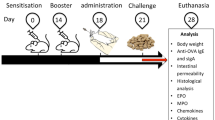
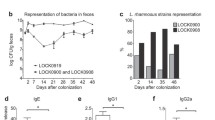
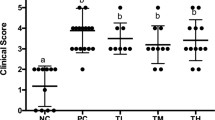
Abstract
Next-generation microorganisms have recently gained prominence in the scientific community, mainly due to their probiotic and postbiotic potentials. However, there are few studies that investigate these potentials in food allergy models. Therefore, the present study was designed to evaluate the probiotic potential of Akkermansia muciniphila BAA-835 in an ovalbumin food allergy (OVA) model and also analyse possible postbiotic potential. To access the probiotic potential, clinical, immunological, microbiological, and histological parameters were evaluated. In addition, the postbiotic potential was also evaluated by immunological parameters. Treatment with viable A. muciniphila was able to mitigate weight loss and serum levels of IgE and IgG1 anti-OVA in allergic mice. In addition, the ability of the bacteria to reduce the injury of the proximal jejunum, the eosinophil and neutrophil influx, and the levels of eotaxin-1, CXCL1/KC, IL4, IL6, IL9, IL13, IL17, and TNF, was clear. Furthermore, A. muciniphila was able to attenuate dysbiotic signs of food allergy by mitigating Staphylococcus levels and yeast frequency in the gut microbiota. In addition, the administration of the inactivated bacteria attenuated the levels of IgE anti-OVA and eosinophils, indicating its postbiotic effect. Our data demonstrate for the first time that the oral administration of viable and inactivated A. muciniphila BAA-835 promotes a systemic immunomodulatory protective effect in an in vivo model of food allergy to ovalbumin, which suggests its probiotic and postbiotic properties.








Similar content being viewed by others
Availability of Data and Material
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Turnbull JL, Adams HN, Gorard DA (2015) The diagnosis and management of food allergy and food intolerances. Aliment Pharmacol Ther 41:3–25. https://doi.org/10.1111/apt.12984
Matsui T, Tanaka K, Yamashita H, Saneyasu KI, Tanaka H, Takasato Y, Sugiura S, Inagaki N, Ito K (2019) Food allergy is linked to season of birth, sun exposure, and vitamin D deficiency. Allergol Int 68:172–177. https://doi.org/10.1016/j.alit.2018.12.003
Aitoro R, Paparo L, Amoroso A, Di Costanzo M, Cosenza L, Granata V, Di Scala C, Nocerino R, Trinchese G, Montella M, Ercolini D, Berni Canani R (2017) Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients 9:672. https://doi.org/10.3390/nu9070672
Sharma G, Im SH (2018) Probiotics as a potential immunomodulating pharmabiotics in allergic diseases: current status and future prospects. Allergy, Asthma Immunol Res 10:575–590. https://doi.org/10.4168/aair.2018.10.6.575
Chernikova DA, Zhao MY, Jacobs JP (2022) Microbiome therapeutics for food allergy. Nutrients 14:5155. https://doi.org/10.3390/nu14235155
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ (2014) Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159–166. https://doi.org/10.1038/nm.3444
Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, Mackay CR (2016) Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 15:2809–2824. https://doi.org/10.1016/j.celrep.2016.05.047
Castellazzi AM, Valsecchi C, Caimmi S, Licari A, Marseglia A, Leoni MC, Caimmi D, Del Giudice MM, Leonardi S, Rosa ML, Marseglia GL (2013) Probiotics and food allergy. Ital J Pediatr 39:1–10. https://doi.org/10.1186/1824-7288-39-47
Kim H, Kwack K, Kim DY, Ji GE (2005) Oral probiotic bacterial administration suppressed allergic responses in an ovalbumin-induced allergy mouse model. FEMS Immunol Med Microbiol 45:259–267. https://doi.org/10.1016/j.femsim.2005.05.005
Lee J, Bang J, Woo HJ (2013) Effect of orally administered Lactobacillus brevis HY7401 in a food allergy mouse model. J Microbiol Biotechnol 23:1636–1640. https://doi.org/10.4014/jmb.1306.06047
Martín R, Langella P (2019) Emerging health concepts in the probiotics field: streamlining the definitions. Front Microbiol 10:1047. https://doi.org/10.3389/fmicb.2019.01047
Souza RO, Miranda VC, Quintanilha MF, Gallotti B, Oliveira SR, Silva JL, Alvarez-leite JI, Jesus LCL, Azevedo V, Vital KD, Fernandes SOA, Cardoso VN, Ferreira E, Nicoli JR, Martins FS (2023) Evaluation of the treatment with Akkermansia muciniphila BAA-835 of chemotherapy-induced mucositis in mice. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-023-10040-2
Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Hupp T, Duchnowska R, Marek-Trzonkowska N, Połom K (2022) Next-generation probiotics - do they open new therapeutic strategies for cancer patients? Gut Microbes 14:2035659. https://doi.org/10.1080/19490976.2022.2035659
Marcial-Coba MS, Cieplak T, Cahú TB, Blennow A, Knøchel S, Nielsen DS (2018) Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage. Food Funct 9:5868–5879. https://doi.org/10.1039/C8FO01331D
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, Park HS, Kim YK, Ryu SH (2018) Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med 50:e450–e450. https://doi.org/10.1038/emm.2017.282
Derrien M, Belzer C, de Vos WM (2017) Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 106:171–181. https://doi.org/10.1016/j.micpath.2016.02.005
Naito Y, Uchiyama K, Takagi T (2018) A next-generation beneficial microbe: Akkermansia muciniphila. J Clin Biochem Nutr 63:33–35. https://doi.org/10.3164/jcbn.18-57
Cuevas-González PF, Liceaga AM, Aguilar-Toalá JE (2020) Postbiotics and paraprobiotics: from concepts to applications. Food Res Int 136:109502. https://doi.org/10.1016/j.foodres.2020.109502
Akter S, Park JH, Jung HK (2020) Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells. J Microbiol Biotechnol 30:477–481. https://doi.org/10.4014/jmb.1911.11019
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, Van der Ark KCH, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD (2017) A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23:107–113. https://doi.org/10.1038/nm.4236
EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA), Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch Ernst KI, Knutsen HK (2021) Safety of pasteurised Akkermansia muciniphila as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J 19:e06780. https://doi.org/10.2903/j.efsa.2021.6780
Reeves P, Nielsen F, Fahey G (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951. https://doi.org/10.1093/jn/123.11.1939
Dourado LPA, Noviello MDLM, Alvarenga DM, Menezes Z, Perez DA, Batista NV, Menezes GB, Ferreira AVM, Souza DG, Cara DC (2011) Experimental food allergy leads to adipose tissue inflammation, systemic metabolic alterations and weight loss in mice. Cell Immunol 270:198–206. https://doi.org/10.1016/j.cellimm.2011.05.008
Miranda VC, Santos SS, Assis HC, Faria AMC, Quintanilha MF, Morão RP, Nicoli JR, Cara DC, Martins FS (2020) Effect of Saccharomyces cerevisiae UFMG A-905 in a murine model of food allergy. Benefic Microbes 11:255–268. https://doi.org/10.3920/BM2019.0113
Saldanha JCS, Gargiulo DL, Silva SS, Carmo-Pinto FH, Andrade MC, Alvarez-Leite JI, Teixeira MM, Cara DC (2004) A model of chronic IgE-mediated food allergy in ovalbumin-sensitized mice. Braz J Med Biol Res 37:809–816. https://doi.org/10.1590/S0100-879X2004000600005
Santos SS, Miranda VC, Trindade LM, Cardoso VN, Reis DC, Cassali GD, Nicoli JR, Cara DC, Martins FS (2021) Bifidobacterium longum subsp. longum 51A attenuates signs of inflammation in a murine model of food allergy. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-021-09846-9
Strath M, Warren DJ, Sanderson CJ (1985) Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods 83:209–215. https://doi.org/10.1016/0022-1759(85)90242-X
Martins FS, Elian SD, Vieira AT, Tiago FC, Martins AK, Silva FC, Souza EL, Sousa LP, Araújo HR, Pimenta PF, Bonjardim CA, Arantes RM, Teixeira MM, Nicoli JR (2011) Oral treatment with Saccharomyces cerevisiae strain UFMG 905 modulates immune responses and interferes with signal pathways involved in the activation of inflammation in a murine model of typhoid fever. Int J Med Microbiol 301:359–364. https://doi.org/10.1016/j.ijmm.2010.11.002
Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, Yuan L, Wang Y, Sun J, Li L, Xiang C (2014) Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol 80:2546–2554. https://doi.org/10.1128/AEM.00003-14
Cara DC, Conde AA, Vaz NM (1997) Immunological induction of flavour aversion in mice. II. Passive/adoptive transfer and pharmacological inhibition. Scand J Immunol 45:16–20. https://doi.org/10.1046/j.1365-3083.1997.d01-363.x
Cardoso CR, Provinciatto PR, Godoi DF, Fonseca MT, Ferreira BR, Teixeira G, Cunha FQ, Pinzan CF, Da Silva JS (2019) The signal transducer and activator of transcription 6 (STAT-6) mediates Th2 inflammation and tissue damage in a murine model of peanut-induced food allergy. Allergol Immunopathol 47:535–543. https://doi.org/10.1016/j.aller.2019.02.006
Kapingidza AB, Kowal K, Chruszcz M (2020) Antigen–Antibody Complexes. In: Hoeger U, Harris J (eds) Vertebrate and invertebrate respiratory proteins, lipoproteins and other body fluid proteins. Subcellular Biochemistry, vol 94. Springer, Cham. https://doi.org/10.1007/978-3-030-41769-7_19
Kelly BT, Grayson MH (2016) Immunoglobulin E, what is it good for? Ann Allergy Asthma Immunol 116:183–187. https://doi.org/10.1016/j.anai.2015.10.026
Meulenbroek LA, de Jong RJ, den Hartog Jager CF, Monsuur HN, Wouters D, Nauta AJ, Knippels LMJ, Joost van Neerven RJ, Ruiter B, Leusen JHW, Hack CE, Bruijnzeel-Koomen CAFM, Knulst AC, Garssen J, van Hoffen E (2013) IgG antibodies in food allergy influence allergen–antibody complex formation and binding to B cells: a role for complement receptors. J Immunol 191:3526–3533. https://doi.org/10.4049/jimmunol.1202398
Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ (1997) Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE-or IgG1-dependent passive anaphylaxis. J Clin Investig 99:901–914. https://doi.org/10.1172/JCI119255
Nunes MPO, van Tilburg MF, Tramontina Florean EOP, Guedes MIF (2019) Detection of serum and salivary IgE and IgG1 immunoglobulins specific for diagnosis of food allergy. PLoS ONE 14:e0214745. https://doi.org/10.1371/journal.pone.0214745
Vidarsson G, Dekkers G, Rispens T (2014) IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5:520. https://doi.org/10.3389/fimmu.2014.00520
Fu L, Xie M, Wang C, Qian Y, Huang J, Sun Z, Zhang H, Wang Y (2020) Lactobacillus casei Zhang alleviates shrimp tropomyosin induced food allergy by switching antibody isotypes through the NF-κB-dependent immune tolerance. Mol Nutr Food Res 64:1900496. https://doi.org/10.1002/mnfr.201900496
Fu L, Peng J, Zhao S, Zhang Y, Su X, Wang Y (2017) Lactic acid bacteria-specific induction of CD4+Foxp3+T cells ameliorates shrimp tropomyosin-induced allergic response in mice via suppression of mTOR signaling. Sci Rep 7:1987. https://doi.org/10.1038/s41598-017-02260-8
Shin HS, Eom JE, Shin DU, Yeon SH, Lim SI, Lee SY (2018) Preventive effects of a probiotic mixture in an ovalbumin-induced food allergy model. J Microbiol Biotechnol 28:65–76. https://doi.org/10.4014/jmb.1708.08051
Acharya KR, Ackerman SJ (2014) Eosinophil granule proteins: form and function. J Biol Chem 289:17406–17415. https://doi.org/10.1074/jbc.R113.546218
Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ (1995) Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med 182:1169–1174. https://doi.org/10.1084/jem.182.4.1169
Lacy P, Latif DA, Steward M, Musat-Marcu S, Man SP, Moqbel R (2003) Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects. J Immunol 170:2670–2679. https://doi.org/10.4049/jimmunol.170.5.2670
Neves JS, Weller PF (2009) Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr Opin Immunol 21:694–699. https://doi.org/10.1016/j.coi.2009.07.011
Ravin KA, Loy M (2016) The eosinophil in infection. Clin Rev Allergy Immunol 50:214–227. https://doi.org/10.1007/s12016-015-8525-4
Rosenberg HF (2016) Eosinophils. Encyclopedia of. Immunobiology 1:334–344. https://doi.org/10.1016/B978-0-12-374279-7.03007-1
Rosenberg HF, Dyer KD, Foster PS (2013) Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 13:9–22. https://doi.org/10.1038/nri3341
Spencer LA, Weller PF (2010) Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol 88:250–256. https://doi.org/10.1038/icb.2009.115
Aratani Y (2018) Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys 640:47–52. https://doi.org/10.1016/j.abb.2018.01.004
Petri B, Sanz MJ (2018) Neutrophil chemotaxis. Cell Tissue Res 371:425–436. https://doi.org/10.1007/s00441-017-2776-8
Sawant KV, Sepuru KM, Lowry E, Penaranda B, Frevert CW, Garofalo RP, Rajarathnam K (2021) Neutrophil recruitment by chemokines Cxcl1/KC and Cxcl2/MIP2: role of Cxcr2 activation and glycosaminoglycan interactions. J Leukoc Biol 109:777–791. https://doi.org/10.1002/JLB.3A0820-207R
Strzepa A, Pritchard KA, Dittel BN (2017) Myeloperoxidase: a new player in autoimmunity. Cell Immunol 317:1–8. https://doi.org/10.1016/j.cellimm.2017.05.002
Muñoz-Cano R, Picado C, Valero A, Bartra J (2016) Mechanisms of anaphylaxis beyond IgE. J Investig Allergol Clin Immunol 26:73–82. https://doi.org/10.18176/jiaci.0046
Yu W, Freeland DMH, Nadeau KC (2016) Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol 16:751–765. https://doi.org/10.1038/nri.2016.111
Demirci M, Tokman HB, Uysal HK, Demiryas S, Karakullukcu A, Saribas S, Cokugras H, Kocazeybek BS (2019) Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol 47:365–371. https://doi.org/10.1016/j.aller.2018.12.009
Méndez CS, Bueno SM, Kalergis AM (2021) Contribution of gut microbiota to immune tolerance in infants. J Immunol Res 2021:7823316. https://doi.org/10.1155/2021/7823316
Nakayama J, Kobayashi T, Tanaka S, Korenori Y, Tateyama A, Sakamoto N, Kiyohara S, Shirakawa T, Sonomoto K (2011) Aberrant structures of fecal bacterial community in allergic infants profiled by 16S rRNA gene pyrosequencing. FEMS Immunol Med Microbiol 63:397–406. https://doi.org/10.1111/j.1574-695X.2011.00872.x
Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E (2017) Dysbiosis and the immune system. Nat Rev Immunol 17:219–232. https://doi.org/10.1038/nri.2017.7
Tsilochristou O, du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, Bahnson HT, Radulovic S, Basting M, Plaut M, Lack G (2019) Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. J Allergy Clin Immunol 144:494–503. https://doi.org/10.1016/j.jaci.2019.04.025
Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, Mandhane SE, Turvey SE, Subbarao P, Becker AB, Scott JA, Kozyrskyj AL (2015) Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy 45:632–643. https://doi.org/10.1111/cea.12487
Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, Mills DA (2010) Consumption of human milk oligosaccharides by gut related microbes. J Agric Food Chem 58:5334–5340. https://doi.org/10.1021/jf9044205
Atarashi K, Honda K (2011) Microbiota in autoimmunity and tolerance. Curr Opin Immunol 23:761–768. https://doi.org/10.1016/j.coi.2011.11.002
Food and Agriculture Organization / World Health Organization (FAO/WHO) Working Group (2002) Guidelines for the evaluation of probiotics in food. FAO/WHO, London, ON, Canada
Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EM, Sanders ME, Shamir R, Swann JR, Szajewska H, Vinderola G (2021) The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol 18:649–667. https://doi.org/10.1038/s41575-021-00440-6
Liong MT (2008) Safety of probiotics: translocation and infection. Nutr Rev 66:192–202. https://doi.org/10.1111/j.1753-4887.2008.00024.x
Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C (2017) Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract Res Clin Gastroenterol 31:637–642. https://doi.org/10.1016/j.bpg.2017.10.001
Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skkurnik M, Boeren S, Satokari R, Mercenier A, Palva A, Smidt H, deVos W, Belzer C (2017) Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 12:e0173004. https://doi.org/10.1371/journal.pone.0173004
Funding
This work was supported by grants from the Brazilian National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES), and the Research Support Foundation of the State of Minas Gerais (FAPEMIG), Brazil. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. VCM received a PhD fellowship from CNPq. AMCF, JRN, and FSM are CNPq fellowship holders.
Author information
Authors and Affiliations
Contributions
VCM and FSM analysed data and wrote the paper. FSM and DCC designed and supervised the research. VCM, ROS, MFQ, BG, and HCA, performed experiments and analysed data. AMCF, JRN, DCC, and FSM provided expertise in laboratory resources, improved the issue, and critically revised the article. All authors approved the final version of the article.
Corresponding author
Ethics declarations
Ethics Approval
All animal procedures were carried out according to the standards of the Brazilian Society of Laboratory Animal Science/Brazilian College for Animal Experimentation (available at http://www.mctic.gov.br/concea). This work was approved by the Ethics Committee in Animal Experimentation of the Federal University of Minas Gerais (CEUA/UFMG, protocol # 110/2019).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miranda, V.C., Souza, R.O., Quintanilha, M.F. et al. A Next-Generation Bacteria (Akkermansia muciniphila BAA-835) Presents Probiotic Potential Against Ovalbumin-Induced Food Allergy in Mice. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10076-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10076-4