperfusion injury of kidney: experiment with rats (Chinese). Zhonghua Yi Xue Za Zhi 2007; 87: 3224-7.
Mukhtar A, Aboulfetouh F, Obayah G, et al. The safety of modern hydroxyethyl starch in living donor liver transplantation: a comparison with human albumin. Anesth Analg 2009; 109: 924-30.
Hanart C, Khalife M, De Ville A, Otte F, De Hert S. Van der Linden P. Perioperative volume replacement in children undergoing cardiac surgery: albumin versus hydroxyethyl starch 130/0.4. Crit Care Med 2009; 37: 696-701.
Kramer GC. Hypertonic resuscitation: physiologic mechanisms and recommendations for trauma care. J Trauma 2003; 54(5 Suppl): S89-99.
Meyer PG, Orliaguet GA, Zerah M, et al. Emergency management of deeply comatose children with acute rupture of cerebral arteriovenous malformations. Can J Anesth 2000; 47: 758-66.
Strandvik GF. Hypertonic saline in critical care: a review of the literature and guidelines for use in hypotensive states and raised intracranial pressure. Anaesthesia 2009; 64: 990-1003.
Acknowledgement
The authors thank Dr. Ana Brett for her assistance with the English language.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
This investigation was supported, in part, by research funds of the School of Medicine of the University of Coimbra (GAPI 08/08).
Rights and permissions
About this article
Cite this article
Catré, D., Viana, J.S., Cabrita, A.M. et al. Hydroxyethyl starch 130/0.4 attenuates early hepatic damage in ischemia/reperfusion injury. Can J Anesth/J Can Anesth 57, 439–445 (2010). https://doi.org/10.1007/s12630-010-9282-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-010-9282-8