Abstract
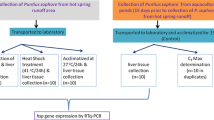
The aim of this study was to elucidate the molecular mechanisms underlying the thermal stress response in the spotted sea bass (Lateolabrax maculatus). Spotted sea basses were exposed to 4 different water temperatures (20, 22, 24, and 28°C) in increasing increments of 2°C/h from 18°C (control) for different time periods (0, 6, 12, 24, 48, 72, and 96 h). Subsequently, 3 tissues (liver, muscle, and gill) were isolated, and the levels of SOD, HSP90, and HSP70 mRNA were assessed. SOD mRNA expression was maintained at baseline levels of control fish at all water temperatures in the liver, while muscle and gill tissue showed an increase followed by a decrease over each certain time with higher water temperature. HSP90 mRNA expression increased in the liver at ≤ 24°C over time, but maintained baseline expression at 28°C. In muscle, HSP90 mRNA expression gradually increased at all water temperatures, but increased and then decreased at ≥ 24°C in gill tissue. HSP70 mRNA expression exhibited an increase and then a decrease in liver tissue at 28°C, but mainly showed similar expression patterns to HSP90 in all tissues. These results suggest the activity of a defense mechanism using SOD, HSP90, and HSP70 in the spotted sea bass upon rapid increases in water temperature, where the expression of these genes indicated differences between tissues in the extent of the defense mechanisms. Also, these results indicate that high water temperature and long-term thermal stress exposure can inhibit physiological defense mechanisms.
Similar content being viewed by others
References
Ackerman PA, Forsyth RB, Mazur CF, Iwama GK (2000) Stress hormones and the cellular stress response in salmonids. Fish Physiol Biochem 23(4):327–336
An KW, Kim NN, Shin HS, Kil GS, Choi CY (2010) Profiles of antioxidant gene expression and physiological changes by thermal and hypoosmotic stresses in black porgy (Acanthopagrus schlegeli). Comp Biochem Phys A 156(2):262–268
Arbeitman MN, Hogness DS (2000) Molecular chaperones activate the Drosophila ecdysone receptor, an RXR heterodimer. Cell 101(1):67–77
Bamber RN, Seaby RM (2004) The effects of power station entrainment passage on three species of marine planktonic crustacean, Acartia tonsa (Copepoda), Crangon crangon (Decapoda) and Homarus gammarus (Decapoda). Mar Environ Res 57(4):281–294
Bermudes M, Glencross B, Austen K, Hawkins W (2010) The effects of temperature and size on the growth, energy budget and waste outputs of barramundi (Lates calcarifer). Aquaculture 306(1):160–166
Bogevik AS, Henderson RJ, Mundheim H, Waagbø R, Tocher DR, Olsen RE (2010) The influence of temperature on the apparent lipid digestibility in Atlantic salmon (Salmo salar) fed Calanus finmarchicus oil at two dietary levels. Aquaculture 309(1):143–151
Cardoso CM, Sartorio PV, Machado AS, Vignardi CP, Rojas DC, Passos MJ, Rocha AJ, Van Ngan P, Gomes V (2015) Hsp70 and p53 expressions and behavior of juvenile pompano, Trachinotus carolinus (Perciformes, Carangidae), at controlled temperature increase. J Exp Mar Biol Ecol 470:34–42
Cheng CH, Yang FF, Liao SA, Miao YT, Ye CX, Wang AL, Tan JW, Chen XY (2015) High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J Therm Biol 53:172–179
Ciavarra RP, Simeone A (1990) T lymphocyte stress response: I. Induction of heat shock protein synthesis at febrile temperatures is correlated with enhanced resistance to hyperthermic stress but not to heavy metal toxicity or dexamethasone-induced immunosuppression. Cell Immunol 129(2):363–376
Clotfelter ED, Lapidus SJ, Brown AC (2013) The effects of temperature and dissolved oxygen on antioxidant defences and oxidative damage in the fathead minnow (Pimephales promelas). J Fish Biol 82(3):1086–1092
Cui Y, Liu B, Xie J, Xu P, Habte-Tsion HM, Zhang Y (2014) Effect of heat stress and recovery on viability, oxidative damage, and heat shock protein expression in hepatic cells of grass carp (Ctenopharyngodon idellus). Fish Physiol Biochem 40(3):721–729
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61(1):243–282
Healy TM, Schulte PM (2012) Factors affecting plasticity in wholeorganism thermal tolerance in common killifish (Fundulus heteroclitus). Comp Biochem Phys B 182(1):49–62
Hightower LE (1991) Heat shock, stress proteins, chaperones and proteotoxicity. Cell 66(2):191–197
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, New York, 480 p
Holloway AC, Leatherland JF (1997) Effect of gonadal steroid hormones on plasma growth hormone concentrations in sexually immature rainbow trout, Oncorhynchus mykiss. Gen Comp Endocr 105(2):246–254
Jakob U, Buchner J (1994) Assisting spontaneity: the role of Hsp90 and small HSPs as molecular chaperones. Trends Biochem Sci 19(5):205–211
Johnston IA (2006) Environment and plasticity of myogenesis in teleost fish. J Exp Biol 209(12):2249–2264
Kashiwagi A, Kashiwagi K, Takase M, Hanada H, Nakamura M (1997) Comparison of catalase in diploid and haploid Rana rugosa using heat and chemical inactivation techniques. Comp Biochem Phys B 118(3):499–503
Iwama GK, Vijayan MM, Forsyth RB, Ackerman PA (1999) Heat shock proteins and physiological stress in fish. Am Zool 39:901–909
Lindquist S, Craig EA (1988) The heat shock proteins. Annu Rev Genet 22(1):631–677
Liu Y, Steinacker JM (2001) Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci 6:12–25
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25(4):402–408
Lund SG., Lund ME, Tufts BL (2003) Red blood cell HSP 70 mRNA and protein as bio-indicators of temperature stress in the brook trout (Salvelinus fontinalis). Can J Fish Aquat Sci 60(4):460–470
Lu X, Wang C, Liu B (2015) The role of Cu/Zn-SOD and Mn- SOD in the immune response to oxidative stress and pathogen challenge in the clam Meretrix meretrix. Fish Shellfish Immun 42(1):58–65
Lushchak VI, Bagnyukova TV (2006) Temperature increase results in oxidative stress in goldfish tissues. 2. Antioxidant and associated enzymes. Comp Biochem Phys C 143(1):36–41
Madeira D, Narciso L, Cabral HN, Vinagre C, Diniz MS (2013) Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp Biochem Phys A 166(2):237–243
Mailhos C, Howard MK, Latchman DS (1994) Heat shock proteins hsp90 and hsp70 protect neuronal cells from thermal stress but not from programmed cell death. J Neurochem 63(5):1787–1795
Mallatt J (1985) Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can J Fish Aquat Sci 42(4):630–648
Mallouk YA, Vayssier-Taussat MU, Bonventre JV, Polla BS (1999) Heat shock protein 70 and ATP as partner in cell homeostasis. Int J Mol Med 4(5):463–474
Martínez-Álvarez RM, Morales AE, Sanz A (2005) Antioxidant defences in fish: biotic and abiotic factors. Rev Fish Biol Fisher 15(1–2):75–88
Meng XL, Liu P, Li J, Gao BQ, Chen P (2014) Physiological responses of swimming crab Portunus trituberculatus under cold acclimation: antioxidant defense and heat shock proteins. Aquaculture 434:11–17
Metz B, Davidson OR (2007) Climate change 2007: mitigation: contribution of Working Group III to the fourth assessment report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change. http://www.ipcc.ch/publications_and_data/ar4/wg3/en/contents.html. Accessed 7 Nov 2016
Minami Y, Kawasaki H, Miyata Y, Suzuki K, Yahara I (1991) Analysis of native forms and isoform compositions of the mouse90-kDa heat shock protein, HSP90. J Biol Chem 266(16): 10099–10103
Moseley PL (1998) Heat shock proteins and the inflammatory response. Ann NY Acad Sci 856(1):206–213
Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B (1997) Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol 17(9):5317–5327
Nordberg J, Arnér ES (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Bio Med 31(11):1287–1312
Pachauri RK, Reisinger A (2007) Climate change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. http://www.ipcc.ch/publications_and_data/ar4/syr/en/contents.html. Accessed 7 Nov 2016
Pandey S, Parvez S, Sayeed I, Haque R, Bin-Hafeez B, Raisuddin S (2003) Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci Total Environ 309(1):105–115
Pratt WB (1997) The role of the HSP90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu Rev Pharmacol 37(1):297–326
Poltronieri C, Maccatrozzo L, Simontacchi C, Bertotto D, Funkenstein B, Patruno M, Radaelli G (2007) Quantitative RT-PCR analysis and immunohistochemical localization of HSP70 in sea bass Dicentrarchus labrax exposed to transport stress. Eur J Histochem 51:125–136
Sanders BM (1993) Stress proteins in aquatic organisms: an environmental perspective. Crit Rev Toxicol 23(1):49–75
Stacey NE (1984) Control of the timing of ovulation by exogenous and endogenous factors. In: Potts GW, Wootton RW (eds) Fish reproduction: strategies and tactics. Academic Press, London, pp 207–222
Sudo R, Tosaka R, Ijiri S, Adachi S, Suetake H, Suzuki Y, Horie N, Tanaka S, Aoyama J, Tsukamoto K (2011) Effect of temperature decrease on oocyte development, sex steroids, and gonadotropin β-subunit mRNA expression levels in female Japanese eel Anguilla japonica. Fisheries Sci 77(4):575–582
Wegele H, Müller L, Buchner J (2004) Hsp70 and Hsp90—a relay team for protein folding. Rev Physiol Bioch P 151:1–44
Weyts FA, Cohen N, Flik G, Verburg-van Kemenade BM (1999) Interactions between the immune system and the hypothalamopituitary- interrenal axis in fish. Fish Shellfish Immun 9(1):1–20
Wuenschel MJ, Jugovich AR, Hare JA (2005) Metabolic response of juvenile gray snapper (Lutjanus griseus) to temperature and salinity: physiological cost of different environments. J Exp Mar Biol Ecol 321(2):145–154
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, MK., Park, HR., Yeo, WJ. et al. Effects of Thermal Stress on the mRNA Expression of SOD, HSP90, and HSP70 in the Spotted Sea Bass (Lateolabrax maculatus). Ocean Sci. J. 53, 43–52 (2018). https://doi.org/10.1007/s12601-018-0001-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12601-018-0001-7