Abstract
Aquafeed quality is the most critical factor for aquaculture sustainability. However, limitations of traditional feed ingredients such as fishmeal (FM) need alternative strategies to ensure the nutritional requirements for aquatic animals. In this trial, four test diets were formulated (2 × 2 factorial design), where FM was incorporated in two diets at 10% with or without Saccharomyces cerevisiae (SC) at 1 g/kg. At the same time, FM was replaced with yellow mealworm (Tenebrio molitor) meal (TM) with or without SC at 1 g/kg. The growth performance indices (final weight, weight gain, and SGR), and the feed utilization indices (FCR and PER) were markedly affected by the protein source (FM or TM) and dietary SC (P < 0.05). The protein source (FM or TM) significantly (P < 0.05) affected the whole-body protein and lipid contents, while the moisture and ash contents were unaffected (P > 0.05) by TM or SC. The growth of the intestinal villi showed a marked increase in both height and branching in the treated groups with SC along the whole length of the intestine. Furthermore, the immune cell infiltration was prominent near the intestinal crypts of the middle intestinal segments in the supplemented groups by SC. Dietary TM and SC revealed improved hepatic parenchyma in the liver tissue better than other groups. The hematological indices, including hemoglobulin, hematocrit, red blood cells, and white blood cells, were markedly affected by dietary SC (P < 0.05). The lysozyme activity and phagocytic index were markedly affected by dietary SC, while phagocytic activity was affected by dietary TM (P < 0.05). The catalase, glutathione peroxidase, and malondialdehyde were markedly affected by the interaction between dietary protein source and SC, while superoxide dismutase was affected by dietary SC (P < 0.05). In conclusion, adding SC could enhance the utilization of TM by Nile tilapia with positive effects on the intestinal and liver histological features and the immune and antioxidative responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is a vital activity for providing healthy seafood and livelihood for humanity [1,2,3]. Several fish species have contributed to farming and presented suitable and feasible solutions worldwide [4]. Carps, salmonoids, and tilapia are the most cultured fish species globally and attracted attention due to their high quality, demand, and commercial value [5, 6]. More specifically, Nile tilapia (Oreochromis niloticus), which is known as “aquatic chicken,” is considered the third most consumed fish species [7]. Nile tilapia offers a high potential to grow under diverse environmental conditions [8]. Besides, it has the ability to grow in intensive systems with a relatively lower cost of production than other farmed fish species [9]. Like other fish species, Nile tilapia require specific amounts of protein that mainly depend on fishmeal (FM), plant proteins, and food byproducts [10, 11]. However, the high prices of these ingredients, especially FM, and the unavailability and high demand for formulating carnivorous fish species feed limited the expansion of Nile tilapia production [12]. Therefore, replacing traditional ingredients with non-traditional nutritional components is crucial for Nile tilapia sustainability [12, 13]. Indeed, FM is a rich source of animal protein, essential amino acids, and flavors; thus, comparable protein sources may offer suitable replacers in Nile tilapia feeds [14, 15].
Recently, circular bio-economy initiatives were implemented to utilize the ever-increasing waste from different human activities [16,17,18,19]. In this regard, insect meal was introduced as an alternative nutritional and cost-effective ingredient for the sustainable aquafeed industry [11, 20,21,22]. More specifically, yellow mealworm (Tenebrio molitor) meal (TM) has been introduced to the aquafeed industry as a friendly environmental strategy to convert food waste into high-value feed ingredients [23]. The meal of TM is a rich source of protein required for formulating nutritionally balanced aquafeed [24]. The protein content of TM is comparable to FM, which is about 60%, and, therefore, could successfully replace FM [25]. Additionally, dietary TM contains chitin and riboflavin involved in various immunological, antioxidative, and antibacterial effects [26, 27]. Therefore, the inclusion of TM was investigated in several fish species as a partial or complete replacer for FM or soybean meal [28, 29]. However, the high chitin content in TM would interrupt the digestibility of nutrients in fish intestines, leading to lowered feed utilization and growth performance [30]. Hence, introducing functional additives such as medicinal herbs and probiotics may protect from the negative impacts and end with positive roles on fish intestines [31,32,33,34].
Undoubtedly, live beneficial microorganisms such as bacteria, fungi, and yeast were proven to enhance aquatic animals' intestinal health and feed digestion [7]. Yeast supplementation has been notably applied in aquaculture as a nutritional and bioactive additive [35]. More specifically, Saccharomyces cerevisiae, which has been used widely in aquaculture, is associated with its high protein content, growth-promoting, immunomodulation, and health benefits [36]. In addition, S. cerevisiae contains functional components in its cell walls, such as polysaccharides, mannan oligosaccharides, β-glucan, and chitin, which activate the entire body’s immunity [37, 38]. In this context, S. cerevisiae can enhance intestinal health through colonization in the mucosal layer, competition with harmful invaders, and inhibiting pathogens [39]. Consequently, S. cerevisiae can protect the intestinal epithelium health and regulate intestinal digestion, physiological function, and immunity [40].
In Nile tilapia, dietary S. cerevisiae enhanced the digestion capacity, intestinal health, immunity, and resistance to harmful pathogens [41,42,43]. In addition, dietary TM was included in tilapia diets without negative impacts [28]. However, the combined beneficial effects of TM and S. cerevisiae still need to be investigated. Thus, this study tested the combined effects of TM and S. cerevisiae on the growth performance, feed utilization, intestinal health, and blood biomarkers of Nile tilapia-fed FM-free diets.
Materials and Methods
Design and Diet Formulation
The trial was conducted at the laboratories and greenhouses for the Faculties of Agriculture and Veterinary Medicine, Kafrelsheikh University, Egypt. Uniform-sized Nile tilapia juveniles were obtained from a private farm located at the international road to Baltim city, Kafrelsheikh, Egypt, and gently transported to the wet laboratory. Carefully, all fish were randomly distributed and stocked in 15 well-cleaned and prepared glass aquaria (100 L) for acclimatization. All aquaria were equipped with electrical aerators and dechlorinated water, which was exchanged at 50% daily. Fish was offered the basal diet at 3% for 15 days, and the remaining feed was siphoned immediately. Subsequently, all fish were redistributed in the glass aquaria at 20 fish per aquarium with an average initial weight of 6.04 ± 0.05 g/fish. The trial was done in four groups, with three glass aquaria replicates representing each group. Every three aquaria were subjected to one of the test diets. Fish were offered the test diets twice daily at 08:00 a.m. and 2:00 p.m., and the amount of feed consumed was recorded. The feed intake was offered until satiation, and when the fish rejected the feed, the feeding was stopped. Feed was offered slowly to avoid excessive feed addition and to calculate the feed intake for each aquarium accurately. The water was regularly exchanged, and feces were siphoned in each aquarium during the 60-day feeding trial. The water quality was checked using the laboratory apparatus and recorded: 27.63 ± 0.29 °C; 7.42 ± 0.28; 6.08 ± 0.23 mg/L; and 0.01 ± 0.001 g/L for temperature, pH, dissolved oxygen, and total ammonia nitrogen, respectively. Fish were kept in 12-h light and 12-h dark intervals during the trial.
According to the NRC [44] and Hassaan and Mohammady [45], four test diets were formulated to meet the needs of Nile tilapia (Table 1). Four test diets were formulated (2 × 2 factorial design), where FM was incorporated in two diets at 10% with or without Saccharomyces cerevisiae (SC) at 1 g/kg. At the same time, FM was replaced with yellow mealworm (Tenebrio molitor) meal (TM) with or without SC at 1 g/kg. The SC (10 × 109 cells/g) was produced by Levitan (Dox-al Italia S.P.A., Italy) and added to the diets at 1 g/kg, according to the recommendation of Abdel-Tawwab and Abdel-Rahman [46]. TM meal was prepared at the Department of Entomology, Faculty of Agriculture, Kafrelsheikh University, and its chemical composition was checked by following the AOAC [47]. The chemical composition (% of dry matter) for TM is 55.82%, 32.24%, 5.42%, and 6.32% for crude protein, total lipids, ash, and fibers, respectively. All ingredients were finely grounded and were thoroughly well mixed using the laboratory food mixer. Water was added to the ingredients at 35–40% to the dry mix for having a dough and then pelleted using the laboratory pelletizer. The pellets were kept at 2 mm size and were air dried inside the laboratory to avoid any possible accumulation threats; then, pellets were appropriately broken to keep suitable sizes for fish. After completely drying, the pellets were collected and kept in plastic bags and then stored in a freezer at − 20 °C. The chemical composition of the test diets was checked by following the AOAC [47]. The actual number of SC in the diets was confirmed by culturing the dilutions on YPD agar plates (2% peptone, 2% glucose, 1% yeast extract, and 1.5% agar) [48].
Final Sampling
At the end of the trial, all fish were satiated for 24 h and then anesthetized with tricaine methane sulfonate (MS-222) (100 mg/L) to avoid handling stress. The individual weight and the total number of fish in each aquarium were recorded; then, randomly, three fish per aquarium were subjected to blood sampling and dissection. Samples for middle intestines and liver tissue from the four groups were dissected and fixed in 10% neutral buffered formalin.
Blood samples were collected from the caudal vein using 5 ml gauge syringes. The collected blood was then divided into two parts: half was stored in EDTA-heparinized tubes for immediate hematological analysis, and the other half was placed in non-heparinized tubes for serum collection. After 2 h, the blood samples in non-heparinized tubes were centrifuged at 1008 × g for 15 min at 4 °C. The serum was then separated and kept at − 20 °C for further analysis.
Histology Study
After 24 h, the collected samples were transferred from 10% neutral buffered formalin to 70% alcohol. The intestine and liver samples were then dehydrated in ascending graded series of ethanol, cleared in xylene, and impregnated and embedded in paraffin wax by following Bancroft and Stevens [49]. Sections of 5 µm were cut using Leica rotatory microtome (RM 20352035; Leica Microsystems, Wetzlar, Germany) and mounted on glass slides. The prepared tissue sections were subjected to conventional staining of hematoxylin and eosin (H&E) according to Gewaily and Abumandour [50]. The stained sections were examined under a light microscope (Olympus, Tokyo, Japan).
Blood Hematology
White blood cell (WBC) and red blood cell (RBC) counts and hemoglobin concentration (Hb) were done following standard procedure [51]. Hematocrit (Hct) was determined by the microhematocrit method while the hemoglobin (Hb) concentration was determined with a spectrophotometer (Model RA 1000, Technicon Corporation, USA) at 540 nm using the Blaxhall and Daisley [52] method.
Blood Immunity and Antioxidative Status
A turbidimetric assay, based on the method of Ellis and Stolen [53], was used to analyze serum lysozyme activity. Leukocyte phagocytic function was assessed following the technique described by Cai and Li [54], where the percentage of leukocytes that engulfed bacteria was determined relative to the total leukocyte count in the smear. The phagocytic activity and index were determined as per Kawahara and Ueda [55].
To measure the levels of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), diagnostic reagent kits from Cusabio Biotech Co., Ltd. (China) were used as per the manufacturer’s instructions. Malondialdehyde (MDA) concentration was detected following the advice of Uchiyama and Mihara [56] and expressed in nmol MDA/mL.
Calculations and Statistical Analysis
The following equations were used to calculate the weight gain (WG), specific growth rate (SGR), and survival rate. Besides, the feed conversion ratio (FCR), the protein efficiency ratio (PER), and feed intake were determined.
All data were tested for homogeneity of variance by the Shapiro–Wilk and Levene tests, and normality was tested by the Kolmogorov–Smirnov test. Data were analyzed as a two-way ANOVA (2 factorial design) using the general linear model (GLM) procedure, the main protein sources (FM or TM), dietary SC, and their interaction. Tukey’s multiple comparison test compared means when interactive effects differed significantly. Values have presented an average of three replicates. Significant differences (P < 0.05) between dietary protein sources (FM or TM) and dietary SC were evaluated by Tukey’s test. All the statistical analyses were done via SPSS version 22 (SPSS Inc., IL, USA).
Results
Growth Performance
The growth performance indices, including final weight (FW), weight gain (WG), and SGR, and the feed utilization indices, including FCR and PER, were markedly affected by the protein source (FM or TM) and dietary SC (P < 0.05) (Table 2). The FW and WG were significantly higher in Nile tilapia-fed dietary FM with SC supplementation than those fed the other diets. In addition, the WG was significantly lower in Nile tilapia-fed TM without SC supplementation than in the other groups. The SGR was significantly higher in fish-fed FM and SC than in fish-fed TM without SC, without significant differences with the other groups. On the other hand, the FCR was significantly higher in Nile tilapia-fed TM without SC supplementation than in the other groups, while fish fed both FM and SC showed the lowest FCR value. The PER was significantly lower in Nile tilapia-fed TM without SC supplementation than in the other groups, while fish fed both FM and SC showed the highest PER value. The survival rate was not impacted (P > 0.05) by dietary TM or SC and was recorded at 95 to 98.33%.
Body Chemical Composition
The protein source (FM or TM) significantly (P < 0.05) affected the whole-body protein and lipid contents, while the moisture and ash contents were unaffected (P > 0.05) by TM or SC (Table 3). The total protein content was significantly lower in Nile tilapia-fed dietary TM without SC supplementation than those fed the other diets. Besides, the total lipid content was significantly higher in Nile tilapia-fed TM with SC supplementation than in the other groups.
Intestinal and Liver Histology
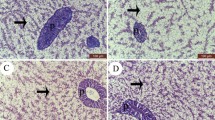
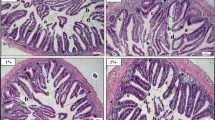
The intestine of Nile tilapia showed intact structures of both intestinal walls and villi in all groups (Fig. 1A–D). The intestinal wall was formed of tunica mucosa internally, propria submucosa, and tunica muscularis, followed by serosa in the outermost layer. The intestinal villi projected within the lumen of the intestine were formed of simple columnar cells with goblet cells arranged around the connective tissue core. The growth of the intestinal villi showed a marked increase in both height and branching in the treated groups with SC along the whole length of the intestine (Fig. 1B–D). Furthermore, the immune cell infiltration was prominent near the intestinal crypts of the middle intestinal segments in the supplemented groups by SC (Fig. 2B, D).
Histomicrograph showing the histological structure of middle segment of Nile tilapia intestine in the fish meal (FM) group (A) as well as other treated groups by Tenebrio molitor (TM) (B), Saccharomyces cerevisiae (SC) (C), and both TM with SC (D). The intestinal wall (IW) intestinal villi (IV) showed apparent growth mainly in group D in addition to immune cell infiltration (black arrow) in groups B and D. Stain H&E. Bar = 100 µm
Histomicrograph showing the histological structure of hepatopancreas in the fish meal (FM) group (A) as well as other treated groups by Tenebrio molitor (TM) (B), Saccharomyces cerevisiae (SC) (C), and both TM with SC (D). The hepatic (H) and pancreatic (black arrow) structures in all groups appeared normal without any deterioration or vacuolation. However, the co-supplementation with both TM and SC stimulated severe glycogen deposition within the hepatocytes’ cytoplasm (D). Stain H&E. Bar 100 µm
The liver in the control fish revealed normal hepatic parenchyma, intact hepatocytes, and pancreatic acinar cells (Fig. 2A). Dietary SC-supplemented groups (Fig. 2B, C) showed similar histomorphology to the control group; however, the co-treatment with both TM and SC revealed improved hepatic parenchyma better than other groups. The hepatocytes showed increased glycogen deposition through irregular glycogen vacuoles inside their cytoplasm (Fig. 2D).
Hematological Indices
The hematological indices, including hemoglobulin (Hb), hematocrit (Hct), red blood cells (RBCs), and white blood cells (WBCs), were markedly affected by dietary SC (P < 0.05) (Table 4). The Hb level was significantly higher in Nile tilapia-fed FM or TM with dietary SC than in fish-fed TM without SC. The RBCs were significantly higher in Nile tilapia-fed FM or TM with dietary SC than fish-fed FM without SC. The Hct level was significantly higher in Nile tilapia-fed FM with dietary SC than fish-fed FM without SC, without significant differences with fish-fed TM with or without SC (P > 0.05). The WBCs were significantly higher in Nile tilapia-fed FM or TM with dietary SC than in fish-fed FM or TM without SC.
Immune Response
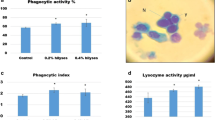
The lysozyme activity and phagocytic index were markedly affected by dietary SC, while phagocytic activity was affected by dietary TM (P < 0.05) (Fig. 3). The lysozyme activity was significantly higher in Nile tilapia-fed FM or TM with dietary SC than in fish-fed TM without SC (Fig. 3A). The phagocytic activity was significantly higher in Nile tilapia-fed FM with dietary SC than in fish-fed FM without SC, without significant differences with the other groups (Fig. 3B). The phagocytic index was significantly higher in Nile tilapia-fed FM or TM with dietary SC than in fish-fed TM without SC (Fig. 3C).
Blood immunity and antioxidative responses of Nile tilapia fed test diets for 60 days. Values are an average of three replicates. Different letters indicate significant differences (P < 0.05) between dietary protein sources (fish meal (FM) or Tenebrio molitor meal (TM)) and Saccharomyces cerevisiae (SC) by Tukey’s test when significant interactions are seen (P < 0.05)
Antioxidative Response
The catalase (CAT), glutathione peroxidase (GPx), and malondialdehyde (MDA) were markedly affected by the interaction between dietary protein source and SC, while superoxide dismutase (SOD) was affected by dietary SC (P < 0.05) (Fig. 4). The SOD was significantly higher in Nile tilapia-fed FM with dietary SC or TM than in fish-fed FM without SC (Fig. 4A). The CAT was significantly higher in Nile tilapia-fed FM or TM with or without dietary SC than in fish-fed FM with or without SC (Fig. 4B). The GPx was significantly higher in Nile tilapia-fed FM or TM with dietary SC than in fish-fed TM without SC (Fig. 4C). However, the MDA was significantly lower in Nile tilapia-fed FM with SC or TM with or without SC than in fish-fed FM without SC.
Blood antioxidative responses of Nile tilapia fed test diets for 60 days. Values are an average of three replicates. Different letters indicate significant differences (P < 0.05) between dietary protein sources (fish meal (FM) or Tenebrio molitor meal (TM)) and Saccharomyces cerevisiae (SC) by Tukey’s test when significant interactions are seen (P < 0.05)
Discussion
Probiotic supplementation can enhance the utilization of non-traditional ingredients in aquafeed [7]. Mealworms such as Tenebrio molitor (TM) are safe and nutritious ingredients with high content of proteins, lipids, and minerals [57]. However, the high chitin component may impact intestinal health if TM is added at high levels [58]. In this consideration, Saccharomyces cerevisiae (SC) may enhance the utilization of TM-based feeds and limit the inclusion of unsustainable ingredients such as fishmeal (FM) [31, 32]. Dietary SC has been illustrated as efficient probiotics involved in regulating intestinal microbiota, mucosal integrity, and digestion capacity [35, 36]. The current study investigated the positive roles of dietary SC on Nile tilapia-fed TM as a replacer for FM.
Regarding the final weight, the results indicated that fish-fed FM and SC had shown the highest final weight. Besides, replacing FM with TM did not impact the final weight of Nile tilapia with or without SC addition. The results are similar to Tubin and Paiano [59], who could feed Nile tilapia with TM without remarkable effects on growth performance. The inclusion of SC also showed similar improvements in the growth performance of Nile tilapia, as stated by Abass and Obirikorang [43], Islam and Rohani [60], and Abdel-Tawwab [61]. The results of this study also indicated that the lowest weight gain and SGR were observed in Nile tilapia-fed TM without SC supplementation. Markedly fish-fed FM or TM with SC recorded high weight gain and SGR. As functional probiotics, dietary SC can enhance the growth performance of fish by enhancing feed utilization through (1) secretion of digestive enzymes (amylase, lipase, and protease) involved in feed degradation and digestion; (2) regulation of intestinal microbial balance through the inhibition of harmful pathogens and fortification of beneficial microorganisms, thereby protecting intestinal integration and absorption capacity [35, 36, 62]. On this occasion, the study also showed enhanced FCR and PER in the Nile tilapia-fed dietary SC in FM or TM-based diets. The improvements in the FCR and PER under the current trial conditions may explain the increased growth performance by dietary SC [43]. It is well-documented that probiotics can enhance the growth performance of aquatic animals and feed utilization by facilitating nutrient digestibility and absorption [60, 63].
In order to facilitate feed digestion and absorption, the intestine and liver histological features have to be considered [62]. Indeed, intestinal health status can always be evaluated by detecting morphological features, especially when testing new feed formulations in aquaculture [64]. Intestines are involved in feed digestion [65], while the liver is the metabolic organ involved in regulating physiological and metabolic functions [63, 66]. Exposure to toxic and harmful compounds that may be present in the feed interrupts liver function and induces hepatic failure [66, 67]. In the current investigation, the growth of the intestinal villi showed a marked increase in both height and branching in the treated groups with SC. Furthermore, the immune cell infiltration was prominent near the intestinal crypts of the middle intestinal segments in the supplemented groups by SC. Previous efforts showed similar results where dietary SC improved the intestinal villi length and width and enhanced the branching and mucosal integration in Nile tilapia [60], rohu (Labeo rohita) [68], and striped catfish (Pangasianodon hypophthalmus) [40]. A detailed explanation of how probiotics could improve the intestinal histological feature needs to be detailed. The high content of nucleotides in yeast walls could be involved in forming intestinal cell walls via the formation of RNA and DNA in the intestinal mucosa [69]. Therefore, the increased villi dimensions by dietary SC can increase the absorption area and effectively help feed utilization by fish. The results also indicated that the liver of fish fed SC revealed improved hepatic parenchyma better than other groups. The hepatocytes showed increased glycogen deposition through irregular glycogen vacuoles inside their cytoplasm. Similarly, Xia and Hao [63] illustrated that channel catfish (Ictalurus punctatus) fed dietary SC revealed improved liver histological features. Indeed, balanced feed formulations, the addition of probiotics, and the absence of xenobiotic hazards could be the main reasons for protecting liver health and, thereby, the entire body’s physiochemical function [7]. In this sense, dietary SC can overcome the negative impacts of high inclusion levels of TM. Besides, the healthy status of the liver confirms the safe use of TM with or without SC addition.
Whole body composition is a vital analysis associated with the effects of different feed ingredients and additives on the nutrient accumulation and weight gain of fish [70]. The results revealed that fish-fed TM showed the lowest protein content, while fish-fed TM and SC showed the highest lipid content. Similarly, Nile tilapia-fed dietary SC showed increased protein and lipid contents, as illustrated by Abdel-Tawwab and Abdel-Rahman [46]. The increased protein and lipid contents can be related to increased feed utilization by SC or FM, which results in a high accumulation of absorbed nutrients in the entire tissues of fish. The increased protein and lipid contents correspond to enhanced feed utilization (FCR and PER) and the growth performance results under the current trial conditions.
The study also exhibited meaningful effects on the hematological indices, which are tightly correlated with expressing fish's metabolic and physiological status [42]. The hemoglobulin, hematocrit, RBCs, and WBCs were enhanced by the addition of SC regardless of the protein source (FM or TM). Parallelly, Abdel-Tawwab and Abdel-Rahman [46], Jahan and Islam [68], and Boonanuntanasarn and Ditthab [40] reported enhanced hemoglobulin, hematocrit, RBCs, and WBCs in Nile tilapia, rohu (Labeo rohita), and striped catfish fed dietary SC. These hematological indices can be escalated due to the high yeast content from vitamin B complex and other hemotoxic substance involved in blood cell production [37, 38]. Additionally, increased WBCs by dietary SC can be related to enhanced immunity in Nile tilapia. Indeed, yeast cell walls contain abundant β-glucan (BG), which contains specific phagocytic cell receptors that contribute to WBC formation through binding with receptor molecules and release signals on the cell surface of the phagocyte [71].
The results also suggested that dietary SC enhanced the immunity of Nile tilapia-fed FM or TM. The immunity of Nile tilapia is evaluated by detecting lysozyme and phagocytic activities. Lysozyme activity is involved in damaging cellular walls for pathogenic bacteria [72], while phagocytic activity can limit the attack of harmful invaders [73]. Generally, probiotic supplementation is known for its role as an immunomodulator due to the direct intestinal immunity activation then mucosa and gut-associated lymphoid tissues [7, 74]. Similarly, dietary SC enhanced the lysozyme activity in Nile tilapia [41, 75] and striped catfish [40]. In addition, dietary SC improved phagocytic activity in Nile tilapia [41, 76], gilthead seabream (Sparus aurata) [77], and grouper (Epinephelus coioides) [76]. The production of inhibitory compounds by probiotics such as lactoferrin, lysozyme, bacteriocins, siderophores, and enzymes can directly inhibit pathogens [35]. Furthermore, yeast contains abundant functional substances such as BG and mannanoligosaccharides that immunomodulate the fish’s entire body [37, 38]. Activated phagocytic activity can also be correlated with increased WBCs by dietary SC, which is evident under the current study conditions.
Malnutrition and the absence of nutrients induce oxidative stress involved in the suppression of immunity, health, and growth performance of fish [62]. Herein, the current study considered evaluating the antioxidative status of Nile tilapia-fed FM or TM-based diets with or without SC. The results indicated marked improvement of the antioxidative responses (SOD, CAT, and GPx) while reducing the lipid peroxidation (low MDA) in Nile tilapia treated with SC. The results are in line with El-Nobi and Hassanin [75], who reported activated antioxidative responses in Nile tilapia-fed dietary SC. Enhanced antioxidative capacity can be related to the activation of the mucosal immunity and the entire body immunity of Nile tilapia-fed SC [78]. Further, the results indicated that Nile tilapia-fed TM showed a higher antioxidative response than those fed FM. Similarly, Zhang and Wu [28] and Sánchez-Muros and de Haro [79] reported that Nile tilapia-fed dietary TM revealed enhanced antioxidative capacity. The abundant chitin content in TM may be the direct reason for enhancing the antioxidative response via inhibiting lipid peroxidation and the formation of MDA [26, 27].
Conclusion
To sum up, mealworms (T. molitor) can replace fishmeal in the diets of Nile tilapia without interrupting the growth performance and health status. Markedly, the addition of S. cerevisiae could enhance the utilization of TM by Nile tilapia with positive effects on the intestinal and liver histological features and the immune and antioxidative responses.
Data Availability
All other relevant data are available from the corresponding authors upon reasonable request.
References
FAO (2022) World Fisheries and Aquaculture. Food and Agriculture Organization, Rome. https://doi.org/10.4060/cc0461en
Amer A-R, Eweedah NM, Amer AA, Gewaily MS, Younis NA, Ahmed HA et al (2023) Dietary effect of soybean lecithin on the growth performance, digestive enzyme activity, blood biomarkers, and antioxidative status of striped catfish (Pangasianodon hypophthalmus). PLOS ONE 18:e0291954
Dawood MAO (2022) Dietary copper requirements for aquatic animals: a review. Biol Trace Elem Res 200:5273–5282
Sankar T (2023) Understanding food safety in fish and fishery products. Adv Fish Proc Technol: Apple Acad Press 395–425
Lagutkina L, Evgrafova E, Ponomarev S, Nevalennyi A, Kriuchkov V (2023) Organic aquaculture: development trends and practical experience. In: Ronzhin A, Kostyaev A (eds) Agriculture Digitalization and Organic Production. Springer Nature Singapore, Singapore, pp 439–447
van Riel A-J, Nederlof MAJ, Chary K, Wiegertjes GF, de Boer IJM (2023) Feed-food competition in global aquaculture: current trends and prospects. Rev Aquac 15:1142–1158
Wang B, Thompson KD, Wangkahart E, Yamkasem J, Bondad-Reantaso MG, Tattiyapong P et al (2023) Strategies to enhance tilapia immunity to improve their health in aquaculture. Rev Aquac 15:41–56
El-Sayed A-FM, Fitzsimmons K (2023) From Africa to the world—the journey of Nile tilapia. Rev Aquacult 15:6–21
MacKinnon B, Debnath PP, Bondad-Reantaso MG, Fridman S, Bin H, Nekouei O (2023) Improving tilapia biosecurity through a value chain approach. Rev Aquac 15:57–91
Mugwanya M, Dawood MAO, Kimera F, Sewilam H (2023) Replacement of fish meal with fermented plant proteins in the aquafeed industry: a systematic review and meta-analysis. Rev Aquac 15:62–88
Somdare PO, Abdul Hamid NK, Abdul KZ (2023) Effect of papaya leaf extract inclusion on growth performance and haematological parameters of red hybrid tilapia, Oreochromis mossambicus × Oreochromis niloticus fed diets formulated with Hermetia meal and Azolla. Agriculture Reports 2:29–45
Midhun SJ, Arun D (2023) Chapter 12 - Alternative feed technology in aquaculture. In: Mathew J, Jose MS, E.K R, Kumar A, editors. Recent Adv Aquacult Microb Technol: Acad Press 291–306
Dawood MAO, Habotta OAE, Elsabagh M, Azra MN, Van Doan H, Kari ZA et al (2022) Fruit processing by-products in the aquafeed industry: a feasible strategy for aquaculture sustainability. Rev Aquacult 14:1945-1965
Kari ZA, Sukri SAM, Rusli ND, Mat K, Mahmud MB, Zakaria NNA et al (2023) Recent advances, challenges, opportunities, product development and sustainability of main agricultural wastes for the aquaculture feed industry – a review. Annals of Animal Science 23:25–38
Soltan NM, Soaudy MR, Abdella MM, Hassaan MS (2023) Partial dietary fishmeal replacement with mixture of plant protein sources supplemented with exogenous enzymes modify growth performance, digestibility, intestinal morphology, haemato-biochemical and immune responses for Nile tilapia, Oreochromis niloticus. Anim Feed Sci Technol 299:115642
Das SK, Mondal B, Sarkar UK, Das BK, Borah S (2023) Understanding and approaches towards circular bio-economy of wastewater reuse in fisheries and aquaculture in India: an overview. Rev Aquac 15:1100–1114
Verreth JAJ, Roy K, Turchini GM (2023) Circular bio-economy in aquaculture. Rev Aquac 15:944–946
Kari ZA, Goh KW, Edinur HA, Mat K, Khalid H-NM, Rusli ND et al (2022) Palm date meal as a non-traditional ingredient for feeding aquatic animals: a review. Aquacult Rep 25:101233
Muhammad Anamul K, Shishir Kumar N, Afrina Yeasmin S, Nik Shahman Nik Ahmad A (2023) Aquatic weeds as functional ingredients for aquaculture feed industry: recent advances, challenges, opportunities, new product development (NPD) and sustainability. Agric Rep 2:1–16
Fawole FJ, Shamna N, Memudu HA, Abdullahi N, Hassaan MS, Gbadamosi OK (2023) Housefly maggot meal complement soybean meal in a fish-free diet for hybrid catfish (Clarias gariepinus ♀ x Heterobranchus longifilis ♂): effect on growth, body composition, blood biochemistry and antioxidant enzyme activity. Anim Feed Sci Technol 295:115543
Prachom N, Boonyoung S, Hassaan MS, El-Haroun E, Davies SJ (2021) Preliminary evaluation of superworm (Zophobas morio) larval meal as a partial protein source in experimental diets for juvenile Asian sea bass. Lates calcarifer Aquaculture Nutrition 27:1304–1314
Tran HQ, von Siebenthal EW, Luce J-B, Nguyen TT, Tomčala A, Stejskal V et al (2024) Complementarity of insect meal and poultry by-product meal as replacement for fishmeal can sustain the production performance of European perch (Perca fluviatilis), reduce economic fish-in fish-out ratio and food-feed competition, and influence the environmental indices. Aquaculture 579:740166
Maroušek J, Strunecký O, Maroušková A (2023) Insect rearing on biowaste represents a competitive advantage for fish farming. Rev Aquac 15:965–975
Khanal P, Pandey D, Næss G, Cabrita ARJ, Fonseca AJM, Maia MRG et al (2023) Yellow mealworms (Tenebrio molitor) as an alternative animal feed source: a comprehensive characterization of nutritional values and the larval gut microbiome. J Clean Prod 389:136104
Alfiko Y, Xie D, Astuti RT, Wong J, Wang L (2022) Insects as a feed ingredient for fish culture: status and trends. Aquaculture and Fisheries 7:166–178
Zhang J, Dong Y, Song K, Wang L, Li X, Tan B et al (2022) Effects of the replacement of dietary fish meal with defatted yellow mealworm (Tenebrio molitor) on juvenile large yellow croakers (Larimichthys crocea) growth and gut health. Animals 12:2659.
Henry MA, Gai F, Enes P, Peréz-Jiménez A, Gasco L (2018) Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 83:308–313
Zhang L, Wu H-X, Li W-J, Qiao F, Zhang W-B, Du Z-Y et al (2023) Partial replacement of soybean meal by yellow mealworm (Tenebrio molitor) meal influences the flesh quality of Nile tilapia (Oreochromis niloticus). Animal Nutrition 12:108–115
Li H, Xue R, Sun J, Ji H (2023) Improving flesh quality of grass carp (Ctenopharyngodon idellus) by completely replacing dietary soybean meal with yellow mealworm (Tenebrio molitor). Animal Nutrition 12:375–387
Gasco L, Biasato I, Enes P, Gai F (2023) Chapter 16 - Potential and challenges for the use of insects as feed for aquaculture. In: Morales-Ramos JA, Rojas MG, Shapiro-Ilan DI, editors. Mass Prod Benefic Org (Second Edition): Academic Press 465–492
Sarker PK (2023) Microorganisms in fish feeds, technological innovations, and key strategies for sustainable aquaculture. Microorganisms 1:439
Hasan I, Rimoldi S, Saroglia G, Terova G (2023) Sustainable fish feeds with insects and probiotics positively affect freshwater and marine fish gut microbiota. Animals 13:1633
Mansour AT, Eldessouki EA, Khalil RH, Diab AM, Selema TAMA, Younis NA et al (2023) In vitro and in vivo antifungal and immune stimulant activities of oregano and orange peel essential oils on Fusarium solani infection in whiteleg shrimp. Aquacult Int 31:1959–1977
Ashry AM, Habiba MM, El-Zayat AM, Badreldeen AH, Younis NA, Ahmed HA et al (2023) Effects of ginger (Zingiber officinale) on the growth performance, digestive enzyme activity, antioxidative response, and antibacterial capacity of striped catfish (Pangasianodon hypophthalmus) reared in outdoor conditions. Aquaculture Reports 33:101760
Diguță CF, Mihai C, Toma RC, Cîmpeanu C, Matei F (2023) In vitro assessment of yeasts strains with probiotic attributes for aquaculture use. Foods 12:124
del Valle JC, Bonadero MC, Gimenez AVF (2023) Saccharomyces cerevisiae as probiotic, prebiotic, synbiotic, postbiotics and parabiotics in aquaculture: an overview. Aquaculture 739342
Machuca C, Méndez-Martínez Y, Reyes-Becerril M, Angulo C (2022) Yeast β-glucans as fish immunomodulators: a review. Animals 12:2154
Agboola JO, Øverland M, Skrede A, Hansen JØ (2021) Yeast as major protein-rich ingredient in aquafeeds: a review of the implications for aquaculture production. Rev Aquac 13:949–970
Jin S, Kong Q, John CK, Wang Z, Zhang T, Li X et al (2023) Natural biota’s contribution to cultured aquatic animals’ growth in aquaculture cannot be ignored. Aquac Res 2023:2646607
Boonanuntanasarn S, Ditthab K, Jangprai A, Nakharuthai C (2019) Effects of microencapsulated Saccharomyces cerevisiae on growth, hematological indices, blood chemical, and immune parameters and intestinal morphology in striped catfish. Pangasianodon hypophthalmus Probiotics and Antimicrobial Proteins 11:427–437
El-Boshy ME, El-Ashram AM, AbdelHamid FM, Gadalla HA (2010) Immunomodulatory effect of dietary Saccharomyces cerevisiae, β-glucan and laminaran in mercuric chloride treated Nile tilapia (Oreochromis niloticus) and experimentally infected with Aeromonas hydrophila. Fish Shellfish Immunol 28:802–808
Owatari MS, da Silva LR, Ferreira GB, Rodhermel JCB, de Andrade JIA, Dartora A et al (2022) Body yield, growth performance, and haematological evaluation of Nile tilapia fed a diet supplemented with Saccharomyces cerevisiae. Anim Feed Sci Technol 293:115453
Abass DA, Obirikorang KA, Campion BB, Edziyie RE, Skov PV (2018) Dietary supplementation of yeast (Saccharomyces cerevisiae) improves growth, stress tolerance, and disease resistance in juvenile Nile tilapia (Oreochromis niloticus). Aquacult Int 26:843–855
NRC (2011) National research council- nutrient requirements of fish and shrimp. National academies press
Hassaan MS, Mohammady EY, Adnan AM, Abd Elnabi HE, Ayman MF, Soltan MA et al (2020) Effect of dietary protease at different levels of malic acid on growth, digestive enzymes and haemato-immunological responses of Nile tilapia, fed fish meal free diets. Aquaculture 522:735124
Abdel-Tawwab M, Abdel-Rahman AM, Ismael NEM (2008) Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for Fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture 280:185–189
AOAC (2012) Official Methods of Analysis of AOAC international. 19th edition. AOAC International, Gaithersburg, Maryland, USA. https://www.eoma.aoac.org
Zaineldin AI, Hegazi S, Koshio S, Ishikawa M, Dawood MAO, Dossou S et al (2021) Singular effects of Bacillus subtilis C-3102 or Saccharomyces cerevisiae type 1 on the growth, gut morphology, immunity, and stress resistance of red sea bream (Pagrus major). Annals Animal Sci 21:589–608
Bancroft J, Stevens A, Turner D (1996) Theory and practice of histological techniques: Churchill Livingstone New York. the text. 766
Gewaily MS, Abumandour MM (2021) Gross morphological, histological and scanning electron specifications of the oropharyngeal cavity of the hooded crow (Corvus cornix pallescens). Anat Histol Embryol 50:72–83
Houston A (1990) Blood and circulation/methods for fish biology. NY. Amer Fish Soc
Blaxhall PC, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:771–781
Ellis A, Stolen J, Fletcher T, Anderson D, Robertson B, Van Muiswinkel W (1990) Lysozyme assay in techniques in fish immunology. Tech Fish Immunol 1:101-103
Cai W-q, Li S-f, Ma J-y (2004) Diseases resistance of Nile tilapia (Oreochromis niloticus), blue tilapia (Oreochromis aureus) and their hybrid (female Nile tilapia×male blue tilapia) to Aeromonas sobria. Aquaculture 229:79–87
Kawahara E, Ueda T, Nomura S (1991) In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathology 26:213–214
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Singh SK, Pawar L, Thomas AJ, Debbarma R, Biswas P, Ningombam A et al (2023) The current state of research and potential applications of insects for resource recovery and aquaculture feed. Environ Sci Pollut Res
Melenchón F, Larrán AM, Hernández M, Abad D, Morales AE, Pula HJ et al (2023) Different diets based on yellow mealworm (Tenebrio molitor)-part b: modification of the intestinal inflammatory response and the microbiota composition of rainbow trout (Oncorhynchus mykiss). Fishes 8:286
Tubin JSB, Paiano D, Hashimoto GSdO, Furtado WE, Martins ML, Durigon E et al (2020) Tenebrio molitor meal in diets for Nile tilapia juveniles reared in biofloc system. Aquaculture 519:734763
Islam SMM, Rohani MF, Shahjahan M (2021) Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquaculture Reports 21:100800
Abdel-Tawwab M (2012) Interactive effects of dietary protein and live bakery yeast, Saccharomyces cerevisiae on growth performance of Nile tilapia, Oreochromis niloticus (L.) fry and their challenge against Aeromonas hydrophila infection. Aquacult Int 20:317–331
Dawood MAO (2021) Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev Aquac 13:642–663
Xia R, Hao Q, Xie Y, Zhang Q, Ran C, Yang Y et al (2022) Effects of dietary Saccharomyces cerevisiae on growth, intestinal and liver health, intestinal microbiota and disease resistance of channel catfish (Ictalurus punctatus). Aquaculture Reports 24:101157
Magouz FI, Shehab El-Din MT, Amer AA, Gewaily MS, El-Dahdoh WA, Dawood MAO (2022) A blend of herbal essential oils enhanced the growth performance, blood bio-immunology traits, and intestinal health of Nile tilapia (Oreochromis niloticus). Annals of Animal Science 22:751–761
Xia Y, Wang M, Gao F, Lu M, Chen G (2020) Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Animal Nutrition 6:69–79
Dong R, Zhou C, Wang S, Yan Y, Jiang Q (2022) Probiotics ameliorate polyethylene microplastics-induced liver injury by inhibition of oxidative stress in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 130:261–272
Ali MF, Soliman AA, Gewaily MS, Abdel-Kader TY, Amer AA, Zaineldin AI et al (2022) Isatis phytogenic relieved atrazine induced growth retardation, hepato-renal dysfunction, and oxidative stress in Nile tilapia. Saudi Journal of Biological Sciences 29:190–196
Jahan N, Islam SMM, Rohani MF, Hossain MT, Shahjahan M (2021) Probiotic yeast enhances growth performance of rohu (Labeo rohita) through upgrading hematology, and intestinal microbiota and morphology. Aquaculture 545:737243
Cerezuela R, Fumanal M, Tapia-Paniagua ST, Meseguer J, Moriñigo MÁ, Esteban MÁ (2012) Histological alterations and microbial ecology of the intestine in gilthead seabream (Sparus aurata L.) fed dietary probiotics and microalgae. Cell Tissue Res 350:477–489
Ahmed I, Jan K, Fatma S, Dawood MAO (2022) Muscle proximate composition of various food fish species and their nutritional significance: a review. J Anim Physiol Anim Nutr 106:690–719
Khanjani MH, Sharifinia M, Ghaedi G (2022) β-glucan as a promising food additive and immunostimulant in aquaculture industry. Annals of Animal Science 22:817–827
Sirimanapong W, Adams A, Ooi EL, Green DM, Nguyen DK, Browdy CL et al (2015) The effects of feeding immunostimulant β-glucan on the immune response of Pangasianodon hypophthalmus. Fish Shellfish Immunol 45:357–366
Meena DK, Das P, Kumar S, Mandal SC, Prusty AK, Singh SK et al (2013) Beta-glucan: an ideal immunostimulant in aquaculture (a review). Fish Physiol Biochem 39:431–457
Siddik MAB, Foysal MJ, Fotedar R, Francis DS, Gupta SK (2022) Probiotic yeast Saccharomyces cerevisiae coupled with Lactobacillus casei modulates physiological performance and promotes gut microbiota in juvenile barramundi, Lates calcarifer. Aquaculture 546:737346
El-Nobi G, Hassanin M, Khalil AA, Mohammed AY, Amer SA, Montaser MM et al (2021) Synbiotic effects of Saccharomy cescerevisiae, Mannan Oligosaccharides, and β-glucan on innate immunity, antioxidant status, and disease resistance of Nile tilapia, Oreochromis niloticus. Antibiotics 10:567
Abu-Elala N, Marzouk M, Moustafa M (2013) Use of different Saccharomyces cerevisiae biotic forms as immune-modulator and growth promoter for Oreochromis niloticus challenged with some fish pathogens. International Journal of Veterinary Science and Medicine 1:21–29
Ortuño J, Cuesta A, Rodrı́guez A, Esteban MA, Meseguer J (2002) Oral administration of yeast, Saccharomyces cerevisiae, enhances the cellular innate immune response of gilthead seabream (Sparus aurata L.). Vet Immunol Immunopathol 85:41–50
Sutthi N, Doan HV (2020) Saccharomyces crevices and Bacillus spp. effectively enhance health tolerance of Nile tilapia under transportation stress. Aquaculture 528:735527
Sánchez-Muros MJ, de Haro C, Sanz A, Trenzado CE, Villareces S, Barroso FG (2016) Nutritional evaluation of Tenebrio molitor meal as fishmeal substitute for tilapia (Oreochromis niloticus) diet. Aquac Nutr 22:943–955
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to this work equally.
Corresponding author
Ethics declarations
Ethical Approval
Experimental procedures followed the local Animal Care and Ethics Committee of Kafrelsheikh University, Egypt.
Competing Interest
The authors declare no competing of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anany, E.M., Ibrahim, M.A., El-Razek, I.M.A. et al. Combined Effects of Yellow Mealworm (Tenebrio molitor) and Saccharomyces cerevisiae on the Growth Performance, Feed Utilization Intestinal Health, and Blood Biomarkers of Nile Tilapia (Oreochromis niloticus) Fed Fish Meal-Free Diets. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10199-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10199-8