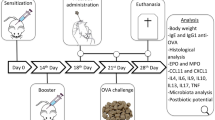
Abstract
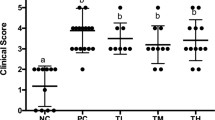
Food allergy is a pathological condition that can lead to hives, swelling, gastrointestinal distress, cardiovascular and respiratory compromise, and even anaphylaxis. The lack of treatment resources emphasizes the necessity for new therapeutic strategies, and in this way, probiotics has been pointed out as an alternative, especially because of its immunomodulatory properties. The goal of this study was to evaluate the probiotic effect of Bifidobacterium longum subsp. longum 51A (BL51A) in a murine model of ovalbumin (OVA) food allergy, as well as to investigate the effect of the dose and viability of the bacteria on the proposed model. For this purpose, the probiotic effect was assessed by clinical, immunological, and histological parameters in mice treated or not with the BL51A and sensitized or not with OVA. Oral administration of BL51A prevented weight loss and reduced serum levels of IgE anti-OVA and of sIgA in the intestinal fluid. Also, it reduced the intestinal permeability, proximal jejunum damage, recruitment of eosinophils and neutrophils, and levels of eotaxin-1, CXCL1/KC, IL4, IL5, IL6, IL13, and TNF. Furthermore, the treatment was able to increase the levels of IL10. Investigating different doses administered, the level of 108 CFU showed the best results in terms of protective effect. In addition, the administration of the inactivated bacteria did not present any beneficial effect. Results demonstrate that BL51A promotes a systemic immunomodulatory protective effect in a murine model of food allergy that depends on the dose and viability of the bacteria, suggesting its use as probiotic in such disease.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kanagaratham C, El Ansari YS, Lewis OL, Oettgen HC (2020) IgE and IgG antibodies as regulators of mast cell and basophil functions in food allergy. Front Immunol 11:603050. https://doi.org/10.3389/fimmu.2020.603050
Liu EG, Yin X, Swaminathan A, Eisenbarth SC (2021) Antigen-presenting cells in food tolerance and allergy. Front Immunol 11:616020. https://doi.org/10.3389/fimmu.2020.616020
Guo Y, Proaño-Pérez E, Muñoz-Cano R, Martin M (2021) Anaphylaxis: focus on transcription factor activity. Int J Mol Sci 22:4935. https://doi.org/10.3390/ijms22094935
Aquino A, Conte-Junior CA (2020) A systematic review of food allergy: nanobiosensor and food allergen detection. Biosensors 10:194. https://doi.org/10.3390/bios10120194
Palosuo K, Karisola P, Savinko T, Fyhrquist N, Alenius H, Mäkelä MJ (2021) A randomized, open-label trial of hen’s egg oral immunotherapy: efficacy and humoral immune responses in 50 children. J Allergy Clin Immunol Pract 9:1892–1901. https://doi.org/10.1016/j.jaip.2021.01.020
Mayorga C, Palomares F, Cañas JA, Pérez-Sánchez N, Núñez R, Torres MJ, Gómez F (2021) New insights in therapy for food allergy. Foods 10:1037. https://doi.org/10.3390/foods10051037
Urisu A, Kondo Y, Tsuge I (2015) Hen’s egg allergy. Ebisawa M, Ballmer-Weber BK, Vieths S, Wood RA (eds): Food Allergy: Molecular Basis and Clinical Practice. Chem Immunol Allergy. Basel, Karger, 101:124–130. https://doi.org/10.1159/000375416
Iweala OI, Choudhary SK, Commins SP (2018) Food allergy. Curr Gastroenterol Rep 20:1–6. https://doi.org/10.1007/s11894-018-0624-y
Ho HE, Bunyavanich S (2019) Microbial adjuncts for food allergen immunotherapy. Curr Allergy Asthma Rep 19:1–11. https://doi.org/10.1007/s11882-019-0859-1
De Martinis M, Sirufo MM, Suppa M, Ginaldi L (2020) New perspectives in food allergy. Int J Mol Sci 21:1474. https://doi.org/10.3390/ijms21041474
FAO/WHO Working Group: guidelines for the evaluation of probiotics in food (2002) London: World Health Organization, ON, Canada: Food and Agriculture Organization
Vieira AT, Teixeira MM, Martins FS (2013) The role of probiotics and prebiotics in inducing gut immunity. Front Immunol 4:445. https://doi.org/10.3389/fimmu.2013.00445
Sharma G, Im SH (2018) Probiotics as a potential immunomodulating pharmabiotics in allergic diseases: Current status and future prospects. Allergy Asthma Immunol Res 10:575–590. https://doi.org/10.4168/aair.2018.10.6.575
Cheng RY, Yao JR, Wan Q, Guo JW, Pu FF, Shi L, Hu W, Yang YH, Li L, Li M, He F (2018) Oral administration of Bifidobacterium bifidum TMC3115 to neonatal mice may alleviate IgE-mediated allergic risk in adulthood. Benef Microbes 9:815–828. https://doi.org/10.3920/BM2018.0005
Vlasova AN, Kandasamy S, Chattha KS, Rajashekara G, Saif LJ (2016) Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet Immunol Immunopathol 172:72–84. https://doi.org/10.1016/j.vetimm.2016.01.003
López P, González-Rodríguez I, Gueimonde M, Margolles A, Suárez A (2011) Immune response to Bifidobacterium bifidum strains support Treg/Th17 plasticity. PLoS One 6:e24776. https://doi.org/10.1371/journal.pone.0024776
Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG (2013) Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol 182:1595–1606. https://doi.org/10.1016/j.ajpath.2013.01.013
Xiao Y, Wang C, Zhao J, Zhang H, Chen W, Zhai Q (2021) Quantitative detection of Bifidobacterium longum strains in feces using strain-specific primers. Microorganisms 9:1159. https://doi.org/10.3390/microorganisms9061159
Souza TC, Silva AM, Drews JRP, Gomes DA, Vinderola CG, Nicoli JR (2013) In vitro evaluation of Bifidobacterium strains of human origin for potential use in probiotic functional foods. Benef Microbes 4:179–186. https://doi.org/10.3920/BM2012.0052
Mendes E, Acetturi BG, Thomas AM, Martins FDS, Crisma AR, Murata G, Braga TT, Camâra NOS, Franco ALDS, Setubal JC, Ribeiro WR, Valduga CJ, Curi R, Dias-Neto E, Tavares-de-Lima W, Ferreira CM (2017) Prophylactic supplementation of Bifidobacterium longum 51A protects mice from ovariectomy-induced exacerbated allergic airway inflammation and airway hyperresponsiveness. Front Microbiol 8:1732. https://doi.org/10.3389/fmicb.2017.01732
Casaro MB, Thomas AM, Mendes E, Fukumori C, Ribeiro WR, Oliveira FA, Crisma AR, Murata GM, Bizzarro B, Sá-Nunes A, Setubal JC, Mayer MPA, Martins FS, Vieira AT, Antiorio ATFB, Tavares-de-Lima W, Camara NOS, Curi R, Dias-Neto E, Ferreira CM (2021) A probiotic has differential effects on allergic airway inflammation in A/J and C57BL/6 mice and is correlated with the gut microbiome. Microbiome 9:1–16. https://doi.org/10.1186/s40168-021-01081-2
Ribeiro WR, Queiroz AG, Mendes E, Casaro MB, Nascimento CM, Coelho LSSF, Martins FS, Leite-Silva VR, Ferreira CM (2021) Preventive oral supplementation with Bifidobacterium longum 51A alleviates oxazolone-induced allergic contact dermatitis-like skin inflammation in mice. Benef Microbes 12:199–209. https://doi.org/10.3920/BM2020.0134
Abrantes FA, Nascimento BB, Andrade MER, de Barros PAV, Cartelle CT, Martins FS, Nicoli JR, Arantes RME, Generoso SV, Fernandes SOA, Cardoso VN (2020) Treatment with Bifidobacterium longum 51A attenuates intestinal damage and inflammatory response in experimental colitis. Benef Microbes 11:47–57. https://doi.org/10.3920/BM2019.0098
Vieira AT, Rocha VM, Tavares L, Garcia CC, Teixeira MM, Oliveira SC, Cassali GD, Gamba C, Martins FS, Nicoli JR (2016) Control of Klebsiella pneumoniae pulmonary infection and immunomodulation by oral treatment with the commensal probiotic Bifidobacterium longum 51A. Microbes Infect 18:180–189. https://doi.org/10.1016/j.micinf.2015.10.008
Souza TC, Zacarías MF, Silva AM, Binetti A, Reinheimer J, Nicoli JR, Vinderola G (2012) Cell viability and immunostimulating and protective capacities of Bifidobacterium longum 51A are differentially affected by technological variables in fermented milks. J Appl Microbiol 112:1184–1192. https://doi.org/10.1111/j.1365-2672.2012.05280.x
Vieira AT, Galvao I, Amaral FA, Teixeira MM, Nicoli JR, Martins FS (2015) Oral treatment with Bifidobacterium longum 51A reduced inflammation in a murine experimental model of gout. Benef Microbes 6:799–806. https://doi.org/10.3920/BM2015.0015
Guerra PV, Lima LN, Souza TC, Mazochi V, Penna FJ, Silva AM, Nicoli JR, Guimarães EV (2011) Pediatric functional constipation treatment with Bifidobacterium-containing yogurt: a crossover, double-blind, controlled trial. World J Gastroenterol 17:3916. https://doi.org/10.3748/wjg.v17.i34.3916
Fonseca JF, Alvim LB, Nunes ÁC, Oliveira FMS, Amaral RS, Caliari MV, Nicoli JR, Neumann E, Gomes MA (2019) Probiotic effect of Bifidobacterium longum 51A and Weissella paramesenteroides WpK4 on gerbils infected with Giardia lamblia. J Appl Microbiol 127:1184–1191. https://doi.org/10.1111/jam.14338
da Silva JGV, Vieira AT, Sousa TJ, Viana MVC, Parise D, Sampaio B, da Silva AL, de Jesus LCL, de Carvalho PKRML, de Castro OL, Aburjaile FF, Martins FS, Nicoli JR, Ghosh P, Brenig B, Azevedo V, Gomide ACP (2021) Comparative genomics and in silico gene evaluation involved in the probiotic potential of Bifidobacterium longum 51A. Gene 795:145781. https://doi.org/10.1016/j.gene.2021.145781
Siciliano RA, Reale A, Mazzeo MF, Morandi S, Silvetti T, Brasca M (2021) Paraprobiotics: a new perspective for functional foods and nutraceuticals. Nutrients 13:1225. https://doi.org/10.3390/nu13041225
Liong MT (2008) Safety of probiotics: translocation and infection. Nutr Rev 66:192–202. https://doi.org/10.1111/j.1753-4887.2008.00024.x
Silva AKS, Silva TRN, Nicoli JR, Vasquez-Pinto LMC, Martins FS (2018) In vitro evaluation of antagonism, modulation of cytokines and extracellular matrix proteins by Bifidobacterium strains. Lett Appl Microbiol 67:497–505. https://doi.org/10.1111/lam.13062
Kwon HS, Yang EH, Lee SH, Yeon SW, Kang BH, Kim TY (2005) Rapid identification of potentially probiotic Bifidobacterium species by multiplex PCR using species-specific primers based on the region extending from 16S rRNA through 23S rRNA. FEMS Microbiol Lett 250:55–62. https://doi.org/10.1016/j.femsle.2005.06.041
Reeves P, Nielsen F, Fahey G (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951. https://doi.org/10.1093/jn/123.11.1939
Dourado LPA, Noviello MDLM, Alvarenga DM, Menezes Z, Perez DA, Batista NV, Menezes GB, Ferreira AVM, Souza DG, Cara DC (2011) Experimental food allergy leads to adipose tissue inflammation, systemic metabolic alterations and weight loss in mice. Cell Immunol 270:198–206. https://doi.org/10.1016/j.cellimm.2011.05.008
Miranda VC, Santos SS, Assis HC, Faria AMC, Quintanilha MF, Morão RP, Nicoli JR, Cara DC, Martins FS (2020) Effect of Saccharomyces cerevisiae UFMG A-905 in a murine model of food allergy. Benef Microbes 11:255–268. https://doi.org/10.3920/BM2019.0113
Saldanha JCS, Gargiulo DL, Silva SS, Carmo-Pinto FH, Andrade MC, Alvarez Leite JI, Cara DC (2004) A model of chronic IgE-mediated food allergy in ovalbumin-sensitized mice. Braz J Med Biol Res 37:809–816. https://doi.org/10.1590/S0100-879X2004000600005
Pedroso SH, Vieira AT, Bastos RW, Oliveira JS, Cartelle CT, Arantes RM, Soares PMG, Generoso SV, Cardoso VN, Teixeira MT, Nicoli JR, Martins FS (2015) Evaluation of mucositis induced by irinotecan after microbial colonization in germ-free mice. Microbiology 161:1950–1960. https://doi.org/10.1099/mic.0.000149
Andrade ME, Santos RD, Soares AD, Costa KA, Fernandes SO, de Souza CM, Cassali GD, de Souza AL, Faria AM, Cardoso VN (2016) Pretreatment and treatment with l-arginine attenuate weight loss and bacterial translocation in dextran sulfate sodium colitis. JPEN J Parenter Enteral Nutr 40:1131–1139. https://doi.org/10.1177/0148607115581374
Arantes RM, Nogueira AM (1997) Distribution of enteroglucagon and peptide YY-immunoreactive cells in the intestinal mucosa of germ-free and conventional mice. Cell Tissue Res 290:61–69. https://doi.org/10.1007/s004410050908
Strath M, Warren DJ, Sanderson CJ (1985) Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods 83:209–215. https://doi.org/10.1016/0022-1759(85)90242-X
Martins FS, Elian SD, Vieira AT, Tiago FC, Martins AK, Silva FC, Souza EL, Sousa LP, Araújo HR, Pimenta PF, Bonjardim CA, Arantes RM, Teixeira MM, Nicoli JR (2011) Oral treatment with Saccharomyces cerevisiae strain UFMG 905 modulates immune responses and interferes with signal pathways involved in the activation of inflammation in a murine model of typhoid fever. Int J Med Microbiol 301:359–364. https://doi.org/10.1016/j.ijmm.2010.11.002
Shi LH, Balakrishnan K, Thiagarajah K, Mohd Ismail NI, Yin OS (2016) Beneficial properties of probiotics. Trop Life Sci Res 27:73–90. https://doi.org/10.21315/tlsr2016.27.2.6
Castan L, Bøgh KL, Maryniak NZ, Epstein MM, Kazemi S, O’Mahony L, Bodinier M, Smit JJ, van Bilsen JHM, Blanchard C, Glogowski R, Kozáková H, Schwarzer M, Noti M, de Wit N, Bouchaud G, Bastiaan S (2020) Overview of in vivo and ex vivo endpoints in murine food allergy models: suitable for evaluation of the sensitizing capacity of novel proteins? Allergy 75:289–301. https://doi.org/10.1111/all.13943
Schoos AMM, Bullens D, Chawes BL, Costa J, De Vlieger L, DunnGalvin A, Epstein MM, Garssen J, Hilger C, Knipping K, Kuehn A, Mijakoski D, Munblit D, Nekliudov N, Ozdemir C, Patient K, Peroni D, Stoleski S, Stylianou E, Tukalj M, Verhoeckx K, Zidarn M, Van De Veen W (2020) Immunological outcomes of allergen-specific immunotherapy in food allergy. Front Immunol 11:568598. https://doi.org/10.3389/fimmu.2020.568598
Akter S, Park JH, Jung HK (2020) Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells. J Microbiol Biotechnol 30:477–481. https://doi.org/10.4014/jmb.1911.11019
Nataraj BH, Ali SA, Behare PV, Yadav H (2020) Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb Cell Fact 19:168. https://doi.org/10.1186/s12934-020-01426-w
Aoki A, Hirahara K, Kiuchi M, Nakayama T (2021) Eosinophils: cells known for over 140 years with broad and new functions. Allergol Int 70:3–8. https://doi.org/10.1016/j.alit.2020.09.002
Francis A, Bosio E, Stone SF, Fatovich DM, Arendts G, Nagree Y, Macdonald SPJ, Mitenko H, Rajee M, Burrows S, Brown SG (2017) Neutrophil activation during acute human anaphylaxis: analysis of MPO and sCD 62L. Clin Exp Allergy 47:361–370. https://doi.org/10.1111/cea.12868
Francis A, Bosio E, Stone SF, Fatovich DM, Arendts G, MacDonald SP, Burrows S, Brown SG (2019) Markers involved in innate immunity and neutrophil activation are elevated during acute human anaphylaxis: validation of a microarray study. J Innate Immun 11:63–73. https://doi.org/10.1159/000492301
Metzemaekers M, Gouwy M, Proost P (2020) Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol 17:433–450. https://doi.org/10.1038/s41423-020-0412-0
Yu W, Freeland DMH, Nadeau KC (2016) Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol 16:751. https://doi.org/10.1038/nri.2016.111
Eisenstein AS, Hilliard B, Silwal S, Wang A (2020) Focus: allergic diseases and type ii immunity: food allergy: searching for the modern environmental culprit. Yale J Biol Med 93:733–747. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7757057/pdf/yjbm_93_5_733.pdf
Zhang LL, Chen X, Zheng PY, Luo Y, Lu GF, Liu ZQ, Huang H, Yang PC (2010) Oral Bifidobacterium modulates intestinal immune inflammation in mice with food allergy. J Gastroenterol Hepatol 25:928–934. https://doi.org/10.1111/j.1440-1746.2009.06193.x
Acknowledgements
This work was supported by grants from the National Council for Scientific and Technological Development (CNPq) and the Coordination for the Improvement of Higher Education Personnel (CAPES). SSS received a PhD fellowship from CAPES and VCM received a PhD fellowship from CNPq. VNC, GDC, JRN, DCC, and FSM are CNPq fellowship holders.
Author information
Authors and Affiliations
Contributions
SSS, DCC, and FSM conceived and designed the work. SSS collected the data, and DCR, LMT, and DCC helped with data collection. SSS, DCC, and FSM analyzed and interpreted the data. VNC, GDC, JRN, DCC, and FSM contributed data and reagents or analysis tools. VCM wrote the paper. SSS, DCC, JRN, and FSM critically revised the article. DCC and FSM co-supervised the work. All the authors approved the final version of the article.
Corresponding author
Ethics declarations
Ethics Approval
All animal procedures were carried out according to the standards of the Brazilian Society of Laboratory Animal Science/Brazilian College for Animal Experimentation (available at http://www.mctic.gov.br/concea). This work was approved by the Ethics Committee in Animal Experimentation of the Federal University of Minas Gerais (CEUA/UFMG, protocol # 268/2015).
Conflict of Interest
The authors declare no competing interests.
Disclaimer.
The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santos, S.S., Miranda, V.C., Trindade, L.M. et al. Bifidobacterium longum subsp. longum 51A Attenuates Signs of Inflammation in a Murine Model of Food Allergy. Probiotics & Antimicro. Prot. 15, 63–73 (2023). https://doi.org/10.1007/s12602-021-09846-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09846-9