Abstract
Objective
To evaluate whether fortification of yogurts with vitamin D and calcium exerts an additional lowering effect on serum parathyroid hormone (PTH) and bone resorption markers (BRM) as compared to iso-caloric and iso-protein dairy products in aged white women at risk of fragility fractures.
Design
A randomized double-blind controlled trial.
Setting
A community dwelling home.
Participants
Forty-eight women over 60 years (mean age 73.4).
Intervention
Consumption during 84 days of two 125 g servings of either vitamin D and calcium-fortified yogurts (FY) at supplemental levels of 10 µg vitamin D3/d and 520 mg/d of calcium (total=800 mg/d), or non fortified control yogurts (CY) providing 280 mg/d of calcium.
Measurements
Serum changes from baseline (D0) to D28, D56 and D84 in 25OHD, PTH and in two BRM: Tartrate-resistant-acid-phosphatase-isoform-5b (TRAP5b) and carboxy-terminal-cross-linked-telopeptide of type-I-collagen (CTX).
Results
The 10 years risk of major and hip fractures were 13.1 and 5.0%, and 12.9 and 4.2 %, in FY and CY groups, respectively. From D0 to D84, serum 25OHD increased (mean±SE) from 34.3±2.4 to 56.3±2.4 nmol/L in FY (n=24) and from 35.0±2.5 to 41.3±3.0 nmol/L in CY (n=24), (P=0.00001). The corresponding changes in PTH were from 64.1±5.1 to 47.4±3.8 ng/L in FY and from 63.5±4.6 to 60.7±4.2 ng/L in CY (P=0.0011). After D84, TRAP5b was reduced significantly (P=0.0228) and CTX fell though not significantly (P=0.0773) in FY compared to CY.
Conclusion
This trial in aged white women living in a community dwelling home at risk for osteoporotic fractures confirms that fortification of dairy products with vitamin D3 and calcium should provide a greater prevention of secondary hyperparathyroidism and accelerated bone resorption as compared to non-fortified equivalent foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D, through its active metabolite 1,25-dihydroxyvitamin D (1,25(OH)2D), plays an essential role for translocating dietary Ca across the intestinal epithelium, thereby raising the extracellular Ca-Pi product to the level required for the adequate mineralization of the bone organic matrix. This physiological link explains why adequate supplies of both vitamin D and Ca are required to support bone health throughout life (1). When their supply is insufficient, homeostatic mechanisms operate to prevent hypocalcaemia by both stimulating the production and secretion of parathyroid hormone (PTH) which, in turn, enhances both the renal conservation and the bone resorption of Ca. Thus, inadequacy in the vitamin D supply, as expressed by a decreased serum 25-hydroxyvitamin D (25OHD), leads to secondary hyperparathyroidism (1), and elevated serum markers of bone resorption (2). The serum biochemical pattern encompassing low 25OHD, high PTH, elevated CTX and TRAP5b characterizes the insufficient supply of vitamin D associated with low Ca intake, as often observed with aging (3). Increasing the supply of both vitamin D and Ca tends to correct these biochemical anomalies within a few weeks or months (3). The long-term impact of such correction is an attenuation of bone loss, a reduced risk of falling and fragility fractures (4–7).
Intervention with pharmacological agents documented that early measurements in serum bone resorption markers predict the rate of bone loss (8) and the incidence of fragility fractures (9). As recently reviewed (10), the predictive value of early change in bone biochemical markers is of particular interest for clinical research in nutrition. Indeed, studies comparing the effects of different foods on bone structural component outcomes, let alone on fragility fractures, are difficult or even impossible to conduct in randomized placebo-controlled trials of long duration (11).
long duration (11). Short-term evaluation is particularly appropriate for assessing the selective effects of food fortification on both skeleton trophic factors and biochemical bone turnover markers (BTMs). Such an assessment implies the comparison of the response to foods that only differ by the addition or not of the tested nutrient(s). This clinical investigation approach can be considered as lying within the conceptual scope of “Comparative Effectiveness Research (CER)” (12). So far, few studies have applied a CER design in the field of clinical nutrition, such as that comparing the effects of two plant-source forms of calcium supplements on bone mineral density (13).
In conformity with the CER concept, we recently published a randomized controlled trial in which consumption of vitamin D and Ca fortified yogurts, as compared to non fortified equivalent dairy products, exerted a greater positive impact on bone trophic factors and BTMs after 8 weeks (3). This randomized controlled trial was carried out in institutionalized elderly women (mean age: 86 years) with quite a low baseline vitamin D status, as documented by a serum level of 25OHD of 18 nmol/L (3). This concentration is well below the level of 50 nmol/L, considered by the Institute of Medicine (IOM) as meeting the requirements of 97.5 % of the North-American population (14), let alone the much higher level of 75 nmol/L estimated as adequate in the Endocrine Society Clinical Practice Guideline (15).
Therefore, it remained to be documented that the same fortified dairy product could also favourably affect skeletal trophic factors and BTMs in women with a vitamin D insufficiency other than the deficiency documented in elderly women living in French nursing homes (3). This hypothesis was tested in a 12 week randomized controlled trial enrolling women over 60 (mean age: ∼ 74 years) living in a community home in England. As compared to the previous study (3), the new cohort displayed a baseline 25(OH)D serum level about twice (∼ 34 nmol/L) as high than that recorded in older French institutionalized women. Despite these substantial differences in age, environmental living conditions and baseline vitamin D status, the results presented here confirm the gain in efficacy of the tested dairy product on bone related factors.
Subjects and methods
Subjects
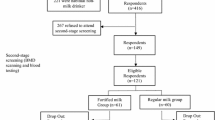
A total of 96 women living in a sheltered accommodation housing in Hull (England) were screened for eligibility. The recruitment for eligibility started after the Research and Development review board of the Hull & East Yorkshire Hospital NHS Trust approved the protocol on April 23, 2012: “Permission for Research R1307”. One single investigator conducted the study, in accordance to the Helsinki Declaration, the Good Clinical Practices (GCPs) and in compliance with English national regulations. Of this initial group, 28 subjects did not fulfill the serum 25OHD and PTH inclusion criteria; 10 subjects withdrew consent and 1 died. Thus, 57 women responded to the inclusion criteria: aged >60 years, with a prior medical check-up suitable for research purposes, serum levels of 25OHD ≤ 20 ng/mL and PTH <150 ng/mL. They were enrolled between May 29, 2012 and July 18, 2012, all having given their informed consent. The age ranged from 61 to 90 years, with mean (±SD) age 73.4 ± 7.5 years (median: 73.4 years).
Exclusion criteria
Exclusion criteria included concomitant diseases or any illness affecting Ca-Pi metabolism such as primary hyperparathyroidism; osteoporotic fracture during the year preceding the study; chronic gastrointestinal disease, chronic renal failure, hepatic and cardiac failure or cancer. Treatment during the last 6 months for osteoporosis or other bone diseases, including pharmaceutical agents such as: bisphosphonates, raloxifen, teriparatide, strontium ranelate and denosumab. Glucocorticoid therapy. Lactose intolerance. Participation in a clinical trial during the 3 months preceding the entry into the study. Confinement to bed.
Design and conducted trial.
It was a monocentric randomized double-blind comparative study conducted in two parallel groups, in which subjects were allocated to consume daily 2 yogurts of 125 g each, providing either 10 μg (400 IU) of vitamin D3 and 800 mg of elemental Ca (Fortified Yogurt = FY) or 0 μg of supplemental vitamin D3 and 280 mg of elemental Ca (Control Yogurt = CY) during 84 days. Before the fortification process, both FY and CY contained 140 mg of milk Ca per serving. To fortify one type of yogurt (FY) vitamin D3 was added in powder form and Ca (+260 mg/serving) as citrate salt. Both vitamin D3 and Ca citrate were mixed with fruit (strawberry, cherry or apricot) preparation used as flavoring components to the two types (FY and CY) of yogurts. Yoplait France provided the yogurts.
A random number table was used with blocks of four to determine the allocation sequence. The random allocation was centralized, computer-generated, and stratified by the investigating center. The two types of yogurts were presented in identical packs. In order to insure the blinding, they were designated with two code letters (A and B) and the code only broken after the statistical analysis had been fully completed. The two servings of 125 g of either type of yogurts daily provided similar amounts of proteins (FY: 7.8 g; CY: 8.0 g), energy (FY: 232 kcal; CY: 248 kcal), lipids (FY: 6.2 g; CY: 6.5 g) and carbohydrates (FY: 33 g; CY: 36 g). They were recommended to be consumed one at lunchtime, the other at dinner.
From the 57 enrolled subjects (CY: 29; FY: 28), 6 (3 in each group) left the study without any follow-up evaluation carried out. From the 51 participants (CY: 26; FY: 25), 3 had to be excluded because of some major deviation from the protocol due to the introduction of either osteoporosis treatment or vitamin D supplementation (CY: 2; FY: 1). Thus, in 48 subjects (24 in each group), serum analysis of 25OHD, PTH, CTX and TRAP5b were measured at all time points (D0, D28, D56 and D84) enabling the assessment of the changes in these 4 biochemical variables from the onset to the end of the intervention.
Compliance and Satisfaction Evaluation
A compliance and satisfaction form on the yogurt consumption was completed daily by each of the participants, and monthly by the dieticians and nurses of the sheltered accommodation center. The questionnaire included a question concerning how much yogurt was consumed and reason for any incomplete intake. At D28, D56 and D84, four questions on the satisfaction of consuming either type of yogurts were asked in relation to: flavor; pot size adapted to appetite; limitations of intake at both lunch and dinner; lack of interest in maintaining intake.
Clinical assessment
Fracture risk evaluation. The 10 year risk of hip and major osteoporotic fracture of each participant was computed by using the probability model FRAX® (16) calibrated to the United Kingdom population. The prediction of fracture with the use of clinical risk factors alone in FRAX, i.e. without a DXA test, is comparable to the BMD measurement alone (17).
Dietary survey at baseline and during the intervention. Ca, proteins and energy intakes were assessed at baseline (D0) and at the end of the intervention (D84). Food consumption was recorded by 3-day dietary diaries (18).
Sun exposure. The participants were asked to report if, at any time, they spent more than 20 minutes daily with uncovered arms exposed to the sun.
Biochemical analysis
The blood samples were collected in the morning after an overnight fast and stored at -70o C until analysis. Serum Ca and inorganic phosphate (Pi) were measured by colorimetry (Roche Diagnostics, Rotkreuz, Switzerland). Serum 25OHD, PTH, CTX and TRAP5b, were measured by enzyme-linked immunosorbent assay (ELISA) on the Bio-Rad Microtech Microplate Reader. The following immunoassay kits were used: 25OHD, CTX and TRAP5b (Immunodiagnostics System, Fountain Hills, AZ, USA); PTH (Human Bioactive PTH Elisa kit, Immunotopics, San Clemente, CA, USA). The intra- assay and inter-assay variations including the variations for TRAP5b were less than 6.0 and 8.0%, respectively. Analytical measurements were made in duplicates for PTH, CTX and TRAP5b.
Statistical analysis
The main aim of the trial was to demonstrate that consumption of fortified as compared to non-fortified yogurts exerted more favorable effects on vitamin D status (increased serum 25OHD), secondary hyperparathyroidism (decreased serum PTH) and decreased BRMs (serum CTX and TRAP5b). Determination of the sample size was estimated from the effects on changes in serum TRAP5b. It was expected that a difference of 10 % in serum TRAP5b changes would be detected between the two groups with a power of 80 % and two-sided alpha of 0.05. Such a 10 % difference in change in TRAP5b required a sample of 15 participants per group, taking into account a standard deviation of inter-individual difference of 9.3% (19). 29 and 28 subjects in CY and FY groups, respectively, were eventually enrolled into the study and randomized. The data are expressed as mean ± SE. Differences between quantitative data measured at D0 and changes from D0 to D84 were evaluated by the Mann-Whitney U test or Student t test to determine whether or not the individual values were normally distributed. The differences in time courses (from D0 to D84), type of yogurts and interactions between time course and type of yogurts for serum 25(OH)D, PTH, CTX and TRAP5b were evaluated by repeated-measure analysis of variance (ANOVA) with adjustment by the Tukey’s test. Statistical analysis of the data was conducted by using SAS V9.2 (SAS Institut Inc, Cary, NC, USA) and STATA software version 9.2 (StataCorp LP, College Station TX, USA). In addition, chi-square tests were used (20) to compare the proportion of FY vs CY, with serum values of 25(OH)D ≥ 50 nmol/L (the IOM sufficiency threshold (14)), and of PTH ≤ 46 ng/L (the normal range limit) after 84 days of intervention. The P value <0.05 was considered statistically significant.
Results
Characteristics at baseline (Day 0)
The demographic characteristics at the inclusion into the trial (D0) did not differ between the CY and FY randomized groups (Table 1). At baseline, using the FRAX questionnaire specifically adapted to the UK population, it appeared that the estimated 10-year risk of major osteoporotic fractures, or hip fracture alone, was not different between the CY and FY group (Table 1). The similarity FRAX scores fracture could be expected given that both quantitative and qualitative risk factors did not differ between the two groups. The dietary intakes of Ca, energy, proteins, carbohydrates and lipids, as recorded in 20 and 16 participants in the CY and FY group, respectively, did not differ significantly (Table 2). At baseline (D0), there was no significant difference in serum 25OHD, PTH, CTX and TRAP5b between the two groups (Table 3).
Response to fortified foods
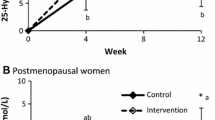
The time-dependent change in serum 25OHD was more pronounced in the FY than in the CY groups (Figure 1). After 84 days it was about 4 times larger (P =0.00001) in FY than CY (Table 3). The rise serum 25(OH)D greater in FY than in CY at D84 was associated with a significantly (P=0.0011) greater decrease in serum PTH (Figure 2). The differences in percent changes between 25(OH)D and PTH from D0 to D84 are illustrated in Figure 3. At D84, the proportion of subjects with a serum level ≥ 50 nmol/L was 70.8% (17/24) and 16.7% (4/24) in the FY and CY groups, respectively (chi square test P<0.001). After the same intervention time, the proportion of subjects with a serum PTH level ≤ 46 ng/L was 58.3% (14/24) and 25% (6/24) in the FY and CY groups, respectively (chi square test P<0.05).
Values are means in percent after 84 days (D84-D0/D0 x 100) of intervention in subjects consuming non-fortified control (CY) and fortified yogurts (FY), respectively. Δ = Difference in percent between CY and FY groups; Number of subjects: 24 in each group; P. Probability levels for the differences D84-D0, calculated either by the two-sided Student t test or by the Mann-Whitney U test, whether the values were normally distributed (PTH and TRAP5b) or not (CTX and 25OH).
The more pronounced fall in serum PTH in the FY group, compared to CY group, was associated with a significant reduction in serum TRAP5b (Figure 3). There was also a reduction in serum CTX, the other marker of bone resorption, though the difference between FY and CY did not reach the level of statistical significance (Figure 3). There was a significant interaction between the time course and the type of yogurt for serum 25OHD (Table 3), but not for serum PTH that also tended to fall in the CY group (Figure 2).
Other monitored variables
The Ca intake recorded by 3-day dietary diaries was 710±45 at D0 and 869±44 mg/d at D84 in the CY group. It was 783±75 at D0 and 1457±57 mg/d at D84 in the FY group. The calcium relative increase was 33 % and 115 % in the CY and FY group, respectively. Meanwhile, in both CY and FY groups, the consumption of energy and protein did not significantly differ between D0 and D84 (data not shown).
The serum Ca, Pi, prealbumin, and albumin did not significantly change between D0 and D84 in either group. Likewise for body weight, systolic and diastolic blood pressure (data not shown).
Sun exposure. During the intervention, none of the subjects spent daily more than 20 minutes outdoors with their uncovered arms exposed to the sun.
Acceptability and adherence
The two types of yogurts were well tolerated. The acceptability in terms of flavor and portion size was considered as appropriate. The mean compliance throughout the intervention was more than 95% in both groups, despite the fact that consuming two yogurts per day is rather unusual in an English diet.
Discussion
The serum biochemical pattern encompassing low vitamin D status, secondary hyperparathyroidism and increased bone resorption indices characterizes the insufficient supply of vitamin D associated with inadequate Ca intake. This pattern tends to be more frequently observed with aging. The related biochemical anomalies can be corrected within a few weeks or months by providing an adequate supply of both vitamin D and Ca. Such correction is associated with an attenuation of bone loss, a reduced risk of falling and osteoporotic fractures (4–7). These beneficial effects of vitamin D supplementation in conditions of deficiency or insufficiency can be observed with much lower-dose regimens than those that can be detrimental to bone health (21). Indeed, vitamin D supply can be inefficient in community-dwelling individuals with vitamin D sufficiency (22). Controversy on the outcome of vitamin D on bone health, fragility fractures and falls can be explained, at least in part, by differences between: supplemental doses; duration of supplementation; ethnic/racial target populations; baseline vitamin D status (21–30). Furthermore, interpretation in terms of inadequacy/adequacy of serum 25OHD baseline levels and-or intervention-induced changes in response to supplemental vitamin D can differ when related to the concept of the “Estimated Average Requirement (EAR) or to the “Recommended Dietary Allowance (RDA)”, as well as to the method of assessment (31, 32). The notion of inadequacy/ adequacy of the vitamin D status is still much more difficult to delineate when putative extraskeletal effects are considered. Indeed, from association findings mostly, it has been suggested that vitamin D insufficiency could be implicated as a causal or at least as a contributing factor to several chronic diseases including type 2 diabetis, cardiovascular disease, some cancers, autoimmune and infectious disorders (15, 33). Cognitive functions have also been positively associated with the vitamin D status (34–37). This link could be implicated in the risk of falling that might be, at least in part, explained by some deficit in both attention and executive functions. The direct causal relationship between vitamin D and the various chronic diseases mentioned above remains to be established in appropriately designed prospective studies.
The foregoing randomized controlled trial was essentially focused on the effects of two nutrients on bone-related metabolism. It confirms the results of a previous similar study (3) showing that fortification of the same dairy product with vitamin D3 and Ca, as compared to the identical non fortified food, enhances vitamin D status, corrects secondary hyperparathyroidism and, concomitantly, reduces biochemical markers of bone resorption. This confirmation, with results supported by intention-to-treat analysis, as in the previous study (3), is of interest since these two trials differed on several important demographic and environmental aspects.
The former trial was carried out in institutionalized women with mean age 86 (3). These participants were entirely dependent on long-term care facilities. The nursing staff provided a comprehensive assistance during the trial, including the regular delivery of meals to the participants. In contrast, in the current study, the participants were younger, with mean age 74. They were free living in sheltered accommodation where tenants are able to look after themselves with a degree of independence. This setting implied that their eating habits could be maintained. Although the new reported outcomes are qualitatively quite similar to those of the previous study (3), there are some quantitative differences between the two trials that deserve to be commented in relation with the distinct demographic and environmental conditions. The better vitamin D status in the current study, as compared to the other “twin” study (3), could be putatively explained by factors such as age (74 vs. 86 years) and-or living conditions (community dwelling vs. nursing home) that may have influenced the supply of vitamin D from both cutaneous and food sources (38).
The changes in the serum 25OHD in response to 10 μg supplemental vitamin D3, as compared to the non fortified yogurts, were at D56 less important in the present study (FY: +19.9; CY: +4.6 nmol/L, difference = +15.3 nmol/L) than in the previous report (3), (FY: +25.3; CY: +5.2: difference = +20.1 nmol/L). This 24% reduced response could be accounted for by the known inverse relationship between the baseline serum level of 25(OH)D and its increment following an oral intake of supplemental vitamin D3 (39, 40). Indeed, at D0, the level of serum 25OHD in the FY group was about 80% higher in the present report (FY Group : 34.1±2.4 nmol/L) than in the previous comparable intervention study (FY Group: 19.2±1.2 nmol/L) testing the same dairy product (3). In the present trial, after 56 days of FY consumption, the mean serum 25OHD (54 nmol/L) was above the level (50 nmol/L) considered by the IOM, at least for skeletal health, as covering the vitamin D requirements of ≥ 97.5 % of the North-American population (14). Figure 1 illustrates the time-dependent changes in serum 25OHD in relation with the 50 nmol/L level for sufficient concentration as stated in the IOM guidelines (14). Accordingly, in the FY group, the mean serum 25OHD concentration overcrosses this level between D28 and D56. At that time, as illustrated in Figure 2, the serum PTH level was still above the upper limit of the normal range. However, at D84, given the additional 4 weeks of FY consumption, serum PTH fell into the normal range, suggesting that correction of secondary hyperparathyroidism was achieved after 3 months of FY consumption (Figure 2). The reduction of the two markers of bone resorption, CTX and TRAP5b, may be interpreted as the functional consequence of the reduced extracellular level of PTH. Besides the 10 μg of supplemental vitamin D3, the 86 % higher Ca intake in the FY group as compared to the CY group (mean: 1457 vs. 783 mg/d) could have substantially contributed to the control of secondary hyperparathyroidism.
As recently reviewed (41), high PTH concentration has been associated with increased risk of cardio-vascular diseases. Among intermediate risk factors, increased blood pressure could be implicated in this association. In our 3 month randomized trial both systolic and diastolic blood presssure remained stable despite a marked fall in serum PTH in the subjects consuming vitamin D an Ca fortified yogurt. Strengths and limitations.
The strengths of this study include a three month randomized controlled trial comparing the effects of Ca and vitamin D fortification of a dairy product in women over 60 living in community dwelling accommodation on the relation between the serum level of 25OHD, PTH and two biochemical markers of bone resorption. The study responds to the concept of comparative effectiveness research, since the fortified yogurts were compared to identical but non-fortified dairy products. Another strength is the intention-to-treat analysis supporting the results with the demonstration of the vitamin D status improvement associated with the reduction of both secondary hyperparathyroidism and bone resorption markers in women at moderate risk of fragility fractures. The good compliance to the consumption of the two compared food products during three months can also be considered as an asset in this clinical trial.
A limitation was the uncomplete data related to the dietary survey. Indeed, the 3-day dietary diaries to assess the spontaneous calcium and macronutrient intakes at baseline and during the intervention were not reliably completed by all of the enrolled participants. Therefore, it cannot be ruled out that uneven bone-related food consumption might have influenced the magnitude of the differences in the reported biochemical variables between the two experimental groups. Another limitation is the difficulty to control the vitamin D supply from both cutaneous and food sources. The rise in the serum level of 25OHD in the control group reflects this difficulty. However, it also underscores the importance of quantifying the actual effect of the tested fortification as compared to an identical non-fortified product in parallel randomized control trials. The reported mean rise in serum 25OHD of 22 nmol/L, modifying the vitamin D status from insufficiency (34 nmol/L) to sufficiency (56 nmol/L) after 3 months of 10 μg/d of vitamin D3 supplied by the consumption of fortified yogurts was determined in white postmenopausal women living in a community dwelling home. This dose-response relationship may not apply to other ethnic or racial population of postmenopausal women living in similar housing communities (30).
Conclusions. The daily consumption of two fortified yogurts for three months providing 520 mg of Ca and 10 μg (400 IU) of vitamin D3 daily, compared to a non fortified equivalent dairy product, increased the mean serum level of 25OHD above 50 nmol/L, almost controlled secondary hyperparathyroidism and reduced serum markers of bone resorption. This comparative effectiveness clinical trial confirms the usefulness of a nutritional approach consisting in enriching dairy products with Ca and vitamin D3 in order to prevent the acceleration of bone turnover in women at risk of fragility fractures.
Abbreviations
- FY:
-
vitamin D and calcium-fortified yogurt
- CY:
-
control (non fortified) yogurt
- Ca:
-
calcium
- Pi:
-
inorganic phosphate
- 25OHD:
-
25-hydroxyvitamin D
- PTH:
-
parathyroid hormone
- BRM:
-
bone resorption marker
- CTX:
-
carboxy-terminal-cross-linked-telopeptide of type-I-collagen
- TRAP5b:
-
tartrate-resistant-acid-phosphatase-isoform-5b
- IGF-I:
-
Insulin-like growth factor-I. BMD, bone mineral density
- BTM:
-
bone turnover markers
- DXA:
-
dual X-ray energy aborptiometry
References
Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501.
Naylor K, Eastell R. Bone turnover markers: use in osteoporosis. NatRevRheumatol. 2012;8(7):379–389.
Bonjour JP, Benoit V, Payen F, Kraenzlin M. Consumption of yogurts fortified in vitamin d and calcium reduces serum parathyroid hormone and markers of bone resorption: a double-blind randomized controlled trial in institutionalized elderly women. J Clin Endocrinol Metab. 2013;98(7):2915–2921.
Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327(23):1637–1642.
Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–676.
Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin d supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92(4):1415–1423.
Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, Wong JB. Effect of Vitamin D on falls: a meta-analysis. Jama. 2004;291(16):1999–2006.
Greenspan SL, Resnick NM, Parker RA. Early changes in biochemical markers of bone turnover are associated with long-term changes in bone mineral density in elderly women on alendronate, hormone replacement therapy, or combination therapy: a three-year, double-blind, placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab. 2005;90(5):2762–2767.
Johnell O, Oden A, De Laet C, Garnero P, Delmas PD, Kanis JA. Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int. 2002;13(7):523–526.
Bonjour JP, Kohrt W, Levasseur R, Warren M, Whiting S, Kraenzlin M. Biochemical markers for assessment of calcium economy and bone metabolism: application in clinical trials from pharmaceutical agents to nutritional products. Nutr Res Rev. 2014:27:252–267.
Heaney RP. Design Considerations for Clinical Investigations of Osteoporosis. In: Marcus M, Feldman D, Nelson DA, Rosen CJ, editors. Osteoporosis. 3rd Edition ed. Amsterdam: Elsevier, Inc; 2008. p. 1598–1620.
Kaats GR, Preuss HG, Leckie RB. Comparative Effectiveness Research (CER): Opportunities and Challenges for the Nutritional Industry. J Am Coll Nutr. 2009;28(3):234–237.
Michalek JE, Preuss HG, Croft HA, Keith PL, Keith SC, Dapilmoto M, Perricone NV, Leckie RB, Kaats GR. Changes in total body bone mineral density following a common bone health plan with two versions of a unique bone health supplement: a comparative effectiveness research study. Nutr J. 2011;10–32.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. JClinEndocrinolMetab. 2011;96(1):53–58.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930.
Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–743.
Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD. FRAX((R)) with and without bone mineral density. Calcif Tissue Int. 2012;90(1):1–13.
Thompson FE, Byers T. Dietary assessment resource manual. J Nutr. 1994;124(11 Suppl):2245S–2317S.
Bonjour JP, Benoit V, Rousseau B, Souberbielle JC. Consumption of Vitamin D-and Calcium-Fortified Soft White Cheese Lowers the Biochemical Marker of Bone Resorption TRAP 5b in Postmenopausal Women at Moderate Risk of Osteoporosis Fracture. J Nutr. 2012;142(4):698–703.
Dawson B, Trapp RG. Basic and Clinical Biostatistics. 3rd ed. New York: Lange Medical Books/McGraw-Hill; 2001.
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–1822.
Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(4):307–320.
Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD0002–7.
Dawson-Hughes B, Harris SS. High-dose vitamin D supplementation: too much of a good thing? JAMA. 2010;303(18):1861–1862.
Grant WB. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes. Lancet Diabetes Endocrinol. 2014;2(5):3–4.
Ng K, Hollis BW, Giovannucci EL, Fuchs CS. Reply to PM Brannon et al. Am J Clin Nutr. 2014;100(3):984–986.
Mak JC. High-dose oral vitamin D supplementation and risk of falls in older women. JAMA. 2010;304(8):854; author reply 856–857.
Bischoff-Ferrari HA, Orav EJ, Willett WC, Dawson-Hughes B. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes. Lancet Diabetes Endocrinol. 2014;2(5):363–364.
Davies JS, Poole CD. Vitamin D and falls. Lancet Diabetes Endocrinol. 2014;2(7):540–541.
Ng K, Scott JB, Drake BF, Chan AT, Hollis BW, Chandler PD, Bennett GG, Giovannucci EL, Gonzalez-Suarez E, Meyerhardt JA, et al. Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebocontrolled trial. Am J Clin Nutr. 2014;99(3):587–598.
Taylor CL, Carriquiry AL, Bailey RL, Sempos CT, Yetley EA. Appropriateness of the probability approach with a nutrient status biomarker to assess population inadequacy: a study using vitamin D. Am J Clin Nutr. 2013;97(1):72–78.
Brannon PM, Mayne ST, Murphy SP, Taylor CL. Vitamin D supplementation in African Americans: dose-response. Am J Clin Nutr. 2014;100(3):982–984.
Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151–1154.
Annweiler C, Montero-Odasso M, Llewellyn DJ, Richard-Devantoy S, Duque G, Beauchet O. Meta-analysis of memory and executive dysfunctions in relation to vitamin D. J Alzheimers Dis. 2013;37(1):147–171.
Bartali B, Devore E, Grodstein F, Kang JH. Plasma vitamin D levels and cognitive function in aging women: the nurses’ health study. J Nutr Health Aging. 2014;18(4):400–406.
Latimer CS, Brewer LD, Searcy JL, Chen KC, Popovic J, Kraner SD, Thibault O, Blalock EM, Landfield PW, Porter NM. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci U S A. 2014;111(41):E4359–4366.
Marcelli C, Chavoix C, Dargent-Molina P. Beneficial effects of vitamin D on falls and fractures: is cognition rather than bone or muscle behind these benefits? Osteoporos Int. 2014.
Rolland Y, de Souto Barreto P, Abellan Van Kan G, Annweiler C, Beauchet O, Bischoff-Ferrari H, Berrut G, Blain H, Bonnefoy M, Cesari M, et al. Vitamin D supplementation in older adults: searching for specific guidelines in nursing homes. J Nutr Health Aging. 2013;17(4):402–412.
Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8(3):222–230.
Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86(3):1212–1221.
van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA. Parathyroid hormone and cardiovascular disease events: A systematic review and meta-analysis of prospective studies. Am Heart J. 2013;165(5):655–664, 664 e651–655.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bonjour, JP., Benoit, V., Atkin, S. et al. Fortification of yogurts with vitamin D and calcium enhances the inhibition of serum parathyroid hormone and bone resorption markers: A double blind randomized controlled trial in women over 60 living in a community dwelling home. J Nutr Health Aging 19, 563–569 (2015). https://doi.org/10.1007/s12603-015-0498-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-015-0498-8