Abstract
Objectives
To perform a systematic review and meta-analysis and quantify the associations of total mortality with calf circumference (CC) in adults 18 years and older via combining various analyses based on empirical dichotomic CC, continuous CC, and dose-response CC.
Methods
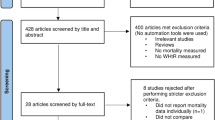
We conducted a systematic search of relevant studies in PubMed, EMBASE, Cochrane Library, and Web of Science published through April 12, 2022. This systematic review includes longitudinal observational studies reporting the relationships of total mortality with CC. We calculated the pooled relative risk (RR) and 95% confidence interval (CI) of total mortality with CC per 1 cm for each study and combined the values using standard meta-analysis approaches. Newcastle-Ottawa scale (NOS), Grading of Recommendations, Assessment, Development and Evaluations approach (GRADE), and the Instrument for assessing the Credibility of Effect Modification Analyses (ICEMAN) were assessed for meta-analyses.
Results
Our analysis included a total of 37 cohort studies involving 62,736 participants, across which moderate heterogeneity was observed (I2=75.7%, P<0.001), but no publication bias was found. Study quality scores ranged from 6 to 9 (mean 7.7), with only three studies awarded a score of 6 (fair quality). We observed an inverse trend between total death risk and CC per 1 cm increase (RR, 0.95, 95% CI, 0.94–0.96; P<0.001; GRADE quality=high). Only a very slight difference was found among residents of nursing homes (6.9% mortality risk reduction per one cm CC increase), community-dwellers (5.4%), and those living in hospitals (4.8%), respectively (P for meta-regression=0.617). Low credible subgroup difference was found based on the ICEMAN tool.
Conclusions
Calf circumference is a valid anthropometric measure for mortality risk prediction in a community, nursing home, or hospital.


Similar content being viewed by others
References
Wickramasinghe K, Mathers JC, Wopereis S, et al. From lifespan to healthspan: the role of nutrition in healthy ageing. J Nutr Sci. 2020;9:e33. doi:https://doi.org/10.1017/jns.2020.26
World Health Organization. Ageing and health. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health [updated 5 February 2018].
Hermans K, van den Brandt PA, Loef C, et al. Anthropometry, physical activity and cancer of unknown primary (CUP) risk: Results from the Netherlands cohort study. Cancer Epidemiol. 2020;69:101836. doi:https://doi.org/10.1016/j.canep.2020.101836
Poon ET, Little JP, Sit CH, et al. The effect of low-volume high-intensity interval training on cardiometabolic health and psychological responses in overweight/obese middle-aged men. J Sports Sci. 2020;38(17):1997–2004. doi:https://doi.org/10.1080/02640414.2020.1766178
Yaprak DS, Yalçın B, Pınar AA, et al. Assessment of nutritional status in children with cancer: Significance of arm anthropometry and serum visceral proteins. Pediatr Blood Cancer. 2020:e28752. doi:https://doi.org/10.1002/pbc.28752
Yasuoka J, Yi S, Okawa S, et al. Nutritional status and dietary diversity of school-age children living with HIV: a cross-sectional study in Phnom Penh, Cambodia. BMC Public Health. 2020;20(1):1181. doi:https://doi.org/10.1186/s12889-020-09238-8
Brand C, Reuter CP, Gaya AR, et al. Association between cardiorespiratory fitness and cardiometabolic risk factors in Brazilianchildren and adolescents: the mediating role of obesity parameters. Paediatr Int Child Health. 2020:1–10. doi:https://doi.org/10.1080/20469047.2020.1838758
Lee MR, Jung SM, Kim HS, et al. Association of muscle strength with cardiovascular risk in Korean adults: Findings from the Korea National Health and Nutrition Examination Survey (KNHANES) VI to VII (2014–2016). Medicine (Baltimore). 2018;97(47):e13240. doi:https://doi.org/10.1097/md.0000000000013240
Rossello X, Fuster V, Oliva B, et al. Association Between Body Size Phenotypes and Subclinical Atherosclerosis. J Clin Endocrinol Metab. 2020;105(12). doi:https://doi.org/10.1210/clinem/dgaa620
Zhu P, Herrington WG, Haynes R, et al. Conventional and Genetic Evidence on the Association between Adiposity and CKD. J Am Soc Nephrol. 2020. doi:https://doi.org/10.1681/asn.2020050679
Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. doi:https://doi.org/10.1136/bmj.i2156
Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–86. doi:https://doi.org/10.1016/s0140-6736(16)30175-1
Jayedi A, Soltani S, Zargar MS, et al. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ. 2020;370:m3324. doi:https://doi.org/10.1136/bmj.m3324
Javed AA, Aljied R, Allison DJ, et al. Body mass index and all-cause mortality in older adults: A scoping review of observational studies. Obes Rev. 2020;21(8):e13035. doi:https://doi.org/10.1111/obr.13035
Winter JE, MacInnis RJ, Wattanapenpaiboon N, et al. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99(4):875–90. doi:https://doi.org/10.3945/ajcn.113.068122
Buckinx F, Croisier JL, Reginster JY, et al. Prediction of the Incidence of Falls and Deaths Among Elderly Nursing Home Residents: The SENIOR Study. J Am Med Dir Assoc. 2018;19(1):18–24. doi:https://doi.org/10.1016/j.jamda.2017.06.014
Mason C, Craig CL, Katzmarzyk PT. Influence of central and extremity circumferences on all-cause mortality in men and women. Obesity (Silver Spring). 2008;16(12):2690–5. doi:https://doi.org/10.1038/oby.2008.438
Sharifi F, Ghaderpanahi M, Fakhrzadeh H, et al. Older people’s mortality index: development of a practical model for prediction of mortality in nursing homes (Kahrizak Elderly Study). Geriatr Gerontol Int. 2012;12(1):36–45. doi:https://doi.org/10.1111/j.1447-0594.2011.00724.x
Tsai AC-H, Lai M-C, Chang T-L. Mid-arm and calf circumferences (MAC and CC) are better than body mass index (BMI) in predicting health status and mortality risk in institutionalized elderly Taiwanese. Archives of Gerontology and Geriatrics. 2012;54(3):443–47. doi:https://doi.org/10.1016/j.archger.2011.05.015
Wijnhoven HAH, van Bokhorst-de van der Schueren MAE, Heymans MW, et al. Low Mid-Upper Arm Circumference, Calf Circumference, and Body Mass Index and Mortality in Older Persons. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2010;65(10):1107–14. doi:https://doi.org/10.1093/gerona/glq100
de Almeida Roediger M, de Fátima Nunes Marucci M, Quintiliano Scarpelli Dourado DA, et al. Body Composition Changes and 10-Year Mortality Risk in Older Brazilian Adults: Analysis of Prospective Data from the SABE Study. J Nutr Health Aging. 2019;23(1):51–59. doi:https://doi.org/10.1007/s12603-018-1118-1
Moon S, Hong GS. Predictive Factors of Mortality in Older Adult Residents of Long-Term Care Facilities. J Nurs Res. 2020;28(2):e82. doi:https://doi.org/10.1097/jnr.0000000000000356
Saka B, Ozkaya H, Karisik E, et al. Malnutrition and sarcopenia are associated with increased mortality rate in nursing home residents: A prospective study. European Geriatric Medicine. 2016;7(3):232–38. doi:https://doi.org/10.1016/j.eurger.2015.12.010
Tsai AC, Chang T-L. The effectiveness of BMI, calf circumference and mid-arm circumference in predicting subsequent mortality risk in elderly Taiwanese. British Journal of Nutrition. 2011;105(2):275–81. doi:https://doi.org/10.1017/s0007114510003429
Weng CH, Tien CP, Li CI, et al. Mid-upper arm circumference, calf circumference and mortality in Chinese long-term care facility residents: a prospective cohort study. BMJ Open. 2018;8(5):e020485. doi:https://doi.org/10.1136/bmjopen-2017-020485
Allison DB, Zhu SK, Plankey M, et al. Differential associations of body mass index and adiposity with all-cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int J Obes Relat Metab Disord. 2002;26(3):410–6. doi:https://doi.org/10.1038/sj.ijo.0801925
Chumlea WC. Is the MNA valid in different populations and across practice settings? J Nutr Health Aging. 2006;10(6):524–7; discussion 27–33.
Landi F, Camprubi-Robles M, Bear DE, et al. Muscle loss: The new malnutrition challenge in clinical practice. Clin Nutr. 2019;38(5):2113–20. doi:https://doi.org/10.1016/j.clnu.2018.11.021
Wei J, Jiao J, Chen CL, et al. The association between low calf circumference and mortality: a systematic review and meta-analysis. Eur Geriatr Med. 2022. doi:https://doi.org/10.1007/s41999-021-00603-3
Yin L, Zhang L, Li N, et al. Several anthropometric measurements and cancer mortality: predictor screening, threshold determination, and joint analysis in a multicenter cohort of 12138 adults. Eur J Clin Nutr. 2021. doi:https://doi.org/10.1038/s41430-021-01009-x
Yang M, Jiang J, Zeng Y, et al. Sarcopenia for predicting mortality among elderly nursing home residents: SARC-F versus SARC-CalF. Medicine (Baltimore). 2019;98(7):e14546. doi:https://doi.org/10.1097/md.0000000000014546
Wu SE, Chen WL. Calf circumference refines sarcopenia in correlating with mortality risk. Age Ageing. 2022;51(2). doi:https://doi.org/10.1093/ageing/afab239
Teixeira PP, Kowalski VH, Valduga K, et al. Low Muscle Mass Is a Predictor of Malnutrition and Prolonged Hospital Stay in Patients With Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Longitudinal Study. JPEN J Parenter Enteral Nutr. 2021;45(6):1221–30. doi:https://doi.org/10.1002/jpen.1998
Sousa IM, Bielemann RM, Gonzalez MC, et al. Low calf circumference is an independent predictor of mortality in cancer patients: A prospective cohort study. Nutrition. 2020;79–80:110816. doi:https://doi.org/10.1016/j.nut.2020.110816
Sobestiansky S, Åberg AC, Cederholm T. Sarcopenia and malnutrition in relation to mortality in hospitalised patients in geriatric care — predictive validity of updated diagnoses. Clin Nutr ESPEN. 2021;45:442–48. doi:https://doi.org/10.1016/j.clnesp.2021.07.002
Sanchez-Rodriguez D, Locquet M, Bruyère O, et al. Prediction of 5-year mortality risk by malnutrition according to the GLIM format using seven pragmatic approaches to define the criterion of loss of muscle mass. Clin Nutr. 2021;40(4):2188–99. doi:https://doi.org/10.1016/j.clnu.2020.09.047
Ren C, Zhang X, Zhu Y, et al. Low calf circumference can predict nutritional risk and mortality in adults with metabolic syndrome aged over 80 years. BMC Endocr Disord. 2022;22(1):47. doi:https://doi.org/10.1186/s12902-022-00964-1
Nishikawa H, Yoh K, Enomoto H, et al. Combined grip strength and calf circumference as a useful prognostic system in patients with liver diseases: a large cohort study. Ann Transl Med. 2021;9(8):624. doi:https://doi.org/10.21037/atm-20-6901
Ge YZ, Ruan GT, Zhang KP, et al. Which anthropometric measurement is better for predicting survival of patients with cancer cachexia? Br J Nutr. 2021:1–9. doi:https://doi.org/10.1017/s0007114521002853
Fernandes DPS, Juvanhol LL, Lozano M, et al. Calf circumference is an independent predictor of mortality in older adults: An approach with generalized additive models. Nutr Clin Pract. 2021. doi:https://doi.org/10.1002/ncp.10780
Dos Santos Rd CO, Burgel CF, Chites Rd VS, et al. Low-cost and fast-performing indicators of muscle mass loss are good predictors of clinical outcomes in hospitalized patients: A longitudinal observational study. JPEN J Parenter Enteral Nutr. 2021. doi:https://doi.org/10.1002/jpen.2268
de Sousa IM, Silva FM, de Carvalho ALM, et al. Accuracy of isolated nutrition indicators in diagnosing malnutrition and their prognostic value to predict death in patients with gastric and colorectal cancer: A prospective study. JPEN J Parenter Enteral Nutr. 2022;46(3):508–16. doi:https://doi.org/10.1002/jpen.2199
Bernardes S, Silva FM, da Costa CC, et al. Reduced calf circumference is an independent predictor of worse quality of life, severity of disease, frequent exacerbation, and death in patients with chronic obstructive pulmonary disease admitted to a pulmonary rehabilitation program: A historic cohort study. JPEN J Parenter Enteral Nutr. 2022;46(3):546–55. doi:https://doi.org/10.1002/jpen.2214
Abreo AP, Bailey SR, Abreo K. Associations between calf, thigh, and arm circumference and cardiovascular and all-cause mortality in NHANES 1999–2004. Nutr Metab Cardiovasc Dis. 2021;31(5):1410–15. doi:https://doi.org/10.1016/j.numecd.2021.01.011
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. doi:https://doi.org/10.1093/oxfordjournals.aje.a116237
Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. doi:https://doi.org/10.1093/aje/kwr265
Shor E, Roelfs D, Vang ZM. The “Hispanic mortality paradox” revisited: Meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants’ mortality. Soc Sci Med. 2017;186:20–33. doi:https://doi.org/10.1016/j.socscimed.2017.05.049
VanderWeele TJ. On a Square-Root Transformation of the Odds Ratio for a Common Outcome. Epidemiology. 2017;28(6):e58–e60. doi:https://doi.org/10.1097/EDE.0000000000000733
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:https://doi.org/10.1136/bmj.n71
World Health Organization, Expert Committee on Physical Status: the Use Interpretation of Anthropometry. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. In. Geneva, Switzerland; 1995. pp. 1–452.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi:https://doi.org/10.1007/s10654-010-9491-z.
Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi:https://doi.org/10.1136/bmj.h870
Wu CJ, Kao TW, Lin YY, et al. Examining the association between anthropometric parameters and telomere length and mortality risk. Oncotarget. 2017;8(21):34057–69. doi:https://doi.org/10.18632/oncotarget.15976
Schandelmaier S, Briel M, Varadhan R, et al. Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ. 2020;192(32):E901–E06. doi:https://doi.org/10.1503/cmaj.200077
Carmelli D, Zhang H, Swan GE. Obesity and 33-year follow-up for coronary heart disease and cancer mortality. Epidemiology. 1997;8(4):378–83. doi:https://doi.org/10.1097/00001648-199707000-00005
Tsai AC, Ku PY. Population-specific Mini Nutritional Assessment effectively predicts the nutritional state and follow-up mortality of institutionalized elderly Taiwanese regardless of cognitive status. Br J Nutr. 2008;100(1):152–8. doi:https://doi.org/10.1017/s0007114507873600
Tsai AC, Yang S-F, Wang J-Y. Validation of population-specific Mini-Nutritional Assessment with its long-term mortality-predicting ability: results of a population-based longitudinal 4-year study in Taiwan. British Journal of Nutrition. 2010;104(1):93–99. doi:https://doi.org/10.1017/s0007114510000188
Ehrlich AC, Smith DA. Abdominal diameter index and 12-year cardiovascular disease incidence in male bridge and tunnel workers. Int J Obes (Lond). 2011;35(3):409–15. doi:https://doi.org/10.1038/ijo.2010.143
Arango-Lopera VE, Arroyo P, Gutiérrez-Robledo LM, et al. Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging. 2013;17(3):259–62. doi:https://doi.org/10.1007/s12603-012-0434-0
Hsu K-H, Shih C-P, Liao P-J. Waist-to-thigh ratio is a predictor of internal organ cancers in humans: findings from a cohort study. Annals of Epidemiology. 2013;23(6):342–48. doi:https://doi.org/10.1016/j.annepidem.2013.04.004
Tsai AC, Chang TL, Wang JY. Short-form Mini-Nutritional Assessment with either BMI or calf circumference is effective in rating the nutritional status of elderly Taiwanese — results of a national cohort study. Br J Nutr. 2013;110(6):1126–32. doi:https://doi.org/10.1017/s0007114513000366
Wang JY, Tsai AC. The short-form mini-nutritional assessment is as effective as the full-mini nutritional assessment in predicting follow-up 4-year mortality in elderly Taiwanese. J Nutr Health Aging. 2013;17(7):594–8. doi:https://doi.org/10.1007/s12603-013-0048-1
Abd-El-Gawad WM, Abou-Hashem RM, El Maraghy MO, et al. The validity of Geriatric Nutrition Risk Index: simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with Mini Nutritional Assessment. Clin Nutr. 2014;33(6):1108–16. doi:https://doi.org/10.1016/j.clnu.2013.12.005
Dent E, Chapman IM, Piantadosi C, et al. Performance of nutritional screening tools in predicting poor six-month outcome in hospitalised older patients. Asia Pac J Clin Nutr. 2014;23(3):394–9. doi:https://doi.org/10.6133/apjcn.2014.23.3.18
Eschbach D, Kirchbichler T, Wiesmann T, et al. Nutritional intervention in cognitively impaired geriatric trauma patients: a feasibility study. Clin Interv Aging. 2016;11:1239–46. doi:https://doi.org/10.2147/cia.S109281
Pan J, Yang X, Zhou X, et al. A prospective study of nutritional status in elderly inpatients with neurological disease. Chinese Journal of Clinical Nutrition. 2016;24(6):338–41. doi:https://doi.org/10.3760/cma.j.issn.1674-635X.2016.06.003
Brown JC, Harhay MO, Harhay MN. Anthropometrically-predicted visceral adipose tissue and mortality among men and women in the third national health and nutrition examination survey (NHANES III). American Journal of Human Biology. 2017;29(1). doi:https://doi.org/10.1002/ajhb.22898
Turusheva A, Frolova E, Hegendoerfer E, et al. Predictors of short-term mortality, cognitive and physical decline in older adults in northwest Russia: a population-based prospective cohort study. Aging Clinical and Experimental Research. 2017;29(4):665–73. doi:https://doi.org/10.1007/s40520-016-0613-7
Hanatani S, Izumiya Y, Onoue Y, et al. Non-invasive testing for sarcopenia predicts future cardiovascular events in patients with chronic kidney disease. Int J Cardiol. 2018;268:216–21. doi:https://doi.org/10.1016/j.ijcard.2018.03.064
Tanaka T, Takahashi K, Akishita M, et al. “Yubi-wakka” (finger-ring) test: A practical self-screening method for sarcopenia, and a predictor of disability and mortality among Japanese community-dwelling older adults. Geriatr Gerontol Int. 2018;18(2):224–32. doi:https://doi.org/10.1111/ggi.13163
Wu CJ, Kao TW, Chang YW, et al. Does the Additional Component of Calf Circumference Refine Metabolic Syndrome in Correlating With Cardiovascular Risk? J Clin Endocrinol Metab. 2018;103(3):1151–60. doi:https://doi.org/10.1210/jc.2017-02320
Gattermann Pereira T, da Silva Fink J, Tosatti JAG, et al. Subjective Global Assessment Can Be Performed in Critically Ill Surgical Patients as a Predictor of Poor Clinical Outcomes. Nutr Clin Pract. 2019;34(1):131–36. doi:https://doi.org/10.1002/ncp.10178
Li M, Kong Y, Chen H, et al. Accuracy and prognostic ability of the SARC-F questionnaire and Ishii’s score in the screening of sarcopenia in geriatric inpatients. Braz J Med Biol Res. 2019;52(9):e8204. doi:https://doi.org/10.1590/1414-431x20198204
Bicakli DH, Uslu R, Güney SC, et al. The Relationship Between Nutritional Status, Performance Status, and Survival Among Pancreatic Cancer Patients. Nutr Cancer. 2020;72(2):202–08. doi:https://doi.org/10.1080/01635581.2019.1634217
Gade J, Quick AA, Beck AM, et al. SARC-F in hospitalized, geriatric medical patients — Feasibility, prevalence of risk of sarcopenia, and characteristics of the risk group, including one-year follow-up. Clin Nutr ESPEN. 2020;37:80–86. doi:https://doi.org/10.1016/j.clnesp.2020.03.016
Tang T, Zhuo Y, Xie L, et al. Sarcopenia index based on serum creatinine and cystatin C is associated with 3-year mortality in hospitalized older patients. Sci Rep. 2020;10(1):1260. doi:https://doi.org/10.1038/s41598-020-58304-z
Yilmaz M, Atilla FD, Sahin F, et al. The effect of malnutrition on mortality in hospitalized patients with hematologic malignancy. Support Care Cancer. 2020;28(3):1441–48. doi:https://doi.org/10.1007/s00520-019-04952-5
Hanatani S, Izumiya Y, Yamamoto M, et al. A simple method of sarcopenia detection can predict adverse cardiovascular events in patients with abdominal obesity. Int J Obes (Lond). 2021;45(10):2214–20. doi:https://doi.org/10.1038/s41366-021-00895-2
Macedo C, Amaral TF, Rodrigues J, et al. Malnutrition and Sarcopenia Combined Increases the Risk for Mortality in Older Adults on Hemodialysis. Front Nutr. 2021;8:721941. doi:https://doi.org/10.3389/fnut.2021.721941
Mainardi LG, Borges TC, Gomes TLN, et al. Association of SARC-F and dissociation of SARC-F + calf circumference with comorbidities in older hospitalized cancer patients. Exp Gerontol. 2021;148:111315. doi:https://doi.org/10.1016/j.exger.2021.111315
Raffield LM, Howard AG, Graff M, et al. Obesity Duration, Severity, and Distribution Trajectories and Cardiovascular Disease Risk in the Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2021;10(24):e019946. doi:https://doi.org/10.1161/jaha.121.019946
Rodrigues FW, Burgel CF, Brito JE, et al. SARC-CalF tool has no significant prognostic value in hospitalized patients: A prospective cohort study. Nutr Clin Pract. 2021;36(5):1072–79. doi:https://doi.org/10.1002/ncp.10675
Vavruk AM, Martins C, Mazza do Nascimento M. Validation of Malnutrition Clinical Characteristics in Critically Ill Patients. Nutr Clin Pract. 2021;36(5):993–1002. doi:https://doi.org/10.1002/ncp.10637
Yin L, Lin X, Li N, et al. Evaluation of the Global Leadership Initiative on Malnutrition Criteria Using Different Muscle Mass Indices for Diagnosing Malnutrition and Predicting Survival in Lung Cancer Patients. JPEN J Parenter Enteral Nutr. 2021;45(3):607–17. doi:https://doi.org/10.1002/jpen.1873
Lee ZY, Hasan MS, Day AG, et al. Initial development and validation of a novel nutrition risk, sarcopenia, and frailty assessment tool in mechanically ventilated critically ill patients: The NUTRIC-SF score. JPEN J Parenter Enteral Nutr. 2022;46(3):499–507. doi:https://doi.org/10.1002/jpen.2194
Lu JL, Xu XY, Chen L, et al. The Predictive Values of Five Sarcopenia Screening Tools on Clinical Outcomes Following Surgery in Patients with Gastric Cancer: A Prospective Cohort Study. J Nutr Health Aging. 2022;26(3):259–65. doi:https://doi.org/10.1007/s12603-022-1751-6
Osuna-Padilla IA, Rodríguez-Moguel NC, Rodríguez-Llamazares S, et al. Low muscle mass in COVID-19 critically-ill patients: Prognostic significance and surrogate markers for assessment. Clin Nutr. 2022. doi:https://doi.org/10.1016/j.clnu.2022.02.019
Ren C, Su H, Tao J, et al. Sarcopenia Index Based on Serum Creatinine and Cystatin C is Associated with Mortality, Nutritional Risk/Malnutrition and Sarcopenia in Older Patients. Clin Interv Aging. 2022;17:211–21. doi:https://doi.org/10.2147/cia.S351068
Allen KL, Miskulin D, Yan G, et al. Association of nutritional markers with physical and mental health status in prevalent hemodialysis patients from the HEMO study. J Ren Nutr. 2002;12(3):160–9. doi:https://doi.org/10.1053/jren.2002.33512
Hsu W-C, Tsai AC, Wang J-Y. Calf circumference is more effective than body mass index in predicting emerging care-need of older adults — Results of a national cohort study. Clinical Nutrition. 2016;35(3):735–40. doi:https://doi.org/10.1016/j.clnu.2015.05.017
Perez-Zepeda MU, Gutierrez-Robledo LM. Calf circumference predicts mobility disability: A secondary analysis of the Mexican health and ageing study. European Geriatric Medicine. 2016;7(3):262–66. doi:https://doi.org/10.1016/j.eurger.2016.01.004
Lidoriki I, Schizas D, Mpaili E, et al. Associations between skeletal muscle mass index, nutritional and functional status of patients with oesophago-gastric cancer. Clinical Nutrition Espen. 2019;34:61–67. doi:https://doi.org/10.1016/j.clnesp.2019.08.012
Hsiang C-H, Wu C-J, Kao T-W, et al. Calf circumference and risk of cardiovascular disease. Geriatrics & Gerontology International. 2020;20(12):1133–37. doi:https://doi.org/10.1111/ggi.14052
Xu JY, Zhu MW, Zhang H, et al. A Cross-Sectional Study of GLIM-Defined Malnutrition Based on New Validated Calf Circumference Cut-off Values and Different Screening Tools in Hospitalised Patients over 70 Years Old. J Nutr Health Aging. 2020;24(8):832–38. doi:https://doi.org/10.1007/s12603-020-1386-4
Basibuyuk GO, Ayremlou P, Saeidlou SN, et al. A comparison of the different anthropometric indices for assessing malnutrition among older people in Turkey: a large population-based screening. Journal of Health Population and Nutrition. 2021;40(1). doi:https://doi.org/10.1186/s41043-021-00228-z
Chang CF, Yeh YL, Chang HY, et al. Prevalence and Risk Factors of Sarcopenia among Older Adults Aged ≥65 Years Admitted to Daycare Centers of Taiwan: Using AWGS 2019 Guidelines. Int J Environ Res Public Health. 2021;18(16). doi:https://doi.org/10.3390/ijerph18168299
Tang M, Ge Y, Zhang Q, et al. Near-term prognostic impact of integrated muscle mass and function in upper gastrointestinal cancer. Clin Nutr. 2021;40(9):5169–79. doi:https://doi.org/10.1016/j.clnu.2021.07.028
Chavarro-Carvajal DA, Ayala AM, Venegas-Sanabria LC, et al. Use of a nutrition-focused quality improvement program for community-living older adults at malnutrition risk is associated with better nutritional outcomes. Clin Nutr ESPEN. 2022;48:291–97. doi:https://doi.org/10.1016/j.clnesp.2022.01.032
Filipovský J, Ducimetière P, Darné B, et al. Abdominal body mass distribution and elevated blood pressure are associated with increased risk of death from cardiovascular diseases and cancer in middle-aged men. The results of a 15- to 20-year follow-up in the Paris prospective study I. Int J Obes Relat Metab Disord. 1993;17(4):197–203.
Marquis K, Debigaré R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):809–13. doi:https://doi.org/10.1164/rccm.2107031
Heitmann BL, Frederiksen P. Thigh circumference and risk of heart disease and premature death: prospective cohort study. Bmj. 2009;339:b3292. doi:https://doi.org/10.1136/bmj.b3292
Otgonsuren M, Stepanova M, Gerber L, et al. Anthropometric and Clinical Factors Associated with Mortality in Subjects with Nonalcoholic Fatty Liver Disease. Digestive Diseases and Sciences. 2013;58(4):1132–40. doi:https://doi.org/10.1007/s10620-012-2446-3
Wu L-W, Lin Y-Y, Kao T-W, et al. Mid-Arm Circumference and All-Cause, Cardiovascular, and Cancer Mortality among Obese and Non-Obese US Adults: the National Health and Nutrition Examination Survey III. Scientific Reports. 2017;7. doi:https://doi.org/10.1038/s41598-017-02663-7
Chen CL, Liu L, Huang JY, et al. Thigh Circumference and Risk of All-Cause, Cardiovascular and Cerebrovascular Mortality: A Cohort Study. Risk Manag Healthc Policy. 2020;13:1977–87. doi:https://doi.org/10.2147/rmhp.S264435
Hirudayakanth K, Gadama L, Dadabhai S, et al. Mid-thigh circumference as an indicator of nutritional status to predict adverse pregnancy outcomes among HIV-infected and HIV-uninfected women in Malawi. Bmc Pregnancy and Childbirth. 2021;21(1). doi:https://doi.org/10.1186/s12884-021-04118-4
Kawamoto R, Kikuchi A, Akase T, et al. Thigh circumference and handgrip strength are significantly associated with all-cause mortality: findings from a study on Japanese community-dwelling persons. Eur Geriatr Med. 2021;12(6):1191–200. doi:https://doi.org/10.1007/s41999-021-00515-2
Rocco MV, Dwyer JT, Larive B, et al. The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: results of the HEMO Study. Kidney Int. 2004;65(6):2321–34. doi:https://doi.org/10.1111/j.1523-1755.2004.00647.x
Albuquerque Silva, Oliveira PFd, Alves da Silva S, et al. Hyperglycemia, clinical evolution and nutritional condition of critically ill patients. Nutricion Clinica Y Dietetica Hospitalaria. 2018;38(2):70–76. doi:https://doi.org/10.12873/382pfrassinette
Cardoso AS, Xavier MO, Dos Santos Costa C, et al. Body mass index and mortality among community-dwelling elderly of Southern Brazil. Prev Med. 2020;139:106173. doi:https://doi.org/10.1016/j.ypmed.2020.106173
Chew STH, Tan NC, Cheong M, et al. Impact of specialized oral nutritional supplement on clinical, nutritional, and functional outcomes: A randomized, placebo-controlled trial in community-dwelling older adults at risk of malnutrition. Clin Nutr. 2021;40(4):1879–92. doi:https://doi.org/10.1016/j.clnu.2020.10.015
Guo J, Shang Y, Fratiglioni L, et al. Individual changes in anthropometric measures after age 60 years: a 15-year longitudinal population-based study. Age and Ageing. 2021;50(5):1666–74. doi:https://doi.org/10.1093/ageing/afab045
Zhang XM, Wu X, Ma Y, et al. Comparing the Performance of Calf Circumference, Albumin, and BMI for Predicting Mortality in Immobile Patients. Risk Manag Healthc Policy. 2021;14:2289–300. doi:https://doi.org/10.2147/rmhp.S311692
Yin L, Lin X, Zhao Z, et al. Is hand grip strength a necessary supportive index in the phenotypic criteria of the GLIM-based diagnosis of malnutrition in patients with cancer? Supportive Care in Cancer. 2021;29(7):4001–13. doi:https://doi.org/10.1007/s00520-020-05975-z
Wijnhoven HA, van Bokhorst-de van der Schueren MA, Heymans MW, et al. Low mid-upper arm circumference, calf circumference, and body mass index and mortality in older persons. J Gerontol A Biol Sci Med Sci. 2010;65(10):1107–14. doi:https://doi.org/10.1093/gerona/glq100
Tsai AC, Chang TL. The effectiveness of BMI, calf circumference and mid-arm circumference in predicting subsequent mortality risk in elderly Taiwanese. Br J Nutr. 2011;105(2):275–81. doi:https://doi.org/10.1017/s0007114510003429
Lin SJ, Hwang SJ, Liu CY, et al. The relationship between nutritional status and physical function, admission frequency, length of hospital stay, and mortality in old people living in long-term care facilities. J Nurs Res. 2012;20(2):110–21. doi:https://doi.org/10.1097/jnr.0b013e318254eac9
Bourdel-Marchasson I, Diallo A, Bellera C, et al. One-Year Mortality in Older Patients with Cancer: Development and External Validation of an MNA-Based Prognostic Score. PLoS One. 2016;11(2):e0148523. doi:https://doi.org/10.1371/journal.pone.0148523
Ho SC, Wang JY, Kuo HP, et al. Mid-arm and calf circumferences are stronger mortality predictors than body mass index for patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2075–80. doi:https://doi.org/10.2147/copd.S107326
Kamiya K, Masuda T, Matsue Y, et al. Prognostic Usefulness of Arm and Calf Circumference in Patients ≥65 Years of Age With Cardiovascular Disease. Am J Cardiol. 2017;119(2):186–91. doi:https://doi.org/10.1016/j.amjcard.2016.09.040
Yajima Y, Kikutani T, Tamura F, et al. Relationship between tongue strength and 1-year life expectancy in elderly people needing nursing care. Odontology. 2017;105(4):477–83. doi:https://doi.org/10.1007/s10266-016-0289-7
Easton JF, Stephens CR, Román-Sicilia H, et al. Anthropometric measurements and mortality in frail older adults. Exp Gerontol. 2018;110:61–66. doi:https://doi.org/10.1016/j.exger.2018.05.011
da Silva JR,Jr., Wiegert EVM, Oliveira L, et al. Different methods for diagnosis of sarcopenia and its association with nutritional status and survival in patients with advanced cancer in palliative care. Nutrition. 2019;60:48–52. doi:https://doi.org/10.1016/j.nut.2018.09.003
Monereo-Muñoz M, Martín-Ponce E, Hernández-Luis R, et al. Prognostic value of muscle mass assessed by DEXA in elderly hospitalized patients. Clin Nutr ESPEN. 2019;32:118–24. doi:https://doi.org/10.1016/j.clnesp.2019.04.001
Rodrigues J, Santin F, Brito F, et al. Nutritional status of older patients on hemodialysis: Which nutritional markers can best predict clinical outcomes? Nutrition. 2019;65:113–19. doi:https://doi.org/10.1016/j.nut.2019.03.002
Aliberti MJR, Szlejf C, Covinsky KE, et al. Prognostic value of a rapid sarcopenia measure in acutely ill older adults. Clin Nutr. 2020;39(7):2114–20. doi:https://doi.org/10.1016/j.clnu.2019.08.026
de Sousa OV, Mendes J, Amaral TF. Nutritional and Functional Indicators and Their Association With Mortality Among Older Adults With Alzheimer’s Disease. Am J Alzheimers Dis Other Demen. 2020;35:1533317520907168. doi:https://doi.org/10.1177/1533317520907168
Tarnowski M, Stein E, Marcadenti A, et al. Calf Circumference Is a Good Predictor of Longer Hospital Stay and Nutritional Risk in Emergency Patients: A Prospective Cohort Study. J Am Coll Nutr. 2020;39(7):645–49. doi:https://doi.org/10.1080/07315724.2020.1723452
Valmorbida E, Trevisan C, Imoscopi A, et al. Malnutrition is associated with increased risk of hospital admission and death in the first 18 months of institutionalization. Clin Nutr. 2020;39(12):3687–94. doi:https://doi.org/10.1016/j.clnu.2020.03.029
Cao L, Morley JE. Sarcopenia Is Recognized as an Independent Condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. J Am Med Dir Assoc. 2016;17(8):675–77. doi:https://doi.org/10.1016/j.jamda.2016.06.001
Kim S, Kim M, Lee Y, et al. Calf Circumference as a Simple Screening Marker for Diagnosing Sarcopenia in Older Korean Adults: the Korean Frailty and Aging Cohort Study (KFACS). J Korean Med Sci. 2018;33(20):e151. doi:https://doi.org/10.3346/jkms.2018.33.e151
Wang L, Yin L, Zhao Y, et al. Muscle Density, but Not Size, Correlates Well With Muscle Strength and Physical Performance. J Am Med Dir Assoc. 2020. doi:https://doi.org/10.1016/j.jamda.2020.06.052
Wang L, Yin L, Zhao Y, et al. Muscle density discriminates hip fracture better than computed tomography X-ray absorptiometry hip areal bone mineral density. J Cachexia Sarcopenia Muscle. 2020. doi:https://doi.org/10.1002/jcsm.12616
Yin L, Xu Z, Wang L, et al. Associations of Muscle Size and Density With Proximal Femur Bone in a Community Dwelling Older Population. Front Endocrinol (Lausanne). 2020;11:503. doi:https://doi.org/10.3389/fendo.2020.00503
Cooper C, Fielding R, Visser M, et al. Tools in the assessment of sarcopenia. Calcif Tissue Int. 2013;93(3):201–10. doi:https://doi.org/10.1007/s00223-013-9757-z
Mijnarends DM, Meijers JM, Halfens RJ, et al. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: a systematic review. J Am Med Dir Assoc. 2013;14(3):170–78. doi:https://doi.org/10.1016/j.jamda.2012.10.009
Shih R, Wang Z, Heo M, et al. Lower limb skeletal muscle mass: development of dual-energy X-ray absorptiometry prediction model. J Appl Physiol (1985). 2000;89(4):1380–86. doi:https://doi.org/10.1152/jappl.2000.89.4.1380
Wang L, Yin L, Cheng X, et al. The association of calcium intake with osteoporotic vertebral fractures in a large Chinese cohort. Aging (Albany NY). 2020;12(6):5500–15. doi:https://doi.org/10.18632/aging.102974
Kawakami R, Miyachi M, Sawada SS, et al. Cut-offs for calf circumference as a screening tool for low muscle mass: WASEDA’S Health Study. Geriatr Gerontol Int. 2020;20(10):943–50. doi:https://doi.org/10.1111/ggi.14025
Hsiang CH, Wu CJ, Kao TW, et al. Calf circumference and risk of cardiovascular disease. Geriatr Gerontol Int. 2020. doi:https://doi.org/10.1111/ggi.14052
Gonzalez MC, Mehrnezhad A, Razaviarab N, et al. Calf circumference: cutoff values from the NHANES 1999–2006. Am J Clin Nutr. 2021;113(6):1679–87. doi:https://doi.org/10.1093/ajcn/nqab029
Lim WS, Lim JP, Chew J, et al. Calf Circumference as a Case-Finding Tool for Sarcopenia: Influence of Obesity on Diagnostic Performance. J Am Med Dir Assoc. 2020;21(9):1359–61. doi:https://doi.org/10.1016/j.jamda.2020.03.033
Asai C, Akao K, Adachi T, et al. Maximal calf circumference reflects calf muscle mass measured using magnetic resonance imaging. Arch Gerontol Geriatr. 2019;83:175–78. doi:https://doi.org/10.1016/j.archger.2019.04.01
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest: No.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, X., Lang, X., Peng, S. et al. Calf Circumference and All-Cause Mortality: A Systematic Review and Meta-Analysis Based on Trend Estimation Approaches. J Nutr Health Aging 26, 826–838 (2022). https://doi.org/10.1007/s12603-022-1838-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-022-1838-0