Abstract
Objectives
We aimed to examine the association of muscle evaluation, including muscle ultrasound, with hospital-associated disability (HAD), focusing on ADL categories.
Design
A prospective observational cohort study.
Setting and Participants
We recruited patients aged 65 years or older who were admitted to the geriatric ward of an acute hospital between October 2019 and September 2021.
Measurements
Handgrip strength, bioimpedance analyzer-determined skeletal muscle mass, bilateral thigh muscle thickness (BATT), and the echo intensity of the rectus femoris on muscle ultrasound were performed as muscle assessments. HAD was evaluated separately for mobility impairments and self-care impairments.
Results
In total, 256 individuals (mean age, 85.2 years; male sex, 41.8%) were analyzed. HAD in mobility was more common than HAD in self-care (37.5% vs. 30.0%). Only BATT was independently associated with HAD in mobility in multiple logistic regression analysis. There was no significant association between muscle indicators and HAD in self-care.
Conclusion
A lower BATT was associated with a higher prevalence of HAD in mobility, suggesting the need to reconsider muscle assessment methods in hospitalized older adults. In addition, approaches other than physical may be required, such as psychosocial and environmental interventions to improve HAD in self-care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional decline is common in older adults, and a strategy for preventing an activity of daily living (ADL) decline is required. In the International Classification of Functioning Disability and Health (ICF) framework established by the World Health Organization (WHO) in 2001, ADLs are also considered a significant part of the “activities and participation” component (1). The evaluation of ADLs is important for independent daily living in older adults living in the community.
An ADL decline due to hospitalization is often referred to as hospital-associated disability (HAD). HAD is commonly defined as the new loss of one or more elements of basic ADLs (2). A recent meta-analysis reported a 30% prevalence rate for HAD (3), and this rate has not changed in the last three decades (4). Risk factors for HAD include multiple domains, such as background factors, acute illness, and factors during hospitalization (2,5). For example, the reported risk factors for HAD include age, mobility, cognitive function, ADL and instrumental ADL (IADL) levels, comorbidities, geriatric syndromes, social factors, depression, malnutrition, polypharmacy, and illness severity. Older hospitalized patients are frailer and share multiple risk factors that would heighten the risk of HAD. HAD is associated with poor prognosis after discharge, including increased mortality, a non-return to pre-illness functional levels (6), an increased readmission rate (7), and institutionalization (8). Therefore, prevention and early intervention of HAD in hospitalized older adults is an urgent clinical task.
In recent years, a relationship between sarcopenia and HAD has also been indicated. Sarcopenia is defined as a progressive skeletal muscle disorder involving decreased muscle mass, muscle strength, and physical function (9). Low handgrip strength at acute hospitalization is associated with ADL dependency (10) and is a risk factor for newly developed ADL disability after discharge (11). Therefore, evaluation of muscle strength, muscle mass, and physical function in hospitalized older adults could be important for preventing HAD. However, it often has some limitations. Muscle strength can be restricted or underestimated by acute illness or comorbidities such as paralysis or cognitive dysfunction. Muscle mass is commonly assessed by dual-energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA), but these modalities are expensive, involve radiation exposure, and can be affected by hydration status. Moreover, the evaluation of physical function in hospitalized older adults is often restricted to bedridden individuals due to acute illness or comorbidity.
Muscle ultrasound of the quadriceps femoris has recently been found useful for evaluating muscle morphology and muscle quality (12). The muscle thickness of the quadriceps femoris shows strong correlations with muscle mass (13), and echo intensity (EI) is an indicator of skeletal muscle quality (14). Muscle ultrasound has been performed in clinical practice to diagnose sarcopenia (15) and to predict mortality (16), and worse recovery of ADLs (17). We have also previously reported that higher corrected EI of the quadriceps femoris was associated with hospital-associated complication (18), and also reported that the thigh muscle thickness tended to be associated with mortality within 3 months after discharge (19).
It would be meaningful to explore the relationship between muscle evaluation, including muscle ultrasound and HAD, but in clinical practice, it may be more useful to classify ADLs by category because changes in ADL during hospitalization are not uniform, and management needs to be changed accordingly. In particular, mobility (ICF chapter: d4) and self-care (ICF chapter: d5) are considered to be key points of ADL assessment by WHO (1). The former comprises four subdomains — changing and maintaining body position; carrying, moving, and handling objects; walking and moving; and moving around using transportation, while the latter comprises seven subdomains—washing oneself; caring for body parts; toileting; dressing; eating; drinking; and looking after one’s health. The classification of ADLs in hospitalized older adults can be used to set goals during hospitalization and to improve quality of daily life after discharge.
Therefore, in the present study of acute hospitalized older adults, we examined the association of muscle evaluation, including muscle ultrasound, with ADL categories. The hypothesis was that muscle thickness and EI would both be related to HAD but that the relationship would differ by ADL categories.
Materials and Methods
Setting and participants
We used data from a prospective observational cohort study conducted in a geriatric ward of an acute hospital, which was very similar to ACE unit (20). Written informed consent was obtained from all participants. If participants were unable to provide consent, family members provided consent on their behalf. The study was approved by the Ethics Committee of Nagoya University Graduate School of Medicine (approval number 2019-0260) and conducted in accordance with the provisions of the Declaration of Helsinki and its later amendments.
We recruited patients aged 65 years or older who were admitted to the geriatric ward of Nagoya University Hospital between October 2019 and September 2021. Participants were excluded if (1) they were discharged within 48 h; (2) they or their family members did not provide written informed consent; (3) their estimated life expectancy was within 1 month, as determined by their attending physician; (4) they were readmitted within 3 months after discharge and were enrolled at the time of their previous admission; (5) they were transferred from other departments; and (6) there was any other reason for the patient’s participation to be reconsidered.
Data collection
Data were first registered in the medical charts within 48 h and also at discharge.
Data collection at admission
Background data were obtained from clinical records, including age, sex, type of admission (emergency or planned), residence before this hospitalization, height, weight, and body mass index (BMI). The attending geriatrician conducted a comprehensive geriatric assessment to determine the cognitive, functional, and nutritional status of each participant. Cognitive function was assessed using the Mini-Mental State Examination (MMSE), which is scored from 0 to 30, with a lower score indicating poorer cognitive status (21). The degree of depressive condition was assessed by the Geriatric Depression Scale-15 (GDS-15), which is scored from 0 to 15, with a higher score indicating more depressed (22). A cutoff value of 6 or higher was considered to indicate depressive symptoms (23). Basic ADLs at baseline (2 weeks before admission) were assessed using the Barthel Index (BI) (24). The BI comprises 10 items (eating, transfers, grooming, toilet use, bathing, walking, stairs, dressing, bowels, and bladder) and is scored from 0 to 100, with a lower score indicating greater dependence. IADLs were assessed using the Lawton and Brody scale, which is scored from 0 to 8, with a lower score indicating greater dependence (25). Nutritional status was assessed using the Mini-Nutritional Assessment-Short Form (MNA-SF), which is scored from 0 to 14, with a lower score indicating poorer nutritional status (26). Comorbidity was evaluated using the Charlson Comorbidity Index (CCI) (27).
Muscle ultrasound
Muscle ultrasound was performed within the first 7 days of admission by the same physician. The procedure was as described previously (18). A B-mode ultrasound system (GE LOGIQ e; GE Healthcare Japan, Tokyo, Japan) with a 5–10 MHz linear-array probe was used. The ultrasound settings were as follows: frequency, 8 MHz; gain, 70 dB; depth, 4.0–6.0 cm; and focus point 1 (top of the image). The depth was unchanged during the measurements of the same participants. The participants were instructed to lie in the supine position, and a sufficient amount of water-soluble transmission gel was applied to the skin to achieve acoustic coupling. Images of the rectus femoris (RF) and vastus intermedius (VI) were obtained at the midpoint between the greater trochanter and proximal border of the patella on both lower limbs. Three images of the quadriceps in each lower limb were taken perpendicularly to the femur bone in the transverse plane, and the mean muscle thickness and subcutaneous fat thickness were obtained. Bilateral thigh muscle thickness (BATT) was defined as the sum of the muscle thickness (right RF + right VI + left RF + left VI) (28). The EI of the RF was measured with ImageJ software, version 1.52k (National Institutes of Health, Bethesda, MD). EI was determined by 8-bit gray scale analysis and is expressed as arbitrary units (a.u.) in the range of 0–255. The EI of the RF was measured in the largest possible rectangular region of interest, avoiding the visible fascia. These methods for measuring BATT and the EI of the RF had high reliability (interclass correlation coefficients [1.1] = 0.995 [0.994–0.996] for BATT and 0.989 [0.986–0.991] for the EI of the RF). Because the EI of the RF is attenuated by the subcutaneous fat thickness, the corrected EI of the RF was also calculated by the following formula: corrected EI = EI + 40.5278 × subcutaneous fat thickness (cm) (29).
Other muscle assessments
Handgrip strength and bioimpedance analyzer-determined skeletal muscle mass were also measured for comparison with muscle ultrasound.
Handgrip strength was measured by a Jamar-type handheld dynamometer (Baseline Hydraulic Hand Dynamometer, Fabrication Enterprises Inc., Elmsford, NY). Two trials were taken with each hand, and the maximum value was recorded. The measurement was taken with the elbows fixed at 90° in the sitting position but, when the participant struggled to achieve the sitting position, it was taken in the supine position. Skeletal muscle mass (SMM) was measured by a portable bioimpedance analyzer (InBody S10; InBody Co., Ltd., Tokyo, Japan), and the skeletal muscle index (SMI) was calculated by dividing SMM by height squared (kg/m2).
Data collection at discharge
Discharge destination (including in-hospital death and transfer to another department), length of hospital stay, and the BI were obtained from medical records.
HAD
The BI was bi-classified into mobility and self-care categories through the application of the ICF[30]. BI (mobility) includes transfers, walking, and stairs (total score, 0–40), whereas BI (self-care) includes eating, grooming, toilet use, bathing, dressing, bowels, and bladder (total score, 0–60). In this study, HAD was evaluated separately for mobility impairments (HAD in mobility) and self-care impairments (HAD in self-care). A previous review using the BI determined that the minimal amount of functional decline was 10% (31). Therefore, in the present study, HAD in mobility and HAD in self-care were defined as a >10% decrease in the BI score at discharge compared with baseline (2 weeks before admission).
Statistical analysis
All statistical analyses were conducted using SPSS software, version 28 (IBM Corp., Armonk, NY). Continuous variables are reported as the mean ± standard deviation or the median (interquartile range), whereas categorical variables are reported as absolute numbers and percentages. BI (mobility) and BI (self-care) were compared between admission and discharge and the prevalence of HAD was calculated. Student’s t-test or Mann—Whitney U test was used to compare muscle indicators (handgrip strength, SMI, BATT, EI, and corrected EI) in two groups (with HAD and without HAD). Multiple logistic regression analysis was conducted to clarify muscle indicators that were independently associated with HAD after adjustment for potential confounders. The confounding factors were age and sex in Model 1, age, sex, MMSE, CCI, and MNA-SF in Model 2, and age, sex, MMSE, CCI, MNA-SF, BI at admission, IADLs, and GDS-15 in Model 3. Spearman’s correlation coefficient was used to examine the relationships between muscle indicators and other related parameters. It was also used to examine the relationships between these related parameters and HAD by Student’s t-test or Mann—Whitney U test (for continuous variables) and χ2 test (for categorical variables). A P-value less than 0.05 was considered statistically significant in all comparisons.
Results
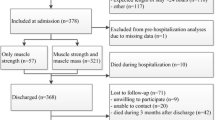
The number of participants was 256, after excluding cases of in-hospital death (n=20), transfer to another department (n=6), and a missing value of the BI (n=18). The median length of hospital stay was 17 (11–28).
Table 1 shows the background characteristics of the participants at admission. The mean age was 85.2 ± 5.9 years, the percentage of men was 41.8%, the median MMSE value was 21 (13–26), the median CCI value was 2 (1–3), the mean MNA-SF was 8.6 ± 3.4, and the median BI was 85 (51.3–100).
Table 2 shows the changes in the BI score (the difference from baseline to discharge) and the prevalence of HAD. The median BI (mobility) and BI (self-care) at baseline were 35 and 55, respectively, and were lower at discharge. HAD in mobility was more common than HAD in self-care (37.5% vs. 30.0%). On the other hand, in 19.9% of cases, the BI score at discharge was higher than at baseline.
Table 3 shows the values of muscle indicators in the two groups (with and without HAD). Handgrip strength was lower in both the HAD in mobility and HAD in self-care groups than in the groups without HAD. BATT was lower only in the HAD in mobility group. In contrast, SMI, EI, and corrected EI were not significantly different between the two groups.
Table 4 illustrates the results of multiple logistic regression analysis conducted to clarify muscle indicators that were independently associated with HAD in mobility. BATT [odds ratio 0.57, 95% confidence interval 0.36–0.89, P=0.013] was independently associated with HAD in mobility in Model 3, whereas handgrip strength, SMI, EI, and corrected EI were not.
Table 5 shows the results of multiple logistic regression analysis to clarify the muscle indicators that were independently associated with HAD in self-care. No significant associations of HAD in self-care were seen for all muscle indicators, but especially in Models 2 and 3.
Supplementary Table 1 details the results of correlations among muscle indicators and related parameters. Handgrip strength was significantly related to age, MMSE, MNA-SF, BI at baseline, and IADLs.
Table 6 shows the values of related parameters compared in groups with and without HAD. Both HAD groups showed a higher age, lower MMSE, and lower IADLs. The prevalence of depressive symptoms was higher in HAD in mobility, whereas the MNA-SF and BI were lower in HAD in self-care.
Discussion
In this study, we classified ADL declines during hospitalization into HAD in mobility and HAD in self-care and examined the association with muscle indicators. To our knowledge, this is the first study to classify HAD into mobility and self-care categories and to investigate their association with muscle indicators. Our results indicated that HAD in mobility was more common than HAD in self-care. In addition, a lower BATT was significantly associated with a higher prevalence of HAD in mobility, unlike handgrip strength and EI. Regarding HAD in self-care, no significant associations were found with muscle indicators.
With regards to the association between muscle mass and ADLs, a meta-analysis reported that a low muscle mass was associated with worsening ADLs in community-dwelling older adults (32), while the Position Statements of the Sarcopenia Definition and Outcomes Consortium (SDSC) concluded that lean muscle mass measured by DXA was not a good predictor of adverse health-related outcomes, including an ADL decline (33). A recent longitudinal study evaluating the annual assessment of ADLs in individuals who experienced hospitalization showed that pre-hospital muscle mass on DXA was not associated with new ADL disabilities at follow-up (11). Moreover, in a recent systematic review including inpatients, most longitudinal studies reported that muscle mass was not associated with ADL scores (34). In the present study of muscle mass assessment, a BIA-based muscle mass indicator (i.e., SMI) was not associated with HAD in mobility, unlike an ultrasound-based muscle mass indicator (i.e., BATT) (Table 4).
The following reasons might explain why the association between muscle mass and HAD in this study differed from that of previous studies. First, there are differences in the evaluation of ADLs. In contrast with the present study, previous studies used the BI as the entire ADL assessment or just a part of the assessment (transferring, bathing, and dressing). In this study, BATT was also associated with HAD in mobility and not associated with HAD in self-care. That may suggest improving muscle mass of lower limbs is essential for prevention of HAD in mobility, which is more closely associated with physical functional decline. BATT could prevent HAD in mobility, which reflects physical function rather that self-care. Second, previous studies targeted community-dwelling individuals or those in rehabilitation hospitals, whereas the participants in the present study were more frail acute inpatients, which may have affected the results by increasing the muscle changes caused by acute inflammation or disuse. Third, muscle mass evaluation using BIA is regarded as one of the standard methods in clinical settings (35), and many studies have used the SMI as an index of muscle mass, which is calculated from both muscles of the upper and lower limbs. Muscle mass evaluation by ultrasound was also reported to be a reliable and valid method for the assessment of muscle size in older adults (36). The anterior thigh muscles are more prone to muscle loss than other muscles and are more commonly and severely affected in sarcopenia (37). These muscles are fundamental to mobility skills. Therefore, BATT, which could directly evaluate them, may be more suitable for assessing mobility skills than SMI. In addition, the BIA method can be affected by hydration status (38), which may influence the results in the case of inpatients with dehydration or overhydration. Muscle ultrasound is a relatively simple and less invasive measurement method, and it is commonly available in clinical practice. The results of the present study may indicate the need for a reconsideration of the assessment of muscle mass or interventions in hospitalized older adults. However, BATT could also be temporarily increased by inflammation or vascular permeability (39). Thus, this method must be used properly and a cutoff value must be established.
Regarding the association between muscle strength and ADLs in hospitalized older adults, previous studies reported that a low handgrip strength at admission was associated with ADL dependency (10) and was a risk factor for newly developed ADL disability after discharge (11). In fact, in the present study, handgrip strength was associated with HAD in univariate analysis, but not in multivariate analysis. The participants of this study had a higher rate of undernutrition or cognitive decline that was related to low handgrip strength (Supplementary Table 1), thereby weakening the association between handgrip strength and HAD in mobility. Furthermore, handgrip strength could be underestimated due to acute illness, and it does not necessarily reflect lower limb muscle strength (40). A recent study showed that knee extension strength was decreased by 11% during hospitalization, while handgrip strength was unchanged (41). There may be challenges in the use of handgrip strength to assess mobility status in hospitalized older adults.
It has been reported that muscle EI is related to muscle strength in older adults (42), therefore, EI may become an important parameter for understanding the physical condition in older adults. Furthermore, in terms of EI, previous studies among subacute and convalescent rehabilitation wards reported that EI of the quadriceps was independently associated with motor Functional Independence Measure scores and was related to the recovery of ADLs (17,43). In contrast with these results, EI was not associated with HAD in mobility in the present study. This is possibly because muscle quality could not be accurately evaluated by EI in the acute phase. A recent review reported that EI is affected by not only muscle damage, but also water balance or glycogen under acute conditions (14). It has been suggested that muscle intracellular hydration status is related to functional capacity (44) and that the glycogen level within skeletal muscle is related to exercise durability 45). It may be thought that factors other than muscle fibers affected EI and its relationship with HAD in the present study. However, in the intensive care unit, a change in EI was associated with intensive care unit-acquired muscle weakness or mortality (46,47). Further research is required to explore the association between EI and clinical outcomes in various settings, such as home medical care and nursing homes.
In contrast to the results of HAD in mobility, no muscle indicators were associated with HAD in self-care. Self-care is commonly defined as the practice of activities that an individual initiates and carries out in order to maintain life, health, and well-being (48), and HAD in self-care has been associated with prolonged functional recovery and increased mortality (6). A previous study indicated that the risk factors for HAD in self-care were grouped into three main themes: patient factors, healthcare provision, and hospital environment (49). The authors suggested that a fear of falls and nurses’ work overload were barriers to functional self-care, while having a positive mindset and an age-friendly environment were facilitators of functional self-care. Another study reported that patients who received a higher amount of ADL/self-care training through occupational therapy had a lower risk of readmission (50). In the present study as well, these environmental factors appear to have been more closely associated with HAD in self-care than muscle indicators. However, a lower MMSE, MNA-SF, BI at baseline, and IADLs and a higher age were found in HAD in self-care (Table 6). The prevention of HAD in self-care may be required to identify the above risk factors early in acute hospitalization and to conduct multidisciplinary interventions with the involvement, for example, of physicians, nurses, dietitians, occupational therapists, and family members.
This study provides important findings, but some limitations should be considered. First, muscle evaluation by ultrasound was conducted by the seventh day after admission (median interval= second days) because our research was performed after medical treatment. In addition, measurements of handgrip strength and BIA were not always performed on the same day as muscle ultrasound. Muscle changes caused by disuse after hospitalization may thus have affected the results. However, the association between BATT, bioimpedance analyzer-determined skeletal muscle mass and HAD in mobility was not changed when we controlled for the measurement date. Second, rehabilitation during hospitalization might have influenced the results. Rehabilitation exercise to prevent deterioration of physical function may affect HAD, but early rehabilitation is commonly conducted in the acute care setting, and most participants had individually undergone rehabilitation. Third, restrictions on family visits on hospital due to COVID-19 pandemic might affect the results. However, access to physiotherapist and dieticians were not restricted during the hospitalization. Before and after the COVID-19 pandemic, BATT and HAD were unchanged, and not significantly different in this study, The association between BATT and HAD in mobility was also unchanged even after adjusting before and after the COVID-19 pandemic.
Fourth, this study was conducted at a single university hospital. Our findings should be verified at other facilities.
Conclusion
We found that only a lower BATT, not other muscle indicators, was significantly associated with a higher prevalence of HAD in mobility. The results of this study suggest muscle ultrasound is useful for evaluations of older adults in acute care settings. There are several modalities for muscle evaluations, and each one of them has strong points and weakness, and clinicians should know these characteristics of modalities for appropriate evaluations and interpretations of the results. Muscle ultrasound can be considered for muscle evaluation in acute care, and may be used more widely. Physical rehabilitation and a nutritional intervention aimed at improving muscle mass could be emphasized to prevent HAD in mobility. However, no muscle indicators were related to HAD in self-care. Thus, psychosocial and environmental intervention approaches may be required to prevent HAD in self-care, rather than physical training.
References
World Health Organization. The International Classification of Functioning, Disability and Health (ICF). Geneva, Switzerland: World Health Organization; 2001.
Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability “She was probably able to ambulate, but i’m not sure.” JAMA — J Am Med Assoc 2011;306(16):1782–93. doi: https://doi.org/10.1001/jama.2011.1556.
Loyd C, Markland AD, Zhang Y, et al. Prevalence of Hospital-Associated Disability in Older Adults: A Meta-analysis. J Am Med Dir Assoc 2020;21(4):455–461.e5. doi: https://doi.org/10.1016/j.jamda.2019.09.015.
Brown CJ. After Three Decades of Study, Hospital-Associated Disability Remains a Common Problem. J Am Geriatr Soc 2020;68(3):465–6. doi: https://doi.org/10.1111/jgs.16349.
Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-Associated Functional Decline: The Role of Hospitalization Processes Beyond Individual Risk Factors. J Am Geriatr Soc 2015;63(1):55–62. doi: https://doi.org/10.1111/jgs.13193.
Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc 2008;56(12):2171–9. doi: https://doi.org/10.1111/j.1532-5415.2008.02023.x.
Tonkikh O, Shadmi E, Flaks-Manov N, Hoshen M, Balicer RD, Zisberg A. Functional status before and during acute hospitalization and readmission risk identification. J Hosp Med 2016;11(9):636–41. doi: https://doi.org/10.1002/jhm.2595.
Portegijs E, Buurman BM, Essink-Bot ML, Zwinderman AH, de Rooij SE. Failure to Regain Function at 3 months After Acute Hospital Admission Predicts Institutionalization Within 12 Months in Older Patients. J Am Med Dir Assoc 2012;13(6):569.e1–569.e7. doi: https://doi.org/10.1016/j.jamda.2012.04.003.
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–46. doi: https://doi.org/10.1016/S0140-6736(19)31138-9.
Meskers CGM, Reijnierse EM, Numans ST, et al. Association of Handgrip Strength and Muscle Mass with Dependency in (Instrumental) Activities of Daily Living in Hospitalized Older Adults — The Empower Study. J Nutr Heal Aging 2019;23:232–8. doi: https://doi.org/10.1007/s12603-019-1170-5.
Andrews JS, Gold LS, Reed MJ, et al. Appendicular Lean Mass, Grip Strength, and the Development of Hospital-Associated Activities of Daily Living Disability Among Older Adults in the Health ABC Study. Journals Gerontol Ser A 2021;XX:1–7. doi: https://doi.org/10.1093/gerona/glab332.
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. doi: https://doi.org/10.1093/ageing/afy169.
Madden KM, Feldman B, Arishenkoff S, Meneilly GS. A rapid point-of-care ultrasound marker for muscle mass and muscle strength in older adults. Age Ageing 2021;50:505–10. doi: https://doi.org/10.1093/ageing/afaa163.
Stock MS, Thompson BJ. Echo intensity as an indicator of skeletal muscle quality: applications, methodology, and future directions. Eur J Appl Physiol 2021;121:369–80. doi: https://doi.org/10.1007/s00421-020-04556-6.
Fukumoto Y, Ikezoe T, Taniguchi M, et al. Cut-off values for lower limb muscle thickness to detect low muscle mass for sarcopenia in older adults. Clin Interv Aging 2021;16:1215–22. doi: https://doi.org/10.2147/CIA.S304972.
Lee ZY, Ong SP, Ng CC, et al. Association between ultrasound quadriceps muscle status with premorbid functional status and 60-day mortality in mechanically ventilated critically ill patient: A single-center prospective observational study. Clin Nutr 2021;40:1338–47. doi: https://doi.org/10.1016/j.clnu.2020.08.022.
Akazawa N, Kishi M, Hino T, et al. Intramuscular adipose tissue in the quadriceps is more strongly related to recovery of activities of daily living than muscle mass in older inpatients. J Cachexia Sarcopenia Muscle 2021;12:891–9. doi: https://doi.org/10.1002/jcsm.12713.
Nagae M, Umegaki H, Yoshiko A, et al. Echo intensity is more useful in predicting hospital-associated complications than conventional sarcopenia-related parameters in acute hospitalized older patients. Exp Gerontol 2021;150:111397. doi: https://doi.org/10.1016/j.exger.2021.111397.
Nagae M, Umegaki H, Yoshiko A, et al. Muscle changes on muscle ultrasound and adverse outcomes in acute hospitalized older adults. Nutrition 2022 in press.
Palmer R, Landefeld C, Kresevic D, et al. A medical unit for the acute care of the elderly. J Am Geriatr Soc 1994; 42: 545–552. doi: https://doi.org/10.1111/j.1532-5415.1994.tb04978.x.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. doi: https://doi.org/10.1016/0022-3956(75)90026-6.
Almeida OP, Almeida SA. Short versions of the Geriatric Depression Scale: A study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry 1999;14:858–65. doi: https://doi.org/10.1002/(SICI)1099-1166(199910)14:10<858::AID-GPS35>3.0.CO;2-8.
Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: A systematic review of screening instruments. Age Ageing 2012;41:148–54. doi: https://doi.org/10.1093/ageing/afr169.
Mahomey FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J 1965;14:61–5.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86.
Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). Journals Gerontol — Ser A Biol Sci Med Sci 2001;56:366–72. doi: https://doi.org/10.1093/gerona/56.6.M366.
Medical C. a New Method of Classifying Prognostic in Longitudinal Studies: Development. J Chronic Dis 1987;40:373–83.
Wilson D V., Moorey H, Stringer H, et al. Bilateral Anterior Thigh Thickness: A New Diagnostic Tool for the Identification of Low Muscle Mass?. J Am Med Dir Assoc 2019;20:1247–1253.e2. doi: https://doi.org/10.1016/j.jamda.2019.04.005.
Young HJ, Jenkins NT, Zhao Q, Mccully KK. Measurement of intramuscular fat by muscle echo intensity. Muscle and Nerve 2015;52:963–71. doi: https://doi.org/10.1002/mus.24656.
Prodinger B, O’Connor RJ, Stucki G, Tennant A. Establishing score equivalence of the functional independence measure motor scale and the barthel index, utilizing the international classification of functioning, disability and health and rasch measurement theory. J Rehabil Med 2017;49:416–22. doi: https://doi.org/10.2340/16501977-2225.
Buurman BM, Van Munster BC, Korevaar JC, De Haan RJ, De Rooij SE. Variability in measuring (instrumental) activities of daily living functioning and functional decline in hospitalized older medical patients: A systematic review. J Clin Epidemiol 2011;64:619–27. doi: https://doi.org/10.1016/j.jclinepi.2010.07.005.
Wang DXM, Yao J, Zirek Y, Reijnierse EM, Maier AB. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle 2020;11:3–25. doi: https://doi.org/10.1002/jcsm.12502.
Bhasin S, Travison TG, Manini TM, et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc 2020;68:1410–8. doi: https://doi.org/10.1111/jgs.16372.
Lunt E, Ong T, Gordon AL, Greenhaff PL, Gladman JRF. The clinical usefulness of muscle mass and strength measures in older people: A systematic review. Age Ageing 2021;50:88–95. doi: https://doi.org/10.1093/ageing/afaa123.
Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300–307.e2. doi: https://doi.org/10.1016/j.jamda.2019.12.012.
Nijholt W, Scafoglieri A, Jager-Wittenaar H, Hobbelen JSM, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 2017;8:702–12. doi: https://doi.org/10.1002/jcsm.12210.
Kara M, Kaymak B, Frontera WR, et al. Diagnosing sarcopenia: Functional perspectives and a new algorithm from ISarcoPRM. J Rehabil Med 2021;53. doi: https://doi.org/10.2340/16501977-2851.
Ceniccola GD, Castro MG, Piovacari SMF, et al. Current technologies in body composition assessment: advantages and disadvantages. Nutrition 2019;62:25–31. doi: https://doi.org/10.1016/j.nut.2018.11.028.
Welch C, Greig CA, Hassan-Smith ZK, Pinkney TD, Lord JM, Jackson TA. A pilot observational study measuring acute sarcopenia in older colorectal surgery patients. BMC Res Notes 2019;12:1–7. doi: https://doi.org/10.1186/s13104-019-4049-y.
Phillipe de Lucena Alves C, Câmara M, Dantas Macêdo GA, et al. Agreement between upper and lower limb measures to identify older adults with low skeletal muscle strength, muscle mass and muscle quality. PLoS One 2022;17:e0262732. doi: https://doi.org/10.1371/journal.pone.0262732.
Hartley P, Romero-Ortuno R, Wellwood I, Deaton C. Changes in muscle strength and physical function in older patients during and after hospitalisation: A prospective repeated-measures cohort study. Age Ageing 2021;50:153–60. doi: https://doi.org/10.1093/ageing/afaa103.
Fukumoto Y, Ikezoe T, Yamada Y, et al. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol 2012;112:1519–25. doi: https://doi.org/10.1007/s00421-011-2099-5.
Akazawa N, Kishi M, Hino T, Tsuji R, Tamura K, Moriyama H. Increased intramuscular adipose tissue of the quadriceps is more strongly related to declines in ADL than is loss of muscle mass in older inpatients. Clin Nutr 2021;40:1381–7. doi: https://doi.org/10.1016/j.clnu.2020.08.029.
Lorenzo I, Serra-Prat M, Carlos Yébenes J. The role of water homeostasis in muscle function and frailty: A review. Nutrients 2019;11:1–15. doi: https://doi.org/10.3390/nu11081857.
Schweitzer GG, Kearney ML, Mittendorfer B. Muscle glycogen: where did you come from, where did you go? J Physiol 2017;595:2771–2. doi: https://doi.org/10.1113/JP273536.
Mayer KP, Thompson Bastin ML, Montgomery-Yates AA, et al. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit Care 2020;24:1–12. doi: https://doi.org/10.1186/s13054-020-03355-x.
Umbrello M, Guglielmetti L, Formenti P, et al. Qualitative and Quantitative Muscle Ultrasound Changes in Covid-19 Related Ards Patients. Nutrition 2021;91–92:111449. doi: https://doi.org/10.1016/j.nut.2021.111449.
Orem D, Taylor S, Renpenning K. Nursing: Concepts of practice, 6th edn. St. Louis: Mosby, 2001.
Chan EY, Samsudin SA, Lim YJ. Older patients’ perception of engagement in functional self-care during hospitalization: A qualitative study. Geriatr Nurs (Minneap) 2020;41:297–304. doi: https://doi.org/10.1016/j.gerinurse.2019.11.009.
Edelstein J, Walker R, Middleton A, Reistetter T, Gary KW, Reynolds S. Higher Frequency of Acute Occupational Therapy Services Is Associated With Reduced Hospital Readmissions. Am J Occup Ther 2022;76:1–9. doi: https://doi.org/10.5014/ajot.2022.048678.
Acknowledgements
We thank the participants and staff members who helped with the current study.
Funding
Funding statement: This work was supported by JSPS KAKENHI (grant numbers JP21H02826). The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Contributions
Author contributions: Masaaki Nagae: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing-original draft preparation. Hiroyuki Umegaki: Writing-review and editing, Supervision, Funding acquisition. Akito Yoshiko: Writing-review and editing. Kosuke Fujita: Validation. Hitoshi Komiya: Project administration. Kazuhisa Watanabe: Software. Yosuke Yamada: Visualization. Tomomichi Sakai: Resources.
Corresponding author
Ethics declarations
Conflict of interest: The authors declare there are no conflicts of interest.
Ethical statement: The study was approved by the Ethics Committee of Nagoya University Graduate School of Medicine (approval number 2019-0260) and conducted in accordance with the provisions of the Declaration of Helsinki and its later amendments.
Electronic supplementary material
Supplementary Table 1:
Spearman’s correlation coefficient between muscle indicators and related parameters
Rights and permissions
About this article
Cite this article
Nagae, M., Umegaki, H., Yoshiko, A. et al. Muscle Evaluation and Hospital-Associated Disability in Acute Hospitalized Older Adults. J Nutr Health Aging 26, 681–687 (2022). https://doi.org/10.1007/s12603-022-1814-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-022-1814-8