Abstract
Background
Probiotic bacteria can induce immune regulation or immune tolerance in patients with allergic diseases, but the underlying mechanisms are still unclear. There has been a growing interest in the use of beneficial bacteria for allergic diseases recently. This study aimed at exploring whether Clostridium butyricum CGMCC0313-1 (C. butyricum) can reduce β-lactoglobulin(BLG)-induced intestinal anaphylaxis in a murine model of food allergy.
Methods
The preventive and therapeutic effects of oral C. butyricum on anaphylactic symptoms induced via BLG in food allergy mice were investigated. Intestinal anaphylaxis, T helper (Th)-specific cytokines and transcription factors, secretory IgA (sIgA), CD4+ CD25+ Foxp3Treg cell and histopathological alterations were examined.
Results
Clostridium butyricum significantly ameliorated intestinal anaphylaxis symptoms in the food allergy mice. sIgA and CD4+ CD25+ Foxp3Treg cell were increased by oral C. butyricum. It also reversed the imbalance of Th1/Th2 andTh17/Treg.
Conclusions
Clostridium butyricum reduces BLG-induced intestinal anaphylaxis in mice and might be an additional or supplementary therapy for food allergy.
Similar content being viewed by others
Background
Food is a foreign antigen that is necessary for nutrition. Inevitably, food antigens present a continuous challenge throughout life. Humans have adapted to food via mechanisms of immune tolerance. Food allergy is defined as abnormal immune responses resulting from breakdown of natural oral tolerance [1]. Food allergy is an increasing public health problem worldwide [2–5] and has been estimated to affect approximately 5% of adults and 8% of children [6]. Among food allergies, cow’s milk allergy is one of the earliest and most prevalent food allergies and β-lactoglobulin (BLG) is the major allergen [7, 8]. Diets based on cow’s milk play a major role in children’s nutrition as it has an essential effect on the patient’s quality of life. Additionally, up to now the current standard for prevention of food allergy is still the strict allergen avoidance and the elimination of the triggering food from the diets [9]. However, accidental ingestion is difficult to be absolutely avoided in our life. Therefore, effectively prevent and manage food allergy to restore immune tolerance is particularly needed.
A previous study shows that the increased prevalence of allergic diseases is associated with decreased microbial exposure and alteration of microbial communities represented in the gut microbiota [10]. It has been demonstrated that the composition and metabolic activity of the microbiota is crucial for both the maintenance and the development of immune homeostasis, as well as the induction of immune tolerance [11, 12]. Intervention strategies targeting the intestinal microbiota include the deliberate administration of probiotic bacteria. Probiotics are live microorganisms that confer a health benefit to the host when administered in adequate amounts [13]. Different bacterial strains or their mixtures have been used to assess their protective effects for allergic diseases in clinical trials, but the results have been controversial [14–16]. Possible mechanisms of their protective action include both the induction of regulatory dendritic cells (regDCs) and T cells and the skewing the imbalances of T helper (Th)1/Th2 as well as Th17/Treg, together with the enhancement of the epithelial barrier function [10, 17–21]. Imbalances in Th responses can also be detected using Th-specific transcription factors: T-bet for Th1 cells, GATA-3 for Th2 cells, retinoic acid orphan receptor-γt (RORγt) for Th17 cells and forkhead box P3 (Foxp3) for Tregs [22]. Nevertheless, the knowledge of molecular mechanisms underlying probiotic-host interactions that shape host immune system in a protective setting is still incomplete. Mouse models of food allergy to clinically relevant allergens could be helpful in providing information difficult to be obtained in human studies.
Clostridium butyricum CGMCC0313-1 (C. butyricum) has been widely used for improving gastrointestinal function as probiotics [23, 24]. However, the benefit of C. butyricum on food allergy is rarely reported. This study aimed to explore whether oral C. butyricum can reduce food allergy in mice. Findings from this study will contribute to a better understanding of the protective effects of the beneficial bacteria in allergic diseases.
Methods
Mice
Male BALB/c mice of 6–8 weeks were purchased from the Laboratory Animal Center of the Fourth Military Medical University and acclimated to their new environment for 1 week. Animals were housed under conventional conditions, fed standard mouse pellets and water adlibitum. Experimental procedures were approved by the Ethics Committee for Animal Studies of the Fourth Military Medical University (20150901) and performed in accordance with their guidelines of the Institutional Animal Care and Use Committee.
The probiotics
The C. butyricum powder (Kexing Biotech Company limited, Weifang, Shandong, China) was stored at −20 °C. Drinks were prepared using normal saline (NS) only or NS plus C. butyricum. The concentration of C. butyricum was 5 × 108 CFU/ml.
Mouse model of food allergy
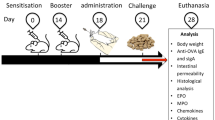
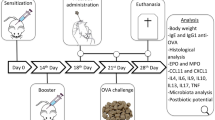
BALB/c mice were randomly assigned into 4 experimental groups with 10 in each group. The 4 groups of mice were treated as follow: the food allergy group received 20 mg BLG (Sigma, St. Louis, MO, USA) plus 10 μg cholera toxin(CTX, Sigma) orally on days 7, 14 and 21, and followed by oral administration of 100 mg BLG challenge on day 28; the preventive and treated groups were approached as the food allergy group, and the animals were given 200 μl C. butyricum feeding from days 1 to 21 or from days 22 to 28 respectively; mice from the control group only received 10 μg CTX orally, and followed by challenge with NS (Fig. 1).
The protocols used for the mouse models of food allergy. Male BALB/c mice were sensitized orally with BLG plus CTX or only CTX on day 7, 14 and 21, and followed by oral administration of 100 mg BLG challenge on day 28. The animals received C. butyricum feeding from day 1 to day 21 or from day 22 to day 28 by oral gavage
Measurement of intestinal anaphylaxis
Intestinal anaphylaxis was assessed in challenged mice by the allergic diarrhea and circulating mouse mast cell protease 1 (mMCP-1) levels as previously described [25, 26]. Briefly, mice were observed for the presence of diarrhea for 1 h after the challenge and were scored as diarrhea positive or negative. Diarrhea and anaphylactic symptoms were scored by visually monitoring mice for 60 min after challenge. Diarrhea—0, normal stools; 1, a few wet and unformed stools; 2, a number of wet and unformed stools with moderate perianal staining of the coat; 3, severe, watery stool with severe perianal staining of the coat. Anaphylactic symptoms—0, no symptoms; 1, reduced activity, trembling of limbs; 2, loss of consciousness, no activity upon prodding; 3, convulsions, death. Serum was obtained 1 h following the final antigen challenge for measurement of mMCP-1 using the commercial enzyme-linked immunosorbentassay (ELISA) (eBioscience, San Diego, CA, USA) according to manufacturer instructions (Table 1).
The measurement of cytokines and immunoglobulin
Serum samples were collected to assay the presence of cytokines and immunoglobulin (Ig). Levels of serum IL-4, IL-5, IL-13, IL-17, INF-γ, IL-10, TGF-β1 and the total IgE were measured by ELISA Kits (RD system, Boston, MA, USA; Uscn Life, Wuhan, Hubei, China) following the manufacture’s protocol.
Secretory IgA
Small intestine was rinsed with 10 ml of cold PBS. Intestinal lavages were centrifuged at 12,000×g for 20 min at 4 °C, and levels of secretory IgA (sIgA) in the supernatants were determined by ELISA (Uscn Life) as previously described [27].
RNA isolation and quantitative real-time PCR
After mice were sacrificed on day 29, the spleen was dissected and immersed in RNAlater® (Ambion, Austin, TX, USA) for PCR. Total RNA (n = 6 mice per group) was carried out using Trizol (Ambion) from whole spleen tissue and reverse-transcribed to cDNA by a PrimeScript™ RT Master Mix Kit (TaKaRa, Tokyo, Japan). cDNA was amplified using SYBR® Premix Ex Taq™ II Kit (TaKaRa) and run in the Real-Time PCR Detection System. Primers for T cell transcription factors were designed and synthesized by TaKaRa Biotechnology (Dalian, Liaoning, China). The sequences were listed in Table 2. The relative levels of gene expression were calculated by reference to the level of β-actin using the ΔΔCT method [ 28 ].
Flow cytometry
Single-cell suspensions isolated from mesenteric lymph nodes (MLN) were stained for FACS analyses as described previously [29]. Cells were first stained for surface markers including CD4-PerCP-Cy5.5, CD25-FITC (BD Pharmingen, San Diego, CA, USA). If required, cells were then fixed and permeabilized by BD Cytofix/Cytoperm reagent (BD Bioscience, San Jose, CA, USA) and stained for intracellular expression markers, Foxp3-PE. Data were acquired with FACSCanto (Beckman Coulter, Miami, FL, USA) and analyzed by FlowJo 10.0.7 software.
Histological analysis
Paraffin-embedded sections of proximal jejunum were stained with hematoxylin and eosin (H&E) for morphological analysis. Villus length was determined by measuring the distance in μm from the crypt neck to the villus tip using Image J software. Six animals from each experimental group were evaluated, and a minimum of 12 well-oriented villi from each section were measured. Data were reported as villus size measured in μm. Morphological analyses were performed in a blinded manner to prevent observer bias.
Statistical analysis
Data were expressed as the mean ± standard error means (SEMs). All data were analyzed with SPSS17.0 software (SPSS Inc, Illinois, Chicago, USA). One-Way ANOVA and Mann–Whitney U non-parametric test was conducted to determine the statistical significance, where appropriate. A P value of <0.05 was considered statistically significant.
Results
Clostridium butyricum ingestion inhibits the development of intestinal anaphylaxis
Oral challenges with BLG in sensitized mice lead to increasing severity of diarrhea and anaphylaxis, and a robust increase in circulating mMCP-1. Administration of C. butyricum obviously inhibited BLG-induced food allergy, namely diarrhea occurrence and scores (Fig. 2a), anaphylactic response and scores (Fig. 2b), and levels of mMCP-1 (Fig. 2c), suggesting a protective effect of C. butyricum on food allergy in mice.
Clostridium butyricum inhibits the development of intestinal anaphylaxis. Diarrhea occurrence, diarrhea scores (a), anaphylactic response, anaphylactic score (b) and levels of mMCP-1 (c) were monitored. Male BALB/c mice were given NS or sensitized/challenged with BLG ± treatment with C. butyricum. Con, n = 10 for the control group; FA, n = 10 for the food allergy group; Pre, n = 10 for the preventive group; and Tre, n = 10 for the treated group. Results are shown as means ± SEMs. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group, and # P < 0.05, ## P < 0.01, ### P < 0.001 versus the food allergy group
Cytokines and immunoglobulin
Cytokines and total IgE were determined using ELISA Kits (Fig. 3). Compared with the control group, the levels of inflammatory cytokines in the serum (Fig. 3a–d: IL-4, IL-5, IL-13, IL-17) were significantly increased in the food allergy group and decreased in the preventive and treated groups. However, the IL-17 of the preventive group was not significant different from that of the food allergy group (P > 0.05). Levels of INF-γ, IL-10 and TGF-β1 (Fig. 3e–g) in the food allergy group were decreased compared with the counterparts of the control group. They increased in the groups received C. butyricum orally except for the IL-10 in the preventive group.
The effect of C. butyricum on cytokines and immunoglobulin. Levels of serum IL-4 (a), IL-5 (b), IL-13 (c), IL-17 (d), INF-γ (e), IL-10 (f), TGF-β1 (g) and the total IgE (h) were measured by ELISA Kits. Male BALB/c mice were given NS or sensitized/challenged with BLG ± treatment with C. butyricum. Con, n = 10 for the control group; FA, n = 10 for the food allergy group; Pre, n = 10 for the preventive group; and Tre, n = 10 for the treated group. Results are shown as means ± SEMs. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group, and # P < 0.05, ## P < 0.01, ### P < 0.001 versus the food allergy group
Compared with the control group, the total IgE (Fig. 3h) was significantly elevated in the food allergy group (P < 0.01). However, they decreased dramatically in the preventive and treated group.
Clostridium butyricum supress the intestinal levels of sIgA
Since sIgA contributes to the barrier function and it is involved in immunological homeostasis [30], we measured levels of this immunoglobulin in small intestinal lavage fluids. sIgA levels were augmented in allergic mice following local inflammation. C. butyricum was effective in suppressing such augment in the preventive and treated group (Fig. 4).
Clostridium butyricum supress the intestinal levels of sIgA. Levels of sIgA were determined by ELISA. Male BALB/c mice were given NS or sensitized/challenged with BLG ± treatment with C. butyricum. Con, n = 10 for the control group; FA, n = 10 for the food allergy group; Pre, n = 10 for the preventive group; and Tre, n = 10 for the treated group. Results are shown as means ± SEMs. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group, and # P < 0.05, ## P < 0.01, ### P < 0.001 versus the food allergy group
Clostridium butyricum results in a strong up-regulation of mRNA for Tbet and Foxp3
To further explore the effects of the different interventions on Th responses in the spleen, the mRNA expression of Th specific transcription factors was measured (Fig. 5). The expression of Th1-(Tbet) and Treg-(Foxp3) transcription factors was significantly decreased in the food allergy group as compared to the control group, whereas the expression of Th2-(Gata3) and Th17-(Rorγt) transcription factors increased dramatically. However, Tbet and Foxp3 expression were significantly increased in the preventive group. Compared to the food allergy group, Tbet expression was increased in the treated group, however, the expression of Foxp3 remained unchanged.
Clostridium butyricum up-regulates Tbet and Foxp3 mRNA expression. The mRNA expression of Th specific transcription factors was measured by quantitative real-time PCR. Male BALB/c mice were given NS or sensitized/challenged with BLG ± treatment with C. butyricum. Con, n = 10 for the control group; FA, n = 10 for the food allergy group; Pre, n = 10 for the preventive group; and Tre, n = 10 for the treated group. Results are shown as means ± SEMs. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group, and # P < 0.05, ## P < 0.01, ### P < 0.001 versus the food allergy group
Clostridium butyricum skews the immune response away from Th2 and towards Treg
To determine the extent of Th response skewing in the spleen, ratios for Gata3/Tbet (Th2/Th1), Foxp3/Rorγt (Treg/Th17), Foxp3/Gata3 (Treg/Th2) and Foxp3/Tbet (Treg/Th1) mRNA expression were calculated (Fig. 6). The food allergy groups showed a Th2-skewed immune response represented by a significant increase in Gata3/Tbet ratio as compared to control group. C. butyricum reduced this ratio in the treated group, but not the preventive group. Interestingly, the Foxp3/Rorγt (except for the treated group)and Foxp3/Gata3 ratios were significantly increased in the groups received C. butyricum orally as compared to the food allergy group indicating an increase in Treg-associated responses. The ratio of Foxp3/Tbet did not differ significantly among the different treatment groups.
Clostridium butyricum skews the immune response away fromTh2 and towards Treg. Ratios for Gata3/Tbet (Th2/Th1), Foxp3/Rorγt (Treg/Th17), Foxp3/Gata3 (Treg/Th2) and Foxp3/Tbet (Treg/Th1) mRNA expression in whole spleen tissue are shown for each C. butyricum treatment. Male BALB/c mice were given NS or sensitized/challenged with BLG ± treatment with C. butyricum. Con, n = 10 for the control group; FA, n = 10 for the food allergy group; Pre, n = 10 for the preventive group; and Tre, n = 10 for the treated group. Results are shown as means ± SEMs. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group, and # P < 0.05, ## P < 0.01, ### P < 0.001 versus the food allergy group
The effect of C. butyricum on Treg cell
We examined the potential induction of Foxp3 by regulatory T cells in the MLN of the mice. Food allergy mice had a lower percentage of CD4+ CD25+ Foxp3+ T cells in the MLN compared to nonsensitized controls. The numbers of CD4+ CD25+ Foxp3+ Treg cells was increased in the preventive group, however, no significant differences in these regulatory T-cell populations were observed in the treated group as compared to the food allergy group (Fig. 7).
The effect of C. butyricum on Treg cell. The gating strategy (a) and effect of oral administration of C. butyricum on populations of CD4+ CD25+ Foxp3+ (b) cells in the MLN. Male BALB/c mice were given NS or sensitized/challenged with BLG ± treatment with C. butyricum. Con, n = 10 for the control group; FA, n = 10 for the food allergy group; Pre, n = 10 for the preventive group; and Tre, n = 10 for the treated group. Results are shown as means ± SEMs. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group, and # P < 0.05, ## P < 0.01, ### P < 0.001 versus the food allergy group
Clostridium butyricum reduces histological changes in allergic mice
Ingestion of BLG induced submucosal edema and increased inflammatory cell infiltration in the gut of sensitized mice (Fig. 8a). Allergic mice had decrease villus height whereas C. butyricum-treatment animals had a similar alteration to the one found in the control mice (Fig. 8b). Thus, oral administration of C. butyricum reduced the development of intestinal inflammation.
Clostridium butyricum reduces histological changes in allergic mice. Representative H&E staining of intestinal sections (a, 200× magnification). Bar graph of villus height (b) determined by measuring vertically well-oriented crypt villus units from H&E-stainedsections of mice. Male BALB/c mice were given NS or sensitized/challenged with BLG ± treatment with C. butyricum. Con, n = 10 for the control group; FA, n = 10 for the food allergy group; Pre, n = 10 for the preventive group; and Tre, n = 10 for the treated group. Results are shown as means ± SEMs. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group, and # P < 0.05, ## P < 0.01, ### P < 0.001 versus the food allergy group
Discussion
Food allergy is an increasing health problem and it has a significant impact on the health and daily activities of allergic individuals. The aim of this study was to investigate the preventive and therapeutic effect of C. butyricum on food allergy in mice. In our study, there were higher scores about diarrhea and anaphylactic symptoms in the food allergy group as compared to the control group. Positive incidence of mice with allergic diarrhea and anaphylactic symptoms was increased in the food allergy mice. Additionally, the expression of mMCP-1 and total IgE were significantly increased in the allergic mice. These were similar to the results observed in a study of ovalbumin allergy [31], however, we lack the data about BLG specific IgE due to the unavailable of the kit from the manufacturer. More importantly, decreased villus length and increased inflammatory reaction were observed in the gut slides of the food allergy mice. Hence, we successfully mimic BLG-induced allergic intestine inflammation in our mouse model.
Various strains of probiotics have been used in animal models of food allergy. Probiotic VSL#3-induced TGF-β ameliorates food allergy inflammation in a mouse model of peanut sensitization through the induction of regulatory T cells in the gut mucosa [32]. A combination of specific immunotherapy with C. butyricum significantly enforces the therapeutic effect on inhibiting the food allergen related inflammation in the intestine, which can be a novel approach for the treatment of food allergy [33]. A recent study suggests that oral supplementation of Lactobacillus paracasei L9 (L9) can reduce the development of allergic sensitization to BLG, likely through regDCs mediated active suppression [34]. In a BALB/c mouse model of BLG allergy, oral administration of Lactobacillus acidophilus (L. acidophilus)can suppress the major allergic symptoms probably due to improve the regulatory T (Treg)/Th17 balance and inhibit the IL-6 production [35]. It has been confirmed that Bifidobacterium longum BBMN68 (BBMN68) may be a suitable therapeutic approach to the alleviation of food allergies likely through the specific induction of CD11c+ CD103+ DCs and semi-mature DCs [36]. Similarly, oral administration of C. butyricum to the mouse models of food allergy is effective in reducing allergic inflammation in our study.
Th2 cells play a key role in the pathogenesis of food allergy, and patients with food allergy were reported to have Th1/Th2 imbalances as well as disturbed Th17/Treg balances. Th2 cytokines including IL-4, IL-5, IL-13 [37]. IFN-γ released by Th1 can inhibit the development of Th2. Th17 cells release IL-17 which is connected with inflammation in the gut [38, 39]. High levels of IL-10, TGF-β expressed by regDCs can directly mediate the conversion of T cells into Foxp3+ Treg cells and induce immune tolerance [17]. Th2 dominance was observed in the food allergy group represented by a significant decrease in Th1 and Treg transcription factors and high Gata3/Tbet ratio. Hence, our model mimics the Th2-responses found in food allergy. Importantly, C. butyricum shifted the immune balance towards Th1 and Treg, with significantly increased Foxp3/Rorγt and Foxp3/Gata ratios and a significantly decreased Gata3/Tbet ratio. These findings are consistent with results of those previous studies. BBMN68 and L9 has been already shown to significantly reduce BLG-specific hypersensitivity reactions by suppressing the aberrant balance of Th1/Th2 responses with increasing the number of CD4+ CD25+ Foxp3+ Treg cells [34, 40]. L. acidophilus supplementation is capable of reducing allergic symptoms in a mouse model of food allergy through reversing the imbalance of regulatory T (Treg)/Th17. More importantly, the above observed effects of beneficial bacteria on the Th responses are mirrored by the detection of CD4+ CD25+ Foxp3+ Tregs in the MLN of the animals. Tregs play a key role in balancing immune responses and it was demonstrated that increased expression of Foxp3 in Tregs is directly associated with increased function of these cells [41]. Preventive ingestion of C. butyricum increase the populations of CD4+ CD25+ Foxp3+ cells in the MLN and induced high levels of TGF-β and IL-10 in the serum, indicating the powerful effect of probiotics on modulating the intestinal immune response. These were similar to results observed in a study of ovalbumin allergy, which showed that LGG only induced the number of CD4+ CD25+ Foxp3+ Treg cells and TGF-β secretion [42]. However, No significant differences in the percentage of CD4+ CD25+ Foxp3+ cells were observed in the treated group as compared with the food allergy group.
sIgA plays a protective role by antigen binding and exclusion [43]. Food allergy may cause impaired epithelial barrier function, including sIgA release into the gut lumen [44]. We observed enhanced levels of sIgA in feces of the food allergy mice. sIgA can mediate a potent anti-inflammatory function following the interaction with SIGNR1 on DC which induces an immune tolerance via regulatory T cell expansion [45]. Therefore, the increase of the protective secretory immunoglobulin might be a regulatory mechanism triggered to counteract allergic inflammation to BLG in the gut mucosa. Levels of sIgA enhanced in allergic mice suggesting that inflammation triggered this modulatory component of immune response. The groups received C. butyricum orally, on the other hand, had little inflammatory consequences and therefore were not accompanied by augmented levels of sIgA.
Conclusions
To our knowledge, this is the first report in which the preventive and therapeutic effects of C. butyricum on food allergy were investigated. The findings reported here indicate that oral probiotics such as C. butyricum, with established anti-inflammatory and anti-allergic activity, can significantly modulate the mucosal immune response and may represent an effective and safe strategy for patients with food allergies, for which no established and effective cures based on the pathogenetic mechanisms are available.
Abbreviations
- C. butyricum :
-
Clostridium butyricum CGMCC0313-1
- BLG:
-
ß-lactoglobulin
- Th:
-
T helper
- sIgA:
-
secretory IgA
- DC:
-
dendritic cells
- RORγt:
-
retinoic acid orphan receptor-γt
- Foxp3:
-
forkhead box P3
- CTX:
-
cholera toxin
- NS:
-
normal saline
- mMCP-1:
-
mouse mast cell protease 1
- ELISA:
-
enzyme-linked immunosorbent assay
- Ig:
-
immunoglobulin
- MLN:
-
mesenteric lymph nodes
- H&E:
-
hematoxylin and eosin
- L9:
-
Lactobacillus paracasei L9
- regDCs:
-
regulatory dendritic cells
- L. acidophilus :
-
Lactobacillus acidophilus
- BBMN68:
-
bifidobacterium longum BBMN68
- SEMs:
-
standard error means
References
Shin HS, Bae MJ, Jung SY, Shon DH. Preventive effects of skullcap (Scutellaria baicalensis) extract in a mouse model of food allergy. J Ethnopharmacol. 2014;153(3):667–73.
Prescott S, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011;22(2):155–60.
Johnston LK, Chien KB, Bryce PJ. The immunology of food allergy. J Immunol. 2014;192(6):2529–34.
Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127(3):594–602.
Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med. 2009;60:261–77.
Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133(2):291–307.
Braun-Fahrlander C, von Mutius E. Can farm milk consumption prevent allergic diseases? Clin Exp Allergy. 2011;41(1):29–35.
Jo J, Garssen J, Knippels L, Sandalova E. Role of cellular immunity in cow’s milk allergy: pathogenesis, tolerance induction, and beyond. Mediat Inflamm. 2014;2014:249784.
Kumar S, Verma AK, Das M, Dwivedi PD. Molecular mechanisms of IgE mediated food allergy. Int Immunopharmacol. 2012;13(4):432–9.
Frei R, Lauener RP, Crameri R, O’Mahony L. Microbiota and dietary interactions: an update to the hygiene hypothesis? Allergy. 2012;67(4):451–61.
Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330(6012):1768–73.
Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10(10):735–44.
Gourbeyre P, Denery S, Bodinier M. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J Leukoc Biol. 2011;89(5):685–95.
Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;4:D6475.
Vliagoftis H, Kouranos VD, Betsi GI, Falagas ME. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101(6):570–9.
Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for the treatment of eczema: a systematic review. Clin Exp Allergy. 2009;39(8):1117–27.
Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+ Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA. 2010;107(5):2159–64.
Lyons A, O’Mahony D, O’Brien F, MacSharry J, Sheil B, Ceddia M, Russell WM, Forsythe P, Bienenstock J, Kiely B, Shanahan F, O’Mahony L. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy. 2010;40(5):811–9.
Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4607–14.
Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8(4):345–50.
Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500.
Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol. 2010;126(6):1081–91.
Shang H, Sun J, Chen YQ. Clostridium butyricum CGMCC0313.1 modulates lipid profile, insulin resistance and colon homeostasis in obese mice. PLoS ONE. 2016;11(4):e154373.
Zhang HQ, Ding TT, Zhao JS, Yang X, Zhang HX, Zhang JJ, Cui YL. Therapeutic effects of Clostridium butyricum on experimental colitis induced by oxazolone in rats. World J Gastroenterol. 2009;15(15):1821–8.
Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112(11):1666–77.
Yamaki K, Yoshino S. Tyrosine kinase inhibitor sunitinib relieves systemic and oral antigen-induced anaphylaxes in mice. Allergy. 2012;67(1):114–22.
Gomes-Santos AC, Moreira TG, Castro-Junior AB, Horta BC, Lemos L, Cruz DN, Guimaraes MA, Cara DC, McCafferty DM, Faria AM. New insights into the immunological changes in IL-10-deficient mice during the course of spontaneous inflammation in the gut mucosa. Clin Dev Immunol. 2012;2012:560817.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45.
Karimi K, Bienenstock J, Wang L, Forsythe P. The vagus nerve modulates CD4+ T cell activity. Brain Behav Immun. 2010;24(2):316–23.
Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12(12):821–32.
Castillo-Courtade L, Han S, Lee S, Mian FM, Buck R, Forsythe P. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy. 2015;70(9):1091–102.
Barletta B, Rossi G, Schiavi E, Butteroni C, Corinti S, Boirivant M, Di Felice G. Probiotic VSL#3-induced TGF-beta ameliorates food allergy inflammation in a mouse model of peanut sensitization through the induction of regulatory T cells in the gut mucosa. Mol Nutr Food Res. 2013;57(12):2233–44.
Shi Y, Xu LZ, Peng K, Wu W, Wu R, Liu ZQ, Yang G, Geng XR, Liu J, Liu ZG, Liu Z, Yang PC. Specific immunotherapy in combination with Clostridium butyricum inhibits allergic inflammation in the mouse intestine. Sci Rep. 2015;5:17651.
Yang J, Ren F, Zhang H, Jiang L, Hao Y, Luo X. Induction of regulatory dendritic cells by Lactobacillus paracasei L9 prevents allergic sensitization to bovine beta-lactoglobulin in mice. J Microbiol Biotechnol. 2015;25(10):1687–96.
Li AL, Meng XC, Duan CC, Huo GC, Zheng QL, Li D. Suppressive effects of oral administration of heat-killed Lactobacillus acidophilus on T helper-17 immune responses in a bovine beta-lactoglobulin-sensitized mice model. Biol Pharm Bull. 2013;36(2):202–7.
Adel-Patient K, Ah-Leung S, Creminon C, Nouaille S, Chatel JM, Langella P, Wal JM. Oral administration of recombinant Lactococcus lactis expressing bovine beta-lactoglobulin partially prevents mice from sensitization. Clin Exp Allergy. 2005;35(4):539–46.
Noval RM, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol. 2016;138(3):639–52.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517.
Tanabe S. The effect of probiotics and gut microbiota on Th17 cells. Int Rev Immunol. 2013;32(5–6):511–25.
Yang J, Zhang H, Jiang L, Guo H, Luo X, Ren F. Bifidobacterium longum BBMN68-specific modulated dendritic cells alleviate allergic responses to bovine beta-lactoglobulin in mice. J Appl Microbiol. 2015;119(4):1127–37.
Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182(1):148–53.
Finamore A, Roselli M, Britti MS, Merendino N, Mengheri E. Lactobacillus rhamnosus GG and Bifidobacterium animalis MB5 induce intestinal but not systemic antigen-specific hyporesponsiveness in ovalbumin-immunized rats. J Nutr. 2012;142(2):375–81.
Faria AM, Gomes-Santos AC, Goncalves JL, Moreira TG, Medeiros SR, Dourado LP, Cara DC. Food components and the immune system: from tonic agents to allergens. Front Immunol. 2013;4:102.
Brandtzaeg P. Update on mucosal immunoglobulin A in gastrointestinal disease. Curr Opin Gastroenterol. 2010;26(6):554–63.
Mkaddem SB, Christou I, Rossato E, Berthelot L, Lehuen A, Monteiro RC. IgA, IgA receptors, and their anti-inflammatory properties. Curr Top Microbiol Immunol. 2014;382:221–35.
Authors’ contributions
JZ, HS, YZ and XS participated in the conception and design of the study. JZ, HS, QL, HW, ML and JH performed laboratory work. JZ, HS, QL, HW and MZ analyzed the data and wrote the manuscript. JZ, HS, XS and YZ contributed to the analysis and helped in the manuscript discussion. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to acknowledge and thank our funding sources.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Experimental procedures were approved by the Ethics Committee for Animal Studies of the Fourth Military Medical University (20150901) and performed in accordance with their guidelines of the Institutional Animal Care and Use Committee.
Funding
This work was supported by the innovation of science and Technology Commission of Shenzhen Municipality (JCYJ20120828092009036) and the National Natural Science Foundation (31371151).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, J., Su, H., Li, Q. et al. Oral administration of Clostridium butyricum CGMCC0313-1 inhibits β-lactoglobulin-induced intestinal anaphylaxis in a mouse model of food allergy. Gut Pathog 9, 11 (2017). https://doi.org/10.1186/s13099-017-0160-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-017-0160-6