Abstract
Given the important functional roles of sponges in the coral reef community, few studies have looked at sponge community assemblages in Singapore. This study was conducted to assess the sponge diversity, species richness, and species abundance of intertidal sponge communities in Singapore’s coral reefs, including Pulau Hantu, P. Subar Laut, Tanjong Rimau, and Labrador Park. Belt transects measuring 10 m by 1 m were established to survey the sponge communities at these reef sites. Based on sponge morphology, a total of 28 morphospecies were identified, belonging to eight different sponge orders, namely Chondrillida, Clionaida, Dictyoceratida, Haplosclerida, Poecilosclerida, Suberitida, Tetractinellida, and Verongiida. Univariate statistical analyses revealed that sponge diversity, species richness, and abundance at P. Hantu site 2 were significantly higher than that in Labrador Park. In addition, multivariate statistical analyses showed that in terms of community structure, there were three distinct clusters found at P. Hantu site 2, Labrador Park, and P. Subar Laut. The formation of these distinct clusters was sponge species specific and shaped by environmental factors and anthropogenic stresses. Preliminary sponge data presented in this study contribute to the regional biogeography of sponges and could serve as baseline data for future studies, including ecological research and biomonitoring of marine invertebrates, and support marine conservation efforts in Singapore.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sponges make up a great percentage of coral reef fauna across the world, playing key functional roles in the reef ecosystem, such as providing habitats and shelters for many reefs fauna, including echinoderms, polychaetes, fishes, and crustaceans (Diaz and Rützler 2001; Fiore and Jutte 2010). For instance, a study by Riberio et al. (2003) found 2235 macrofauna of 75 invertebrate species inhabiting specimens of Mycale (Carmia) microsigmatosa Arndt, 1927, an encrusting sponge. In addition, sponges have great potential to be used in bioremediation and biomonitoring of marine habitats (Longo et al. 2010; Aresta et al. 2015; Orani et al. 2018, 2022). In comparison with other filter-feeders, like bivalves and tunicates, sponges are efficient in filtering large amounts of water rapidly during feeding and retaining microorganisms and other particles ranging from 0.1 to 50 μm (Osinga et al. 1999; Ribes et al. 1999). Sponge compositions are found to be highly associated with environmental conditions and hence, they could be used as indicators in biomonitoring of the marine environment (Cleary and de Voogd 2007; de Voogd and Cleary 2007). Moreover, studies have reported that bioactive specialized metabolites obtained from sponges and their microbial symbionts are potential drug leads for the treatment of microbial infections and cancers (Sipkema et al. 2005; Laport et al. 2009; Thomas et al. 2010).
While several studies have been conducted in Singapore on the biodiversity of sponges in Singapore reefs, the sponge community composition in Singapore reefs has yet to be extensively studied despite their ecological and biopharmaceutical importance (Lim et al. 2009, 2012; de Voogd and Cleary 2009). Since the description of the first sponge species, Cliona (as Spongia) patera (Hardwicke, 1822) from Singapore in the nineteenth century, more than 190 sponge species have been recorded in local waters (Lim et al. 2012, 2016). The first sponge diversity survey, with voucher specimens, by Hooper et al. (2000) listed about 80 species. This was followed by a sponge diversity and community composition study by de Voogd and Cleary (2009) where a total of 82 species were identified. At the same time, a publication by Lim et al. (2009) recorded 62 fouling sponge species found on navigational buoys. These publications provided mainly qualitative information on sponge diversity and no quantitative data related to sponge species-specific abundance were measured. In addition to sponge diversity related publications, at least six new sponge species, including Tethycometes radicosa Lim and Tan 2008, Suberites diversicolor Becking and Lim 2009, Theonella laena Lim and Tan 2016, Forcepia (Forcepia) vansoesti Lim et al. 2012, Clathrina sororcula van Soest and de Voogd 2015, and Anamixilla singaporensis van Soest and de Voogd 2015, have been described from Singapore.
Singapore, with a population of about 5.6 million, consists of a highly urbanized main island with numerous offshore islands to the south (Chou 2006). Extensive land reclamation over the last century significantly expanded Singapore’s total land area by more than 50% and changed the coastline dominated by concrete, artificial seawalls and lagoons with few remaining original marine habitats (Corlett 1992). The increased turbidity and sedimentation due to land reclamation and coastal constructions resulted in a highly compacted coral cover restricted to a narrow strip between the reef crest and upper reef slope of 3–6 m depth (Huang et al. 2009; Guest et al. 2016). Several studies conducted on coral communities in Singapore revealed that coral genera typically found in deeper waters, such as Leptoseris and Oxypora, are now at shallow depths while the most common hard corals belong to sediment-tolerant genera, including Montipora, Pectinia and Porites (Dikou and van Woesik 2006). In contrast, research focusing on the community composition of sponges in Singapore’s territorial waters are understudied. As such, the current research aims to fill the gap by analyzing the abundance and distribution of relatively common intertidal sponge species associated with coral reefs at various distances from mainland Singapore, namely, Tanjong Rimau, Labrador Park, Pulau (P = Island) Hantu and P. Subar Laut. These sites were chosen to examine the impact of relative anthropogenic activities and environmental stresses on sponge communities. Moreover, baseline information from this study on the abundance and diversity of sponges at selected reefs in Singapore can support marine conservation efforts as well as future studies on ecological and possible biotechnological roles that sponges and their associated microbes play.
2 Materials and Methods
2.1 Study Area
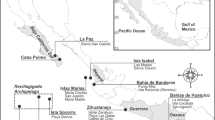
Sponge surveys were conducted at four distinct intertidal reef flats located south of Singapore, namely P. Hantu (1° 13′ 23.98″ N, 103° 45′ 6.29″ E, 1° 13′ 28.01″ N, 103° 45′ 3.40″ E, 1° 13′ 26.81″ N, 103° 44′ 49.02″ E), Tanjong Rimau (1° 15′ 28.52″ N, 103° 48′ 28.99″ E), P. Subar Laut (1° 12′ 45.08″ N, 103° 50′ 9.48″ E), and Labrador Park (1° 16′ 1.53″ N, 103° 48′ 1.11″ E). These sites were chosen based on their distances from the city of Singapore with Tanjong Rimau and Labrador Park located closest to the mainland. The surveys were conducted over a period of 5 months from June to October 2016. A total of six study sites were surveyed with three sites from P. Hantu and one site each from Tanjong Rimau, Labrador Park and P. Subar Laut (Fig. 1).
Map of the Southern Islands of Singapore showing the locations of six study sites, namely P. Hantu site 1 (H1, 1° 13′ 23.98″ N, 103° 45′ 6.29″ E), P. Hantu site 2 (H2, 1° 13′ 28.01″ N, 103° 45′ 3.40″ E), P. Hantu site 3 (H3, 1° 13′ 26.81″ N, 103° 44′ 49.02″ E), P. Subar Laut (S, 1° 12′ 45.08″ N, 103° 50′ 9.48″ E), Tanjong Rimau (R, 1° 15′ 28.52″ N, 103° 48′ 28.99″ E), and Labrador Park (L, 1° 16′ 1.53″ N, 103° 48′ 1.11″ E)
2.2 Survey Method
As Singapore experiences semi-diurnal tides with a maximum tidal range of 3 m, the intertidal sponge surveys were done during low spring tides, usually below 0.5 m. At each survey site, a total of three replicate belt transects of 10 m by 1 m each were established along the intertidal reef flat, parallel to the shore. The length of each sponge encountered along the belt transects was measured (in cm) and photographed for identification purposes. Small samples of the sponge tissues were also collected to help in species identification. Sponge samples of 1 cm by 1 cm were obtained via sectioning in situ and placed in separate Ziploc® bags labeled with a number code that corresponded with the photograph taken. The samples were then transported to the laboratory at the National Institute of Education (NIE), Singapore, to be stored at − 20 °C.
The morphology of sponges was photographed during surveys and compared against photographs and descriptions of sponges from taxonomic related publications (Lim et al. 2008, 2012, 2016; Lim and Tan 2008). Photographs/samples were also examined by sponge taxonomy expert, Lim Swee Cheng, at the St. John’s Island National Marine Laboratory, Singapore, for confirmation of taxonomic identifications. Samples that were difficult to identify to the specific level based on their morphology were represented at either the order or generic level.
2.3 Statistical Analysis
The abundance of each sponge species encountered along each belt transect was enumerated based on the total linear cover (in cm) of each specific sponge. Sponge diversity for each site surveyed was represented by the Shannon–Wiener index of diversity (Hutcheson 1970). This index of diversity was computed for each replicate belt transect based on species richness and abundance (linear cover, in cm) of sponge species. One-way Analysis of Variance (ANOVA) was conducted on the Shannon–Weiner diversity index, species richness and abundance data to determine if species diversity, species richness, and sponge abundance varied between survey sites (Quinn and Keough 2002). A post hoc Tukey’s multiple comparison test was carried out to determine significant differences between sites in terms of species diversity, species richness, and sponge abundance (Zar 2010). The univariate analysis of data was conducted using Minitab 17 Statistical Software.
Multivariate analyses were also carried out using the PRIMER v6 (Plymouth Routines in Multivariate Ecological Research) software package (Clarke and Green 1988; Clarke 1993) to analyze the sponge community structure at the various survey sites. The Bray–Curtis similarity index was performed, and results were illustrated using a non-parametric multi-dimensional scaling (MDS) ordination plot (Clarke and Warwick 2001). The distances of the points on the MDS plot correspond to the similarities of the sites based on their sponge community compositions. Similar sites are represented by points that are close to each other while dissimilar sites are represented by points that are far apart. To illustrate the abundance of selected sponge species at the different sites, a bubble plot of abundance was overlaid on the two-dimensional (2D) MDS plot. The size of the bubble illustrated is proportional to the abundance of the specific sponge species. To determine if the sponge community structures among the six sites were statistically dissimilar, a one-way Analysis of Similarity (ANOSIM) was performed. The pairwise R value (statistic) was used to compare with the Global R value to determine if there was significant dissimilarity in community structure between the sites (p < 0.02). Pairwise R value statistics greater than the Global R showed significant dissimilarity in sponge community structures between the two sites. To determine which species contributed to Bray–Curtis dissimilarity and clusters observed, Similarity Percentage (SIMPER) analysis was performed (Clarke and Warwick 2001).
3 Results
3.1 Sponge Species Diversity
All sponges encountered at the six study sites surveyed consisted of members from the order Chondrillida, Clionaida, Dictyoceratida, Haplosclerida, Poecilosclerida, Suberitida, Tetractinellida, and Verongiida. Of a total of 28 distinct sponge species, 15 sponges were identified to the species (Table 1), eight sponges were identified to the genera and one to the order. The identity of one sponge could not be determined, and hence was classified under the group, keratose sponge, based on its physical morphology. The remaining three sponges were classified as unknown as their identities could not be determined based on their morphology.
3.2 ANOVA Analysis of Sponge Communities
The diversity index and the species richness of sponges were found to be highest in P. Hantu site 1, while the abundance of sponges was highest at P. Hantu site 2. Univariate analyses indicated that the sponge diversity (Fig. 2), species richness (Fig. 3A), and species abundance (Fig. 3B) in P. Hantu site 2 were significantly higher compared to that of Labrador Park where the lowest ecological indices were measured (p < 0.05). However, results of ANOVA and Tukey’s test did not indicate significant differences in sponge diversity indices between all other study sites.
3.3 MDS Analysis of Sponge Communities
The 2D MDS plot derived from untransformed sponge abundance data at all survey sites showed three distinct clusters of sponge communities, namely Pulau Hantu site 2 cluster, Labrador Park cluster, and P. Subar Laut cluster (circles indicate > 40% similarity; Fig. 4). Analysis of Similarities (ANOSIM) test results indicated that there were significant dissimilarities in the sponge community structure between P. Subar Laut and the other sites (p < 0.02; Table 2). ANOSIM results also indicated that apart from P. Hantu site 3, the sponge community at P. Hantu site 2 was significantly dissimilar with that of all other study sites (p < 0.02; Table 2).
2D MDS configuration of sponge communities at P. Hantu 1 (H1), P. Hantu 2 (H2), P. Hantu 3 (H3), Tanjong Rimau (R), Labrador Park (L) and P. Subar Laut (S). A stress value of 0.12 on untransformed sponge abundance data gave a potentially useful 2D picture of the data. Circled clusters indicate 40% similarity
The pattern of the bubble plot overlaid on the 2D MDS plot showed that the sponge species, Spheciospongia cf. vagabunda, could be the determining factor resulting in the clusters seen in the 2D MDS plot (Fig. 5). The size of the bubbles indicating abundance showed a distinct cluster at Pulau Hantu site 2 which contained the highest abundance of S. cf. vagabunda, while the cluster at P. Subar Laut plotted the furthest distance from other sites contained the smallest bubbles, indicating the lowest observed abundance of S. cf. vagabunda. The trend observed in the bubble plot strengthens the ANOSIM results where P. Hantu site 2 and P. Subar Laut clusters were plotted furthest apart, indicating dissimilarity in sponge communities at the two sites.
Bubble plot of abundance of Spheciospongia cf. vagabunda (in cm) overlaid on the 2D MDS plot of sponge communities at P. Hantu 1 (H1), P. Hantu 2 (H2), P. Hantu 3 (H3), Tanjong Rimau (R), Labrador Park (L) and P. Subar Laut (S). H2 site contained the highest abundance of Spheciospongia cf. vagabunda
3.4 SIMPER Analysis
The SIMPER analysis showed that Spheciospongia cf. vagabunda, Halichondria cartilaginea, and Haliclona cymaeformis were the main species that accounted for the distinct clustering of sponge communities of P. Hantu site 2 and other study sites. SIMPER results also showed that S. cf. vagabunda was the main species that influenced the Labrador Park sponge community cluster. Additionally, the presence of both S. cf. vagabunda and Pseudoceratina purpurea contributed to the sponge community of P. Subar Laut being clustered separately from others sampling sites.
4 Discussion
In this study, a total of six intertidal reef flat sites, namely P. Hantu site 1, P. Hantu site 2, P. Hantu site 3, Tanjong Rimau, Labrador Park, and P. Subar Laut, in Singapore were surveyed for sponge abundance and communities. In total, 28 distinct sponge morphospecies were identified based on their morphology (Table 1). Many of the intertidal sponges identified in this study have been previously recorded in Singapore (Lim et al. 2012). In comparison, a higher number of intertidal sponge species has been recorded by local surveys conducted by Lim et al. (2012). For instance, about 20, 14, 12, and 10 intertidal sponges, identified to the species, have been recorded from P. Hantu, Sisters’ Island, Labrador and T. Rimau, respectively (Lim et al. 2012). Studies from several Southeast Asian regions revealed higher sponge diversities. This includes 33 species from Cebu, Philippines (Longakit et al. 2005), 118 species from Jakarta Bay, Indonesia (de Voogd and Cleary 2008), 126 species from the Eastern Gulf of Thailand (Kritsanapuntu et al. 2001) and 299 species from Vietnam (Quang 2013). The higher number of intertidal sponge species recorded in these regions is probably due to the extensive nature of the survey coverage and longer sampling duration, such as study by Lim et al. (2012) carried out over a period of seven years on Singapore sponge diversity. Consistent with previous studies, the intertidal sponge communities found in this study were dominated by sponges, such as Spheciospongia cf. vagabunda, that are able to tolerate high sediment load (Lim and Tan 2016). In addition, nine morphological species observed in this study belonged to the Haplosclerida, an order of shallow-water sponges known to tolerate more variable and extreme environmental conditions (Setiawan et al. 2021).
In this study, both the identified and unidentified sponge species were included in the analysis of sponge diversity, species richness, abundance and community structures using one-way ANOVA, MDS, ANOSIM, and SIMPER as the unidentified species were morphologically distinct from all other species sampled. Such inclusion of unidentified sponges is supported by Pos et al. (2014) who indicated that having a large number of unidentified species in a dataset did not potentially affect the conclusions of ecologically important patterns.
The univariate analyses indicated that the sponge diversity, species richness and abundance in P. Hantu site 2 were significantly higher compared to that of Labrador Park where the lowest ecological indices were measured (p < 0.05; Figs. 2 and 3). However, the results of univariate analyses did not indicate significant differences of P. Hantu site 2 sponge communities and all other sites.
On the other hand, multivariate analysis using MDS and ANOSIM were able to determine that, with the exception of P, Hantu site 3, sponge communities at P. Hantu site 2 were significantly dissimilar with communities at the four other study sites. Equally, MDS and ANOSIM results revealed that P. Subar Laut and Labrador Park sponge communities were significantly dissimilar with each other, and also with P. Hantu sponge communities in terms of community structure (p < 0.02).
Multivariate analysis in this case was more robust as it takes into account all the variables in terms of sponge diversity, species richness, and relative abundance in the analysis of community characteristics. The 2D MDS plot showed three distinct clusters of sites, P. Hantu site 2, Labrador Park, and P. Subar Laut (Fig. 4). The bubble plot of Spheciospongia cf. vagabunda overlaid on 2D MDS plot also seemed to correspond with the trend in clustering patterns (Fig. 5). Similarly, the SIMPER analysis indicated that S. cf. vagabunda was one of the main species that resulted in the three distinct clusters.
Of the six study sites surveyed, Labrador Park was the only site located on mainland Singapore. Interestingly, Labrador Park is also the last remaining natural rocky shoreline on the entire southern coast of mainland Singapore (Todd and Chou 2005). Singapore has engaged in extensive land reclamation and coastal development projects in the past four decades and it was predicted that about 60% of the total coral reef area was lost due to land reclamation (Dikou and van Woesik 2006). As such, shores of mainland Singapore have been exposed to greater anthropogenic stresses, such as sedimentation due to the land reclamation and dredging operations. In particular, the coral reef of Labrador Park suffered from anthropogenic impacts, such as thermal effluent from a power plant situated at one end of the reef, as well as sedimentation caused by reclamation of part of the reef flat and an oil tanker jetty in the middle of the reef flat (Todd and Chou 2005). In fact, by 1968 coral diversity was reduced to eight from 30 species (Chuang 1973) due to these impacts. Studies have shown that sediments can adversely affect sponges in numerous ways such as clogging the filtering apparatus of the sponge (Bakus 1968) and preventing the settlement of larvae on suitable substrate covered in settled sediment (Maldonado and Uriz 1999; Maldonado et al. 2008). The effects of high sedimentation can also impact sponges of certain morphological types, including mortality of cup-shaped sponge types and tissue necrosis in species with massive, encrusting and wide cup morphologies (Pineda et al. 2016). As such, sponges of such morphologies are not commonly found at Labrador Park. In addition, high water temperature can result in the death of sponges as the increased temperature can disrupt their symbiotic relationship with microbes (Webster et al. 2008; Ramsby et al. 2018). Therefore, such anthropogenic stresses could be contributing factors explaining the differences in sponge community characteristics of Labrador Park site with the other sites.
Among all six study sites, P. Hantu site 2 was the only site situated in a lagoon. From the results, it was found that P. Hantu site 2 had the highest abundance of S. cf. vagabunda. Even though this species is found at all sites, its linear coverage (in cm) at P. Hantu site 2 was about two to three times to that recorded at P. Hantu sites 1/2 and about 19 times to that at Labrador Park (Table 1). In addition, sponges from the genus Spheciospongia have been reported to form one of the most common sponges in Singapore (Lim et al. 2008, 2012). In a study conducted by Beepat et al. (2013), it was reported that S.cf. vagabunda, unlike most other sponges, was found mostly anchored in sand and not on hard substratum. This species had been previously described as a burrowing sponge (Barruca et al. 2007). In addition, S. cf. vagabunda was found to be most abundant in shallow water in the middle of the lagoon not subjected to high wave action (Beepat et al. 2013). These environmental conditions are similar to that of P. Hantu site 2, where the endopsammic S. cf. vagabunda was found on sandy substrata, and were most abundant in the middle of the lagoon (Schönberg 2021). As such, this species thrives in such conditions, thereby giving rise to the high abundance recorded, which resulted in the sponge community at P. Hantu site 2 being clustered away from other sites.
Interestingly, another endopsammic sponge, Coelocarteria singaporensis, was recorded along the transact line at P. Hantu site 2 and not at other study sites, providing evidence that the habitat at P. Hantu site 2 is conducive for growth of psammobiotic sponge species. This sponge was recently investigated by Schönberg and Lim (2019) and found that it is unlikely to bioerode calcareous matter as compared to Spheciospongia species. Their study also included photographs of fistular and non-fistular Spheciospongia specimens in Singapore which were identified as S. cf. inconstans (Dendy, 1887). We are unable to verify if the specimens we worked on in our study are S. cf. inconstans and not S. cf. vagabunda (Ridley, 1884).
SIMPER analysis showed that Halichondria cartilaginea and Haliclona cymaeformis also contributed to the clustering pattern of P. Hantu site 2. It was observed that these two species were only found in P. Hantu site 2 and not at other sites. Pulau Hantu site 2, which is located in an intertidal lagoon, is exposed to abiotic stresses, such as longer exposure to air during low tide (about 3–4 h), varying temperatures and ultra-violet (UV) radiation. When exposed during low tide, sponges living in the lagoon are unable to filter-feed and may need alternative food sources. Halichondria cartilaginea and Haliclona cymaeformis have photosymbionts which are able to complement the nutrient uptake of the sponges with their photosynthetic activity (van Soest and Verseveldt 1987; Steindler et al. 2002; Freeman et al. 2013). In addition, photosymbionts may produce UV-screening substances which protect the sponges from UV radiation (Steindler et al. 2002). Therefore, having photosymbionts could possibly be one of the reasons Halichondria cartilaginea and Haliclona cymaeformis were able to inhabit P. Hantu site 2. In a sponge diversity survey conducted along coastal and islands of Chanthaburi and Trat Provinces in Thailand, Halichondria cartilaginea was commonly found in the upper subtidal zone of coral reefs exposed to sunlight (Sumaitt 2011). On the other hand, P. Subar Laut was observed to have the lowest abundance of S. cf. vagabunda. The intertidal reef flat of P. Subar Laut is exposed to higher wave action (measured in knots) due to its geographical location compared to the other sites (Maritime and Port Authority 2015). Hence, this could possibly be the reason for the low abundance of S. cf. vagabunda, causing P. Subar Laut to be clustered away from the other sites. In addition, SIMPER analysis also revealed that Pseudoceratina purpurea was abundant only in P. Subar Laut. This could be because P. purpurea can survive current-swept turbid water conditions in P. Subar Laut. Hence, this could be another reason the sponge community at P. Subar Laut was clustered away from the other sites.
In conclusion, this study has provided information on sponge diversity, species richness and abundance, and has documented sponge community characteristics at six coral reef sites of Singapore, namely, P. Hantu, Tanjong Rimau, Labrador Park, and P. Subar Laut. Of the six sites surveyed, multivariate analysis showed three distinct sites, namely P. Hantu site 2, Labrador Park, and P. Subar Laut, that were clustered away from the other sites. Based on the bubble plot patterns as well as the SIMPER analysis, the abundance of S. cf. vagabunda was a likely factor determining the pattern of distinct clusters. Preliminary community data presented in this study could potentially provide useful baseline information for future studies on biomonitoring of intertidal sponges as well as support marine conservation efforts in Singapore.
Data availability
Processed data that support the findings of this study are available from the corresponding author, Goh, B.P.L., upon reasonable request.
References
Aresta A, Nonnis Marzano C, Lopane C, Corriero G, Longo C, Zambonin C, Stabili L (2015) Analytical investigations on the lindane bioremediation capability of the demosponge Hymeniacidon perlevis. Mar Pollut Bull 90(1–2):143–149. https://doi.org/10.1016/j.marpolbul.2014.11.003
Bakus G (1968) Sedimentation and benthic invertebrates of Fanning Island, central Pacific. Mar Geol 6(1):45–51. https://doi.org/10.1016/0025-3227(68)90008-X
Barruca M, Azzini F, Bavestrello G, Biscotti M, Calcinai B, Canapa A, Cerrano C, Olmo E (2007) The systematic position of some boring sponges (Demospongiae, Hadromerida) studied by molecular analysis. Mar Biol 151:529–535. https://doi.org/10.1007/s00227-006-0486-y
Becking LE, Lim SC (2009) A new Suberites (Demospongiae: Hadromerida: Suberitidae) from the tropical Indo-West Pacific. Zool Meded Leiden 83(29):853–862
Beepat SS, Appadoo C, Marie DEP, Paula J, Sivakumar K (2013) Distribution and abundance of the sponge Spheciospongia vagabunda (Ridley, 1884) (Phylum: Porifera, Class: Demospongiae) in a shallow Mauritian lagoon. West Indian Ocean J Mar Sci 12(1):15–23
Chou LM (2006) Marine habitats in one of the world’s busiest harbours. In: Wolanksi E (ed) The environment in Asia Pacific harbours. Springer, Amsterdam, pp 377–391
Chuang SH (1973) Animal life and nature in Singapore. Singapore University Press, Singapore, p 340
Clarke KR (1993) Non-parametric multivariate analysis of changes in community structure. Aust J Ecol 18(1):117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Clarke KR, Green RH (1988) Statistical design and analysis for a ‘biological effects’ study. Mar Ecol-Prog Ser 46:213–226. https://doi.org/10.3354/meps046213
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth, p 176
Cleary DFR, de Voogd NJ (2007) Environmental determination of sponge assemblages in the Spermonde Archipelago, Indonesia. J Mar Biol Assoc UK 87(6):1669–1676. https://doi.org/10.1017/S0025315407052770
Corlett RT (1992) The ecological transformation of Singapore, 1819–1990. J Biogeogr 19(4):411–420. https://doi.org/10.2307/2845569
de Voogd NJ, Cleary DFR (2007) Relating species traits to environmental variables in Indonesian coral reef assemblages. Mar Freshw Res 58(3):240–249. https://doi.org/10.1071/MF06125
de Voogd NJ, Cleary DFR (2008) An analysis of sponge diversity and distribution at three taxonomic levels in the Thousand islands/Jakarta Bay reef complex, West-Java, Indonesia. Mar Ecol 29(2):205–215. https://doi.org/10.1111/j.1439-0485.2008.00238.x
de Voogd NJ, Cleary DFR (2009) Variation in sponge composition among Singapore reefs. Raff Bull Zool 22:59–67
Diaz MC, Rützler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69(2):535–546
Dikou A, van Woesik R (2006) Survival under chronic stress from sediment load: spatial patterns of hard coral communities in the southern islands of Singapore. Mar Pollut Bull 52(1):7–21. https://doi.org/10.1016/j.marpolbul.2006.02.011
Fiore CL, Jutte PC (2010) Characterization of macrofaunal assemblages associated with sponges and tunicates collected off the southeastern United States. Invert Biol 129(2):105–120. https://doi.org/10.1111/j.1744-7410.2010.00184.x
Freeman CJ, Thacker RW, Baker DM, Fogel ML (2013) Quality or quantity: is nutrient transfer driven more by symbiont identity and productivity than by symbiont abundance? ISME J 7:1116–1125. https://doi.org/10.1038/ismej.2013.7
Guest JR, Tun KPP, Low J, Vergés A, Marzinelli EM, Campbell AH, Bauman AG, Feary DA, Chou LM, Steinberg PD (2016) 27 years of benthic and coral community dynamics on turbid, highly urbanised reefs off Singapore. Sci Rep 6:36260. https://doi.org/10.1038/srep36260
Hooper JNA, Kennedy JA, van Soest RWM (2000) Annotated checklist of sponges (Porifera) of the South China Sea region. Raff Bull Zool 8:125–207
Huang D, Tun KPP, Chou LM, Todd PA (2009) An inventory of zooxanthellate scleractinian corals in Singapore, including 33 new records. Raff Bull Zool 22:69–80
Hutcheson K (1970) A test for comparing diversities based on the Shannon formula. J Theor Biol 29(1):151–154. https://doi.org/10.1016/0022-5193(70)90124-4
Kritsanapuntu S, Chaitanawisuti N, Yeemin T, Putchakan S (2001) First investigation on biodiversity of marine sponges associated with reef coral habitats in the eastern Gulf of Thailand. Asian Mar Biol 18:105–115
Laport MS, Santos OC, Muricy G (2009) Marine sponges: potential sources of new antimicrobial drugs. Curr Pharm Biotechnol 10(1):86–105. https://doi.org/10.2174/138920109787048625
Lim SC, de Voogd NJ, Tan KS (2008) A guide to sponges of Singapore. Singapore Science Centre, Singapore, p 173
Lim SC, de Voogd NJ, Tan KS (2009) Fouling sponges (Porifera) on navigation buoys from Singapore waters. Raff Bull Zool 22:41–58
Lim SC, de Voogd NJ, Tan KS (2012) Biodiversity of shallow-water sponges (Porifera) in Singapore and description of a new species of Forcepia (Poecilosclerida: Coelosphaeridae). Contr Zool 81(1):55–71. https://doi.org/10.1163/18759866-08101004
Lim SC, Putchakarn S, Thai M-Q, Wang D, Huang YM (2016) Inventory of sponge fauna from the Singapore Strait to Taiwan Strait along the western coastline of the South China Sea. Raff Bull Zool 34:104–129
Lim SC, Tan KS (2008) A new species of Tethycometes Sarà, 1994 (Porifera: Hadromerida: Tethyidae) from Singapore. Zootaxa 1841:65–68. https://doi.org/10.5281/zenodo.183204
Lim SC, Tan KS (2016) Description of a new species of sponge encrusting on a sessile gastropod in the Singapore Strait. Raff Bull Zool 34:97–103
Longakit MB, Sotto F, Kelly M (2005) The shallow water marine sponges (Porifera) of Cebu, Philippines. Sci Diliman 17:52–74
Longo C, Corriero G, Licciano M, Stabili L (2010) Bacterial accumulation by the Demospongiae Hymeniacidon perlevis: a tool for the bioremediation of polluted seawater. Mar Pollut Bull 60(8):1182–1187. https://doi.org/10.1016/j.marpolbul.2010.03.035
Maldonado M, Giraud K, Carmona C (2008) Effects of sediment on the survival of asexually produced sponge recruits. Mar Biol 154:631–641. https://doi.org/10.1007/s00227-008-0956-5
Maldonado M, Uriz MJ (1999) An experimental approach to the ecological significance of microhabitat-scale movement in an encrusting sponge. Mar Ecol Prog Ser 185:239–255. https://doi.org/10.3354/meps185239
Maritime and Port Authority (2015) Year 2016 Singapore tide tables. Hydrographic Division, Maritime and Port Authority of Singapore, Singapore, p 271
Orani AM, Barats A, Vassileva E, Thomas OP (2018) Marine sponges as a powerful tool for trace elements biomonitoring studies in coastal environment. Mar Pollut Bull 131(Part A):633–645. https://doi.org/10.1016/j.marpolbul.2018.04.073
Orani AM, Vassileva E, Thomas OP (2022) Marine sponges as coastal bioindicators of rare earth elements bioaccumulation in the French Mediterranean Sea. Environ Pollut 304:119172. https://doi.org/10.1016/j.envpol.2022.119172
Osinga R, Tramper J, Wijffels RH (1999) Cultivation of marine sponges. Mar Biotechnol 1(6):509–532. https://doi.org/10.1007/pl00011807
Pineda M, Duckworth A, Webster N (2016) Appearance matters: sedimentation effects on different sponge morphologies. J Mar Biol Assoc UK 96(2):481–492. https://doi.org/10.1017/S0025315414001787
Pos E, Guevara Andino J, Sabatier D, Molino J, Pitman N, Mogollón H, Neill D, Cerón C, Rivas G, Di Fiore A, Thomas R, Tirado M, Young K, Wang O, Sierra R, García-Villacorta R, Zagt R, Palacios W, Aulestia M, ter Steege H (2014) Are all species necessary to reveal ecologically important patterns? Ecol Evol 4(24):4626–4636. https://doi.org/10.1002/ece3.1246
Quang TM (2013) A review of the diversity of sponges (Porifera) in Vietnam. In: The proceedings of the 2nd international workshop on marine bioresources of Vietnam, Hanoi, pp 109–115
Quinn G, Keough M (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge, p 553
Ramsby BD, Hoogenboom MO, Whalan S, Webster NS (2018) Elevated seawater temperature disrupts the microbiome of an ecologically important bioeroding sponge. Mol Ecol 27(8):2124–2137. https://doi.org/10.1111/mec.14544
Riberio SM, Omena EP, Muricy G (2003) Macrofauna associated to Mycale microsigmatosa (Porifera, Demospongiae) in Rio de Janeiro State, SE Brazil. Estuar Coast Shelf Sci 57(5–6):951–959. https://doi.org/10.1016/S0272-7714(02)00425-0
Ribes M, Coma R, Gili J-M (1999) Natural diet and grazing rate of the temperate sponge Dysidea avara (Demospongiae, Dendroceratida) throughout an annual cycle. Mar Ecol Prog Ser 176:179–190. https://doi.org/10.3354/meps176179
Schönberg CHL (2021) No taxonomy needed: Sponge functional morphologies inform about environmental conditions. Ecol Indic 129:107806. https://doi.org/10.1016/j.ecolind.2021.107806
Schönberg CHL, Lim SC (2019) Psammobiosis and bioerosion: examining ecological strategies in sponges using the case example Coelocarteria singaporensis. Facies 65:14. https://doi.org/10.1007/s10347-019-0556-5
Setiawan E, Relex D, Marshall DJ (2021) Shallow-water sponges from a high-sedimentation estuarine bay (Brunei, Northwest Borneo, Southeast Asia). J Trop Biodivers Biotechnol 6(3):jtbb66435. https://doi.org/10.22146/jtbb.66435
Sipkema D, Granssen MCR, Osinga R, Tramper J, Wijffels RH (2005) Marine sponges as pharmacy. Mar Biotechnol 7:142–162. https://doi.org/10.1007/s10126-004-0405-5
Steindler L, Beer S, Ilan M (2002) Photosymbiosis in intertidal and subtidal tropical sponges. Symbiosis 33(3):263–274
Sumaitt P (2011) Species diversity of marine sponges along Chanthaburi and Trat Provinces, the eastern coast of the Gulf of Thailand. Publ Seto Mar Biol Lab 41:17–23. https://doi.org/10.5134/159486
Thomas TRA, Kavlekar DP, LokaBharathi PA (2010) Marine drugs from sponge–microbe association: a review. Mar Drugs 8(4):1417–1468. https://doi.org/10.3390/md8041417
Todd PA, Chou LM (2005) A tale of survival: Labrador Park, Singapore. Coral Reefs 24:391. https://doi.org/10.1007/s00338-005-0022-4
van Soest RWM, de Voogd NJ (2015) Calcareous sponges of Indonesia. Zootaxa 3951(1):1–105. https://doi.org/10.11646/zootaxa.3951.1.1
van Soest RWM, Verseveldt J (1987) Unique symbiotic octocoral-sponge association from Komodo. Indo-Malayan Zool 4:27–32
Webster NS, Cobb RE, Negri AP (2008) Temperature thresholds for bacterial symbiosis with a sponge. ISME J 2:830–842. https://doi.org/10.1038/ismej.2008.42
Zar JH (2010) Biostatistical analysis, 5th edn. Pearson Prentice-Hall, Upper Saddle River, p 944
Acknowledgements
The authors would like to acknowledge Dr. Lim Swee Cheng (SJINML, National University of Singapore, Singapore) for assistance in sponge taxonomic identification as well as Jasmine Tong and David Toh (NIE, Singapore) for their technical support. This research was conducted under NParks research permit number NP/RP14-096-1a.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, S.F., Tan, L.T. & Goh, B.P.L. Distribution and Abundance of Intertidal Sponge (Porifera) Communities in Coral Reefs of Singapore. Ocean Sci. J. 58, 30 (2023). https://doi.org/10.1007/s12601-023-00123-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12601-023-00123-0