Abstract
Objectives
We aimed to evaluate the role of SARC-F and SARC-CalF scores as risk factors for mortality in adults over 60 years of age with cancer of the Centro Médico Naval (CEMENA) in Callao, Peru during 2012–2015.
Methods
We performed a secondary analysis of data from a prospective cohort carried out from September 2012 to February 2013 in the Geriatrics Department of CEMENA. The outcome variable was mortality at two years of follow-up, while the exposure variable was the risk of sarcopenia assessed using the SARC-F and SARC-CalF scales. We carried out Cox proportional-hazards models to assess the role of SARC-F and SARC-CalF scores as risk factors for mortality. We estimated crude (cHR) and adjusted (aHR) hazard ratios (HR) with their respective 95% confidence intervals (95%CI). Likewise, we calculated the area under the curve (AUC) of both exposure variables in relation to mortality.
Results
We analyzed data from 922 elderly men with cancer; 43.1% (n=397) were between 60 and 70 years old. 21.5% (n=198) and 45.7% (n=421) were at risk of sarcopenia according to SARC-F and SARC-CalF, respectively, while the incidence of mortality was 22.9% (n=211). In the adjusted Cox regression model, we found that the risk of sarcopenia measured by SARC-F (aHR=2.51; 95%CI: 1.40–2.77) and SARC-CalF (aHR=2.04; 95%CI: 1.55–4.02) was associated with a higher risk of death in older men with cancer. In the diagnostic performance analysis, we found that the AUC for mortality prediction was 0.71 (95%CI: 0.68–0.75) for SARC-F and 0.80 (95%CI: 0.78–0.82) for SARC-CalF.
Conclusions
The risk of sarcopenia evaluated by SARC-F and SARC-CalF scores was associated with an increased risk of mortality in older men with cancer. Both scales proved to be useful and accessible instruments for the identification of groups at risk of mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a major public health problem globally, with an estimated 19.3 million new cases and 10 million deaths in 2020 (1). The highest incidence worldwide occurs in the elderly and represents the fourth most frequent cause of death in older adults (2). It is estimated that by 2035, the incidence of cancer in older adults will double (3) and by 2050 the number of older adults with cancer will triple, reaching 446 million (4).
Cancer can cause a decrease in food intake, energy expenditure at rest and alterations in the metabolism of nutrients, being one of the main inflammatory diseases related to age (5, 6). On the other hand, inflammation affects cell metabolism, muscle strength and energy regulation, predisposing the development of sarcopenia (6). In this way, cancer is frequently associated with rapid weight loss as well as a significant deterioration of muscle mass that may be mediated by sarcopenia or by the development of cachexia (7, 8). In this sense, cancer-induced sarcopenia is associated with an increase in the adverse effects of treatment, unfavorable outcomes and lower survival (5). Sarcopenia represents a problem related to various long-term diseases and older adults with cancer have a higher risk of presenting this disease (9).
The diagnosis of sarcopenia is made by measuring grip strength, physical performance, and muscle mass. Ideally, the latter is determined by dual energy X-ray absorptiometry (DXA); however, there are several techniques that have different cut-off points, producing variability of results and, consequently, difficulty in diagnosing sarcopenia (9) and the need for different tools for adequate diagnosis.
SARC-F is an instrument that assesses the risk of sarcopenia and can be used both in clinical settings and in community health care. It has a moderate to low sensitivity and a very high specificity (10), for which an improved variant was proposed.
SARC-CalF is an instrument to which the measurement of calf circumference was added, increasing the sensitivity from 29.5–33.3% to 60.7–66.7% (11, 12). Previous studies have been conducted using the SARC-F and/or SARC-CalF scales for screening of the risk of sarcopenia in older adults with cancer in the United States (13) and Brazil (14, 15). However, these studies had limitations due to the short follow-up time and study population (13–15), in addition to having a cross-sectional design that did not allow causal association to be evaluated in one of these studies (15).
Cancer increases the risk of sarcopenia, and both diseases are associated with important complications such as morbidity, disability and mortality (16, 17). It is necessary to evaluate the risk of sarcopenia as a risk factor for mortality, especially in this vulnerable group. However, there are no previous studies that evaluate the association of interest using both scales or their diagnostic performance. In addition, it is relevant to have accessible and low-cost tools that allow screening for sarcopenia in primary care in rural areas (18, 19), considering the lack of health personnel and infrastructure (20). For this reason, this study aims to evaluate the role of SARC-F and SARC-CalF scores as risk factors for mortality in older adults with cancer in Peru.
Methods
Study design, population and sample
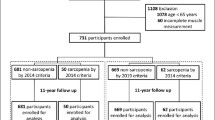
We performed a secondary analysis of data from a prospective cohort including older adults (60 years and older) of retired military men diagnosed with cancer and receiving palliative treatment. The participants were evaluated and enrolled from September 2012 to February 2013 and were followed until 2015, in the Geriatrics Service of the Naval Medical Center of Peru (CEMENA), located in Callao, Peru. We have developed previous articles (21–23) with the present database evaluating geriatric syndromes and their risk of adverse outcomes. The initial evaluation included 1,178 eligible older adults among which 121 were subsequently excluded due to a Mini Mental State Examination (MMSE) score less than or equal to 23, 81 for having a diagnosis of dementia, nine due to a diagnosis without curative intent, four because they discontinued their treatment, four due to incomplete medical records, six due to loss to follow-up, and 31 did not agree to participate in the study (Figure 1). For this secondary analysis we excluded participants with incomplete data on our variables of interest. The calculation of statistical power was based on a previous study by Yang et al. (24), considering a hazard ratio (HR) of 2.08, a proportion of deaths with risk of sarcopenia of 29% and a proportion of deaths without the risk of sarcopenia of 17.6%. Thus, we obtained a statistical power of 98% for a sample of 922 older adults included.
Procedures
In the original study, older adults were invited to participate in the study during outpatient evaluation after confirmation of the diagnosis of cancer. After voluntarily agreeing to participate in the study and signing the informed consent, the evaluating physician collected the sociodemographic variables, medical history, and functional and performance-based measures, including the SARC-F and SARC-CalF scores.
Variables
Outcome variable: mortality
The outcome variable was all-cause mortality, which we defined by the patient’s vital status at the end of follow-up. We obtained information on mortality from the CEMENA Epidemiological Surveillance Office registry.
Exposure variables: SARC-F and SARC-CalF scores
SARC-F is a questionnaire that is a quick and simple diagnostic tool to assess the risk of sarcopenia. It includes five components: aid for walking, falls, getting up from a chair, climbing stairs, and strength. The scores for each item range from zero to two, obtaining a total score between zero to 10 points. Thus, a score greater than or equal to four was defined as risk of sarcopenia (25).
SARC-CalF is a modified SARC-F, which adds calf circumference measurement, improving the diagnostic performance of the instrument for the evaluation of the risk of sarcopenia. This scale defines calf circumference in men greater than 34 cm with a score of 0 points and less than or equal to 34 with a score of 10. The total score with the previously mentioned components plus the calf circumference can be of up to 20, with a score of 11 to 20 being defined as an indicator of risk of sarcopenia (26, 27).
Other variables
Sociodemographic characteristics
We included age (60–70, ≥71 years) and marital status (single, married/cohabitant, divorced/separated, widowed). These variables were collected by self-reporting.
Medical and personal history
We evaluated the following comorbidities: hypertension, type 2 diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, osteoporosis, dyslipidemia, urinary incontinence (assessed using the Edmonton Frailty Scale (28)), sedentary lifestyle (defined by a score less than or equal to 64 on the Physical Activity Scale for the Elderly (PASE) (29, 30) and overweight or obesity (defined by a body mass index greater than or equal to 25 kg/m2 and 30 kg/m2, respectively). We collected data from the patient’s clinical history and generated a variable that included the previously described comorbidities (0, 1, ≥2). Likewise, polypharmacy, defined as the consumption of five or more prescribed medications (31), use of health services, defined as at least one admission to the CEMENA hospitalization or emergency service and smoking habit (yes, no) were collected by self-reporting.
Functional evaluation
We used the Barthel index to assess dependence of basic activities of daily living (ABVD), considering a score less than 100 as positive (32). Likewise, we evaluated exhaustion with three questions: a) Do you feel full of energy? (yes, no); b) Do you feel that everything you do requires effort? (yes, no); c) Do you feel that you can no longer move forward? (yes, no). These questions evaluated how the older adult felt in the last two weeks and a score greater than or equal to two was considered as positive (33). Dynapenia was defined as grip strength less than 26 kg (10). We evaluated self-reported weight loss using the Edmonton Frailty Scale (yes, no) (28).
Statistical analysis
We performed the statistical analysis using the statistical package Stata v16.0 (StataCorp, Tx). The descriptive analysis was carried out using absolute and relative frequencies for the qualitative variables. Bivariate analysis between covariates of interest and all-cause mortality was performed using the Pearson’s Chi-square test. We performed Cox proportional hazards modeling to assess the role of the SARC-F and SARC-CalF scores as risk factors for sarcopenia and mortality. We developed a crude model for each exposure variable and three models adjusted for potential confounders as described in the literature (11, 24). In addition, we elaborated a supplementary Cox regression model including both SARC-F and SARC-CalF. We estimated crude HR (cHR) and adjusted HR (aHR), with their respective 95% confidence intervals (95%CI).
Similarly, a receiving operating characteristic curve analysis was performed to evaluate the diagnostic performance of SARC-F and SARC-CalF as predictors of mortality in in the study sample of older males with cancer. We calculated the sensitivity, specificity, positive predictive value, negative predictive value, percentage of correctly classified, Youden index and area under the curve (AUC) of both exposure variables. We performed the diagnostic performance analysis considering cut-off points for SARC-F (greater than or equal to four) (25) and SARC-CalF (greater than or equal to 11) (26, 27) described in previous studies to define risk of sarcopenia.
Ethical aspects
The primary study was approved by the Institutional Research Ethics Committee of CEMENA (Memorandum No. CEI-CMN-134-2009). The older male adults provided signed informed consent prior to enrollment in the primary study. For this secondary analysis, we did not carry out additional evaluations and the integrity or confidentiality of the participants was not compromised.
Results
General characteristics of the sample and bivariate analysis according to mortality
We included 922 older men with cancer in the analysis, with a mean follow-up of 589 days. Of these, 43.1% (n=397) were between 60 and 70 years old, 55.6% (n=513) were married or cohabiting, 59.2% (n=546) had two or more comorbidities, and 20% (n= 184) had a smoking habit. In addition, 27% (n=249) had functional dependence for ABVD, 55.4% (n=511) reported polypharmacy, and 46.8% (n=431) reported weight loss, while 41.1% (n=379) and 48.7% (n=449) presented exhaustion and dynapenia, respectively.
In addition, according to the SARC-F and SARC-CalF scores, 21.5% (n=198) and 45.7% (n=421) were at risk of sarcopenia, respectively, while the incidence of mortality was 22.9% (n=211). We found that 62.1% (n=123) of older adults considered at risk of sarcopenia by the SARC-F score died during follow-up, while only 12.2% (n=88) of those without risk of sarcopenia died. On the other hand, 47% (n=198) of the group at risk of sarcopenia, defined by SARC-CalF, died during follow-up, while only 2.6% (n=13) of those without risk of sarcopenia died. There were statistically significant differences between the study covariates and mortality, except for age groups (Table 1).
Frequency of cancer types according to risk of sarcopenia and mortality
We found that the most frequent types of cancer in the group at risk of sarcopenia according to SARC-F were lungs and airways (40.9%), liver and bile ducts (21.7%), lymphomas and leukemias (12.1%), multiple myeloma (11.1%) and colorectal (8.6%). In addition, in the group at risk of sarcopenia by SARC-CalF we found lungs and airways (25.6%), liver and bile ducts (23.0%), multiple myeloma (15.7%), lymphomas and leukemia (15.4%) and colorectal (10.4%) were the most frequent types of cancer. Likewise, the most frequent types of cancer in the group that died were lungs and airways (26.1%), liver and bile ducts (23.2%), lymphomas and leukemias (16.6%), multiple myeloma (16.1%) and colorectal (9.0%) (Table 2).
SARC-F and SARC-CalF scores as risk factors for mortality
In the crude Cox regression model, the risk of sarcopenia measured by SARC-F (cHR=4.31; 95%CI: 2.12–5.23) and SARC-CalF (cHR=2.98; 95%CI: 1.14–6.91) increased the risk of death in older men with cancer. Likewise, this association was maintained in the adjusted models (models 1, 2 and 3). In model 3, the association persisted between the risk of sarcopenia measured by SARC-F (aHR=2.51; 95%CI: 1.40–2.77) and SARC-CalF (aHR=2.04; 95%CI: 1.55–4.02) and a greater risk of mortality, respectively (Table 3). In addition, in the supplementary Cox regression model, we found that after including SARC-CalF as a confounder, SARC-F remained associated with an increased risk of mortality (aHR=2.11; 95%CI: 1.34–2.44). Likewise, after including SARC-F as a confounder, SARC-CalF remained associated but with a greater magnitude (aHR=3.98; 95%CI: 2.28–6.56) (Table S1).
Diagnostic performance analysis of SARC-F and SARC-CalF scores as predictors of mortality
We performed a diagnostic performance analysis for both tools, finding a sensitivity of 58% (95%CI: 0.51–0.65) and a specificity of 89% (95%CI: 0.86–0.92) for the prediction of mortality for SARC-F. On the other hand, SARC-CalF had a sensitivity of 94% (95%CI: 0.90–0.97) and a specificity of 69% (95%CI: 0.65–0.72). In addition, the percentage of correct classification was 82% (95%CI: 0.80–0.85) and 74% (95%CI: 0.71–0.77) for SARC-F and SARC-CalF, respectively. Finally, the AUC calculated for the prediction of mortality was 0.71 (95%CI: 0.68–0.75) for SARC-F and 0.80 (95%CI: 0.78–0.82) for SARC-CalF (Table 4).
Discussion
Main results
We found that approximately two out of 10 participants were at risk of sarcopenia defined by SARC-F and five out of 10 by SARC-CalF. In addition, two out of 10 died during follow-up. We found that SARC-F and SARC-CalF scores were associated with an increased risk of mortality, regardless of sociodemographic characteristics, history, and functional assessment. The latter tool presented the best diagnostic performance. Both scales are useful and rapid instruments to identify groups at higher risk of death.
Comparison with previous studies
We found only one study in older Chinese adults that evaluated the same association of interest, but this did not include cancer patients (24). This comparison of instruments was also evaluated in another Chinese study, but the incidence of mortality was not evaluated (11). Studies in the United States (13) and Brazil (14) evaluated the role of the SARC-F score as a risk factor for mortality, while previous studies in India (34), and Brazil (35) evaluated SARC-F in adults from 30 years and older adults with cancer. Likewise, the role of the SARC-CalF score as a risk factor for mortality was evaluated in a study in Brazil (36); however, this study included older adults without cancer. On the other hand, another study evaluated the role of the SARC-CalF score as a risk factor for mortality in adults with cancer in Brazil, but included participants from the age of 20, with only 52.9% being older adults (26).
We evaluated the risk of sarcopenia only in older men, while previous studies included both sexes, thus, reporting a higher frequency of risk of sarcopenia in men compared to women (11, 13). This is important as a previous study described that adults with cancer and at risk of sarcopenia were more likely to die after major surgery, especially gastrointestinal operations (14). In addition, the risk of sarcopenia in older men may increase in the short term after receiving chemotherapy, radiotherapy or chemo-radiotherapy with curative intent (34), as well as predict lower survival (13).
On the other hand, SARC-CalF demonstrated a higher sensitivity and better diagnostic performance but a lower specificity than SARC-F for screening for the risk of sarcopenia. This finding is similar to that reported in a study conducted in China, which however, only included adults from 18 years of age with advanced cancer (11).
Interpretation of results
Among the most common changes that accompany aging, the decrease in muscle strength and mass can lead to adverse health outcomes and decreased survival (8, 37). Muscle tissue changes as the person ages, producing cell reduction, decreased volume of the sarcoplasmic reticulum and calcium pumping, disorganization of the sarcomere spaces, less excitability in the muscle of the plasma membrane, greater storage of fat around and inside muscle cells, reduced time and force of muscle contraction, fewer motor neurons, and a reduced ability to regenerate nerve tissue (8). In addition, the gradual decline in testosterone concentrations leads to decreased muscle mass and muscle protein synthesis (38).
Sarcopenia is the pathological decline in the function and structure of muscle mass, being affected by aging, due to changes in muscle structures such as a decrease in the number and size of type 1 and 2 fibers, together with a loss of motor units. This leads to disorganization of the Z lines and myofilaments, in addition to an accumulation of lipofuscin inside these fibers (8, 39, 40). Sarcopenia is commonly accompanied by disability, functional impairment, decreased quality of life, and an increased risk of death (41).
One of the most common causes of sarcopenia is neoplastic diseases (8). The high prevalence of sarcopenia in older adults with cancer, in addition to the age-related risk, represents an even greater threat to this population, making them especially vulnerable. Therefore, after receiving treatments such as systemic chemotherapy, radiotherapy and surgery, they can develop lower tolerance to treatment, a higher risk of toxicity to chemotherapy treatments, a greater number of postoperative complications, a lower survival and, finally, higher mortality rates independently of age, sex, type and stage of cancer (41, 42).
Clinical relevance of the findings
According to estimates by the World Health Organization (WHO) in Latin America and the Caribbean (LAC) there were more than 900 thousand new cases of cancer in older adults during 2020, being the fourth continent with the highest incidence of cancer in the older age group, behind Oceania, North America and Europe. Furthermore, more than 500,000 cancer deaths were recorded in 2020 in older Latin American adults alone (43).
It should be noted that while low- and middle-income countries only represent 51.8% of all older adults with cancer worldwide, they account for 65% of all deaths (43). In addition, cancer is the second leading cause of mortality in the Americas with 1.4 million deaths in 2018 and with an expected increase to 2.1 million by 2030 (44, 45). This is due to demographic aging (46) and the high prevalence of cancer related to infectious diseases (47). Consequently, oncological pathology is an important and growing health challenge that requires preventive interventions, especially in the elderly.
Although efforts have been made to address this problem through universal insurance, there is still a significant gap in access to health services due to long waiting times, out-of-pocket costs, geographical and cultural barriers in LAC (48). Likewise, there is a limited supply of specialized care and oncology drugs, exacerbated by the COVID-19 pandemic, a context that overshadows predictions of cancer mortality in LAC (49).
Due to its pathophysiological correlation (including high levels of energy consumption due to inflammation, malnutrition and decreased physical activity) (8), the prevalence of sarcopenia associated with cachexia in older cancer patients is high and varies from 16% to 71% according to the type of cancer. There are numerous reports that identify the presence of sarcopenia as a poor prognostic factor for survival independent of cancer location (50–54). However, definitive diagnosis of sarcopenia requires the use of medical imaging technologies such as DXA, magnetic resonance imaging or computed tomography (9), which are difficult to access in developing countries and, therefore, little applied in the first level of care.
We found that both SARC-F and SARC-CalF have prognostic value (they have an AUC greater than 0.5), however, by themselves they are not good prognostic markers. It is necessary to develop future studies including both scores into pre-existing models or designing new ones considering them. In this sense, the use of rapid and low-cost diagnostic tools such as SARC-F and SARC-CalF is important for low- and middle-income countries such as those located in LAC due to their applicability in daily clinical practice, without requiring specialized centers, and good performance for the identification of individuals with a higher risk of death.
Limitations and strengths
This study has several limitations: 1) The 2-year follow-up time could have limited the recording of a greater number of events; 2) Only male participants were included, which means that the association in females may vary; 3) The study population was exclusively made up of retired sailors, and thus, the results of the study cannot be extrapolated to the general population; 4) We did not obtain a record of the dose of antineoplastic treatment received by the participants; 5) Data of relevant variables such as urea and creatinine for diagnosing renal failure were not available, which would serve to identify patients with a higher risk of adverse effects to antineoplastic treatment inducing a higher risk of mortality; 6) BMI, albumin and other variables related to nutrition in older adults with cancer were not available, since this is an important topic, we consider it as a limitation. Despite these limitations, this study evaluated the role of SARC-F and SARC-CalF scores as risk factors for mortality in older Peruvian adults with cancer. In addition, their usefulness as low-cost and easily accessible diagnostic tools allow the identification of groups of older adults with cancer at increased risk of mortality due to increased risk of sarcopenia.
Conclusions
The risk of sarcopenia assessed by SARC-F and SARC-CalF was associated with a higher risk of mortality in older men with cancer. To our knowledge, this is the first study in Latin America that evaluates this association. However, more studies are required to determine the association between the risk of sarcopenia and mortality in older adults of both sexes and with a longer follow-up time.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians. 2021;71(1):7–33; doi: https://doi.org/10.3322/caac.21654.
Hashim D, Carioli G, Malvezzi M, Bertuccio P, Waxman S, Negri E, et al. Cancer mortality in the oldest old: a global overview. Aging (Albany NY). 2020;12(17):16744–58; doi: https://doi.org/10.18632/aging.103503.
Pilleron S, Sarfati D, Janssen-Heijnen M, Vignat J, Ferlay J, Bray F, et al. Global cancer incidence in older adults, 2012 and 2035: A population-based study. International Journal of Cancer. 2019;144(1):49–58; doi: https://doi.org/10.1002/ijc.31664.
He W, Goodkind D, Kowal P. An Aging World: 2015. U.S. Census Bureau, International Population Reports. Vol. P95/16-1. Washington, DC: U.S. Government Publishing Office; 2016.
Zhang X, Tang T, Pang L, Sharma SV, Li R, Nyitray AG, et al. Malnutrition and overall survival in older adults with cancer: A systematic review and meta-analysis. Journal of Geriatric Oncology. 2019;10(6):874–83; doi: https://doi.org/10.1016/j.jgo.2019.03.002.
Bleve A, Motta F, Durante B, Pandolfo C, Selmi C, Sica A. Immunosenescence, Inflammaging, and Frailty: Role of Myeloid Cells in Age-Related Diseases. Clin Rev Allergy Immunol. 2022;1–22; doi: https://doi.org/10.1007/s12016-021-08909-7.
Peterson SJ, Mozer M. Differentiating Sarcopenia and Cachexia Among Patients With Cancer. Nutrition in Clinical Practice. 2017;32(1):30–9; doi: https://doi.org/10.1177/0884533616680354.
Colloca G, Capua BD, Bellieni A, Cesari M, Marzetti E, Valentini V, et al. Muscoloskeletal aging, sarcopenia and cancer. Journal of Geriatric Oncology. 2019;10(3):504–9; doi: https://doi.org/10.1016/j.jgo.2018.11.007.
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. The Lancet. 2019;393(10191):2636–46; doi: https://doi.org/10.1016/S0140-6736(19)31138-9.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31; doi: https://doi.org/10.1093/ageing/afy169.
Fu X, Tian Z, Thapa S, Sun H, Wen S, Xiong H, et al. Comparing SARC-F with SARC-CalF for screening sarcopenia in advanced cancer patients. Clinical Nutrition. 2020;39(11):3337–45; doi: https://doi.org/10.1016/j.clnu.2020.02.020.
Mo Y-H, Zhong J, Dong X, Su Y-D, Deng W-Y, Yao X-M, et al. Comparison of Three Screening Methods for Sarcopenia in Community-Dwelling Older Persons. Journal of the American Medical Directors Association. 2021;22(4):746–750.e1; doi: https://doi.org/10.1016/j.jamda.2020.05.041.
Williams GR, Al-Obaidi M, Dai C, Bhatia S, Giri S. SARC-F for screening of sarcopenia among older adults with cancer. Cancer. 2021;127(9):1469–75; doi: https://doi.org/10.1002/cncr.33395.
Behne TEG, Dock-Nasimento DB, Sierra JC, Rodrigues HHNP, Palauro ML, Andreo FO, et al. Association between preoperative potential sarcopenia and survival of cancer patients undergoing major surgical procedures. Rev Col Bras Cir. 2020;47:e20202528; doi: https://doi.org/10.1590/0100-6991e-20202528.
Mainardi LG, Borges TC, Gomes TLN, Pichard C, Laviano A, Pimentel GD. Association of SARC-F and dissociation of SARC-F + calf circumference with comorbidities in older hospitalized cancer patients. Experimental Gerontology. 2021;148:111315; doi: https://doi.org/10.1016/j.exger.2021.111315.
Landi F, Calvani R, Cesari M, Tosato M, Martone AM, Ortolani E, et al. Sarcopenia: An Overview on Current Definitions, Diagnosis and Treatment. Curr Protein Pept Sci. 2018;19(7):633–8; doi: https://doi.org/10.2174/1389203718666170607113459.
Anjanappa M, Corden M, Green A, Roberts D, Hoskin P, McWilliam A, et al. Sarcopenia in cancer: Risking more than muscle loss. Tech Innov Patient Support Radiat Oncol. 2020;16:50–7; doi: https://doi.org/10.1016/j.tipsro.2020.10.001.
Messina C, Albano D, Gitto S, Tofanelli L, Bazzocchi A, Ulivieri FM, et al. Body composition with dual energy X-ray absorptiometry: from basics to new tools. Quant Imaging Med Surg. 2020;10(8):1687–98; doi: https://doi.org/10.21037/qims.2020.03.02.
Lera L, Albala C, Ángel B, Sánchez H, Picrin Y, Hormazabal MJ, et al. Predicción de la masa muscular apendicular esquelética basado en mediciones antropométricas en Adultos Mayores Chilenos. Nutrición Hospitalaria. 2014;29(3):611–7; doi: https://doi.org/10.3305/nh.2014.29.3.7062.
Huicho L, Canseco FD, Lema C, Miranda JJ, Lescano AG. Incentivos para atraer y retener personal de salud de zonas rurales del Perú: un estudio cualitativo. Cad Saude Publica. 2012;28(4):729–39; doi: https://doi.org/10.1590/S0102-311X2012000400012.
Runzer-Colmenares FM, Urrunaga-Pastor D, Aguirre LG, Reategui-Rivera CM, Parodi JF, Taype-Rondan A. Frailty and vulnerability as predictors of radiotoxicity in older adults: A longitudinal study in Peru. Medicina Clínica (English Edition). 2017;149(8):325–30; doi: https://doi.org/10.1016/j.medcli.2017.02.022.
Runzer-Colmenares FM, Urrunaga-Pastor D, Roca-Moscoso MA, De Noriega J, Rosas-Carrasco O, Parodi JF. Frailty and Vulnerability as Predictors of Chemotherapy Toxicity in Older Adults: A Longitudinal Study in Peru. J Nutr Health Aging. 2020;24(9):966–72; doi: https://doi.org/10.1007/s12603-020-1404-6.
Runzer-Colmenares F, Chambergo-Michilot D, Espinoza-Gutiérrez G, Corcuera-Ciudad R, Patiño-Villena A, Paima-Olivari R, et al. Type 2 Diabetes mellitus and chemotherapy toxicity in older adults with prostate cancer. Rev Habanera Ciencias Médicas. 2019;18(1):74–87.
Yang M, Jiang J, Zeng Y, Tang H. Sarcopenia for predicting mortality among elderly nursing home residents: SARC-F versus SARC-CalF. Medicine. 2019;98(7):e14546; doi: https://doi.org/10.1097/MD.0000000000014546.
Malmstrom TK, Morley JE. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. Journal of the American Medical Directors Association. 2013;14(8):531–2; doi: https://doi.org/10.1016/j.jamda.2013.05.018.
Souza VF de, Ribeiro T de SC, Marques R de A, Petarli GB, Pereira TSS, Rocha JLM, et al. SARC-CalF-assessed risk of sarcopenia and associated factors in cancer patients. Nutr Hosp. 2020;37(6):1173–8; doi: https://doi.org/10.20960/nh.03158.
Barbosa-Silva TG, Menezes AMB, Bielemann RM, Malmstrom TK, Gonzalez MC. Enhancing SARC-F: Improving Sarcopenia Screening in the Clinical Practice. Journal of the American Medical Directors Association. 2016;17(12):1136–41; doi: https://doi.org/10.1016/j.jamda.2016.08.004.
Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35(5):526–9; doi: https://doi.org/10.1093/ageing/afl041.
Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of Frailty Using Eight Commonly Used Scales and Comparison of Their Ability to Predict All-Cause Mortality. Journal of the American Geriatrics Society. 2013;61(9):1537–51; doi: https://doi.org/10.1111/jgs.12420.
Menant JC, Weber F, Lo J, Sturnieks DL, Close JC, Sachdev PS, et al. Strength measures are better than muscle mass measures in predicting health-related outcomes in older people: time to abandon the term sarcopenia? Osteoporos Int. 2017;28(1):59–70; doi: https://doi.org/10.1007/s00198-016-3691-7.
Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2007;63(2):187–95; doi: https://doi.org/10.3109/09638288809164105.
Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability. Int Disabil Stud. 1988;10(2):64–7; doi: https://doi.org/10.3109/09638288809164105.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in Older Adults: Evidence for a Phenotype. The Journals of Gerontology: Series A. 2001;56(3):M146–57; doi: https://doi.org/10.1093/gerona/56.3.m146.
Chauhan NS, Samuel SR, Meenar N, Saxena PP, Keogh JWL. Sarcopenia in male patients with head and neck cancer receiving chemoradiotherapy: a longitudinal pilot study. PeerJ. 2020;8:e8617.; doi: https://doi.org/10.7717/peerj.8617
Gomes TLN, Borges TC, Pichard C, Pimentel GD. Correlation Between SARC-F Score and Ultrasound-Measured Thigh Muscle Thickness in Older Hospitalized Cancer Patients. J Nutr Health Aging. 2020;24(10):1128–30; doi: https://doi.org/10.1007/s12603-020-1524-z.
Rodrigues FW, Burgel CF, Brito JE, Baumgardt E, de Araújo BE, Silva FM. SARC-CalF tool has no significant prognostic value in hospitalized patients: A prospective cohort study. Nutrition in Clinical Practice. 2021;36(5):1072–9; doi: https://doi.org/10.1002/ncp.10675.
Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A, et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res. 2017;29(1):35–42; doi: https://doi.org/10.1007/s40520-016-0705-4.
Tenover JS, Matsumoto AM, Clifton DK, Bremner WJ. Age-Related Alterations in the Circadian Rhythms of Pulsatile Luteinizing Hormone and Testosterone Secretion in Healthy Men. Journal of Gerontology. 1988;43(6):M163–9; doi: https://doi.org/10.1093/geronj/43.6.m163.
Rosenberg IH. Sarcopenia: Origins and Clinical Relevance. The Journal of Nutrition. 1997;127(5):990S–991S; doi: https://doi.org/10.1093/jn/127.5.990S.
Kamel HK. Sarcopenia and Aging. Nutrition Reviews. 2003;61(5):157–67; doi: https://doi.org/10.1301/nr.2003.may.157-167.
Williams GR, Rier HN, McDonald A, Shachar SS. Sarcopenia & Aging in Cancer. J Geriatr Oncol. 2019;10(3):374–7; doi: https://doi.org/10.1016/j.jgo.2018.10.009.
Kazemi-Bajestani SMR, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Seminars in Cell & Developmental Biology. 2016;54:2–10; doi: https://doi.org/10.1016/j.semcdb.2015.09.001.
Cancer today [Internet]. World Health Organization. [cited 2021 Dec 21]. Available from: http://gco.iarc.fr/today/home
Cancer — PAHO/WHO Pan American Health Organization [Internet]. [cited 2022 Mar 14]. Available from: https://www.paho.org/en/topics/cancer
Cayon A. PAHO/WHO Country cancer profiles 2020 [Internet]. Pan American Health Organization/World Health Organization. 2020 [cited 2022 Mar 14]. Available from: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=15716:country-cancer-profiles-2020&Itemid=72576&lang=en
Lence JJ, Camacho R. Cáncer y transición demográfica en América Latina y el Caribe. Revista Cubana de Salud Pública. 2006;32(3):0–0.
Stewart BW, Wild CP. World Cancer Report 2014. International Agency for Research on Cancer (IARC); 2014.
Báscolo E, Houghton N, Del Riego A. Lógicas de transformación de los sistemas de salud en América Latina y resultados en acceso y cobertura de salud. Rev Panam Salud Publica. 2018;42:e126; doi: https://doi.org/10.26633/RPSP.2018.126.
Barrios CH, Werutsky G, Mohar A, Ferrigno AS, Müller BG, Bychkovsky BL, et al. Cancer control in Latin America and the Caribbean: recent advances and opportunities to move forward. The Lancet Oncology. 2021;22(11):e474–87.; doi: https://doi.org/10.1016/S1470-2045(21)00492-7
Dohzono S, Sasaoka R, Takamatsu K, Hoshino M, Nakamura H. Low paravertebral muscle mass in patients with bone metastases from lung cancer is associated with poor prognosis. Supportive Care in Cancer. 2020;28(1):389–95; doi: https://doi.org/10.1007/s00520-019-04843-9.
Chargi N, Bril SI, Emmelot-Vonk MH, de Bree R. Sarcopenia is a prognostic factor for overall survival in elderly patients with head-and-neck cancer. Eur Arch Otorhinolaryngol. 2019;276(5):1475–86; doi: https://doi.org/10.1007/s00405-019-05361-4.
Kuwada K, Kuroda S, Kikuchi S, Yoshida R, Nishizaki M, Kagawa S, et al. Clinical Impact of Sarcopenia on Gastric Cancer. Anticancer Research. 2019;39(5):2241–9; doi: https://doi.org/10.21873/anticanres.13340.
Villaseñor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6(4):398–406; doi: https://doi.org/10.1007/s11764-012-0234-x.
Stene GB, Helbostad JL, Amundsen T, Sørhaug S, Hjelde H, Kaasa S, et al. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncologica. 2015;54(3):340–8; doi: https://doi.org/10.3109/0284186X.2014.953259.
Acknowledgments
We thank the staff of the Center for Research on Aging — Faculty of Medicine of the University of San Martin de Porres, Peru and the staff of the Geriatric Service of the Naval Medical Center of Peru for the logistical support provided. We thank the Universidad Científica del Sur and Donna Pringle for English editing support.
Funding
Funding: Self-funded.
Author information
Authors and Affiliations
Contributions
Authors’ contributions: All authors participated in this research and contributed to the final version of the manuscript. Kimi Ururi-Cupi, Fiorella Oliva-Zapata, Leslie Salazar-Talla, Sofia Cuba-Ruiz, Diego Urrunaga-Pastor, Fernando M. Runzer-Colmenares, Jose F. Parodi participated in concept design and supervising the study. Diego Urrunaga-Pastor and Fernando M. Runzer-Colmenares conducted the statistical analysis. In addition, all the authors participated in manuscript writing, editing, final revision and have read and agreed on the submitted manuscript.
Corresponding authors
Ethics declarations
Conflict of interests: The authors declare no conflict of interest.
Electronic supplementary material
12603_2022_1844_MOESM1_ESM.docx
Table S1. Supplementary Cox regression model including both SARC-F and SARC-CalF scores as risk factors for mortality in the study sample (n=922).
Rights and permissions
About this article
Cite this article
Ururi-Cupi, K., Oliva-Zapata, F., Salazar-Talla, L. et al. SARC-F and SARC-CalF Scores as Mortality Risk Factors in Older Men with Cancer: A Longitudinal Study from Peru. J Nutr Health Aging 26, 856–863 (2022). https://doi.org/10.1007/s12603-022-1844-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-022-1844-2