Abstract
The sorghum aphid Melanaphis sorghi (Theobald, 1904) is the most critical pest in sorghum crops grown in the USA and Mexico. The cultivated area of sorghum was reduced by more than 30% between 2016 and 2019 in northern Mexico, Guanajuato, Morelos, and other regions. This study provides essential information to support an Integrated Pest Management of this insect. M. sorghi field colonies from Guanajuato, Mexico, were identified by morphometric criteria and reared on Sorghum bicolor var. UPM-219 leaf discs using a bioclimatic chamber under different temperatures: 5, 10, 15, 20, 25, 30, 35 and 40 °C. The highest mortality occurred in extreme temperatures: 40 °C (100%). The lowest temperature assessed (5 °C) shows no reproduction, but the pre-reproductive period spread to 62.5 days, allowing an average of 88.2 days of the insects' survival. At 20 °C, M. sorghi produced a supernumerary N5 stage that prolonged the development by 7.1 days, while at the highest temperature (40 °C), N1s died after 0.9 days. The most prolonged reproductive period was 33.2 days (0.6 nymphs/day) at 10 °C, in contrast to 4.1 days (0.4 nymphs/day) at 35 °C. The most extended post-reproductive period (22.4 days) was observed at 10 °C and the shortest (4.2 days) at 35 °C. The highest fertility average (79.06 nymphs) was reached at 25 °C. The heat units required for each generation were 158.9, while theoretical thermal thresholds were 2.0 and 40 °C. Population parameters show that 25 °C is the optimal temperature for this aphid, estimating Ro (79.06), Rm (0.44), T (10.01), DT (1.59), and λ (1.55).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2013, a species of the genus Melanaphis affected sorghum crops [Sorghum bicolor (L.) Moench] of North America. Named as Melanaphis sacchari during the period 2013 to 2020 (Zehntner, 1897) (Nibouche et al., 2014, 2018) and as Melanaphis sorghi later from 2021 (Theobald, 1904) (Nibouche et al., 2021), this aphid was recognized as the most critical pest of sorghum crops in the USA (Bowling et al., 2016) and Mexico (Rodríguez-del-Bosque & Terán, 2015). The damage in Southern Texas, from the invasion´s beginning (2013) to the evaluation period (2015), was estimated at US$40.95 million (Zapata et al., 2018). Meanwhile, in Guanajuato, Mexico, there was an average of 40 to 60% plot loss during 2015 and 2016, respectively, and occurring some total lost cases (Quijano-Carranza et al., 2017).
The genetic diversity of aphids is a relevant factor that may be related to crop damage severity (Bournoville et al., 2000). In Guanajuato, M. sorghi populations have shown obligate parthenogenesis. However, there are some co-existing populations with sexual forms (Peña-Martínez et al., 2016, 2018b), as occurs in the affected areas of the United States. The population parameters of these insects may vary according to biotic factors related to the host plant (species, variety, alternate hosts (Du et al., 2018)), aphid characteristics (physiology and biotype), abiotic conditions (photoperiod and temperature) (Wu et al., 2018), and even certain symbiotic microorganisms (Blackman & Eastop, 2018; Simon & Peccoud, 2018).
The temperature is a key factor in the physiology, survival, fertility, and population density of sorghum aphids. Therefore, laboratory studies under controlled temperatures provide significant information to understand its biology, population dynamics, and restraints for its establishment in defined geographical areas, which are the bases for implementing any Integrated Pest Management (IPM) program (De Souza et al., 2018). Several works focused on these parameters have been carried out with U.S. populations (Lopes-da-Silva et al., 2014; Hinson, 2017; de Souza et al., 2018; Du et al., 2018); however, studies are required on Mexican populations due to the different characteristics of clones, as field data suggest (Ibarra et al., 2016; Quijano-Carranza et al., 2017; Peña-Martínez et al., 2018a, b). Therefore, this study evaluates the effect of temperature on the viviparous M. sorghi females’ life parameters from the state of Guanajuato at different temperatures on a tolerant variety of Sorghum bicolor.
Material and methods
Sorghum of the variety UPM-219 was used for the rearing and experiments performed in this work. M. sorghi colonies were initially collected in the municipality Juventino Rosas (from 20°33’ to 20°49’N, and from 100°51’to 101°08’W, 1700 msl). These aphid populations show obligated and cyclical parthenogenesis, morphologically indistinguishable by their viviparous apterous phase (Peña-Martínez et al., 2018a, b). Controlled conditions rear began in July 2017 at the Bajío Agricultural Experimental Field (INIFAP) in Celaya, Guanajuato. Then, part of the colony was moved to greenhouses at Colegio de Postgraduados in Texcoco, State of Mexico.
The effect of temperature on the aphid life cycle was evaluated using eight conditions: 5, 10, 15, 20, 25, 30, 35 and 40 °C. The experiments were performed in environmental rearing chambers (Shellab Model LI15), with a 12/12 h constant photoperiod and relative humidity (RH) of 60 ± 10%. Each treatment was replicated 20 times, complemented by a second identical experiment (n = 40).
Each experimental unit comprised a petri dish with a layer of agar–agar gel at the bottom and a leaf disk (circular cuts from 20 to 25 days S. bicolor plants) on the top of the gel (Li & Akimoto, 2018; Salas-Monzón et al., 2018). An adult female aphid was placed in each container and withdrawn after four hours when newborn nymphs (N1) were present. Then, individual nymphs were moved to experimental units, recording molting twice daily (9:00 and 16:00 h) until the adult stage (genital plate appeared and the complete development of the cauda), recording the pre-reproductive period. The reproductive period was recorded along with the number of new N1s per female, total fertility, and the average number of nymphs per day. Finally, the post-reproductive period was registered from the end of reproduction to death. Longevity was the sum of all periods mentioned above. Some individuals were processed to prepare micro-assembly slides using the Blackman and Eastop (2018) technique. The rest of the aphids were kept in the Aphidomorpha Collection at Facultad de Estudios Superiores Iztacala (FES-I), UNAM.
Data analysis
The quantitative comparison of pre-reproductive, reproductive, and post-reproductive periods, the daily and total fertility, and the longevity were performed by a Kruskal–Wallis’s test (p = 0.5) since the data did not meet the assumptions of normality. The development rate was determined using the inverse of the development data, applying a linear adjustment to get the minimum threshold development temperature where this line intersects the X-axis. Heat units (degree-days) required for development were estimated with the formula: DD = (e.t – m.t.t) (d.c) (e.t – m.t.t.) (d.c.),
where e.t. = evaluated temperature, m.t.t. = minimum threshold temperature, d.c. = development cycle per female. The population dynamics parameters of M. sorghi at different temperatures were calculated with the LifeTable package, including a comparison through a Jackknife estimate (Maia et al., 2000; SAS Institute, n.d.).
Results
Life table
The M. sorghi experimental individuals completed their development at temperatures of 5 to 35 °C, while at 40 °C, all died during the first incubation day (0.9 d). Aphids showed important differences in the pre-reproductive (χ = 141.17, p = 0.000), reproductive (χ = 128.66, p = 0.000) and post-reproductive (χ = 140.37, p = 0.000) periods. Low temperatures delayed the development of M. sorghi and extended the fertility period, changing accordingly with the thermal gradient (Table 1). Therefore, the shortest pre-reproductive period was recorded at 35 °C with an average of 4.5 ± 13.7 days. The longest period was recorded at 5 °C, in which the nymphs took 62.5 ± 8.1 days to reach adulthood and lived 25.7 days (88.2 ± 8.7 days, in total).
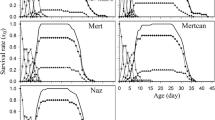
The extreme thermal conditions affected the reproductive capacity of the aphids (Figs. 1 and 2). The lowest fertility rate (1.7 nymphs/day) was observed at 35 °C, while no fertility was observed at 5 °C. Females who grew at 20, 25, and 30ºC showed 96.5, 99.4, and 99.4% of total fertility in the second week, respectively (Fig. 2). Under these same temperatures, total fertility values per female observed were 77.5, 76.5, and 77.5%, respectively, with no statistical difference (χ2 = 0.908, gl = 2, p = 0.635). Additionally, the most extensive reproductive period was observed at 10 °C, corresponding to 33.2 days (0.67 nymphs/day), while the shortest period was observed at 35 °C with 4.1 days (0.41 nymphs/day).
The lowest longevity observed on individuals still able to reproduce was 8.3 days at 35 °C; in contrast, the highest was 113 days at 10 °C. The later data was observed in a single specimen that showed a fertility record of 33 days but with 0.5 to 0.1 nymphs per day. The cohort developed at 15ºC lasted 43 days to register 50% survival, followed by individuals growing at 20ºC (35 days), 25ºC (30 days), and 30ºC (23 days). The minimum projected threshold was 2 °C, with an estimated 158.9 heat units required for its complete development (Fig. 1). Population parameters of M. sorghi varied throughout the tested temperatures, except for the reproductive rate (Ro) from 20 to 35ºC. Individuals developed at 10ºC had the lowest values in all the estimated parameters, in contrast to those evaluated at 30ºC (Table 2).
Outliers
As expected, the genetic diversity of this species was evident as the number of individuals within each cohort showed outlier data of the measured parameters. For example, a supernumerary nymphal stage 5, grown at 20 °C, showed a pre-reproductive period of 7.1 days, a reproductive rate of 16.2, 77.5% fecundity with 4.78 nymphs/day, and a longevity of 40.9 days. Also, spontaneous abortions (nymphoposition of dead individuals) were observed at a temperature range of 10 to 35 °C, a phenomenon previously recorded in Mexico (Peña-Martínez et al., 2018b).
The presence of two males was detected at 15 °C in early September and oviparous females at 10 °C in December. Color variation was observed on some individuals grown at 5, 10, and 15 °C, varying from brown to grayish yellow. Besides, a tendency to develop pigmented dorsal sclerites on males, while their nymphs were purple. Normal intense yellow colorations dominated in most of the studied stages at temperature range from 20 to 40 °C. All treatments showed old or senile females to turn purple.
Discussion
Suggestions by Blackman (personal communication), fundamental aspects of the biology commented by Blackman and Eastop (2018), and the morphometric criteria proposed to Nibouche et al. (2021) allowed to consider the aphid species used in this work as M. sorghi. The morphometric and molecular assays on the Mexican aphid populations, and descriptions of alates and sexual forms, are on process by our work group (Peña-Martínez et al., unpublished data).
The influence of temperature and host plants on the basic biology of M. sacchari (= Longiunguis sacchari) was previously described in Japan (Setokuchi, 1973, 1974, 1975). These works found alike results using used similar methodology as the reported in this paper. For example, the rearing period used and bioassays performed, the use of sorghum leaves, and treatment temperatures of 15, 20, 25 and 30 °C employed. The author found that the optimal fertility was observed at 20 °C with a slightly decrease with temperature increase at 25 and 30 °C, besides the presence of sexual forms.
The comparison with a more recent study conducted by De Souza et al. (2018) with sorghum aphid populations from Oklahoma, USA (OKL), shows similar pre-reproductive period and fertility at 20 °C. However, it also shows important differences in some biological aspects (Fig. 3), as sexual forms (Peña-Martínez et al., 2016) and spontaneous abortion occurrence (Peña-Martínez et al., 2018b). Furthermore, individuals from Guanajuato (GTO) population showed higher longevity than OKL populations, 61% higher at 10 °C, 16% higher at 15 °C, 25% higher at 20 °C, 9% higher at 25 °C, and 33% higher at 30 °C. However, longevity is 5% lower than OKL at 35 °C. Unfortunately, authors showed no fertility records and consider the temperature of 35 °C as lethal.
Comparison of longevity and fecundity results of M. sorghi of this work and that from De Souza et al. (2018)
Interestingly, a GTO supernumerary fifth nymphal stage was observed at 20 °C, similar to that reported for Rhopalosiphum nymphaea (L.) at 18.3 °C (Ballou et al., 1986),), and to the sixth nymphal instar at different temperatures found with a Diuraphis noxia (Mordvilko ex Kurdjumov) (Nowierski et al., 1995). Regarding fertility, GTO clones registered 6 times higher values at 10 °C than those of OKL populations, also higher values were recorded (21, 12, and 47%) at similar temperatures (20, 25 and 30 °C, respectively). Moreover, the GTO clones exhibited a pre-reproductive period of 5.9 days at 35 °C and reproduced at a minimum average level of 1.7, with spontaneous abortion cases. At the same temperature, being considered as lethal for the OKL population, the latter population showed a pre-reproductive period of 8.7 days with no reproduction. The lethal temperature for the GTO was 40 °C, where most individuals died in less than 24 h.
Abortion is a rare event in aphids, and it is unknown if this phenomenon prevails in clones from other parts of the world or in a species of the same genus. These events may be likely to occur but go unnoticed. An example of this is the abortion of M. pyraria (Passerini) shown by Chaubet in 2010 in a digital image but went unnoticed by the author and editors of the website Encyclop’Aphid (Hullé et al., 2020).
The heat units required to develop M. sorghi (158.9 HU) are slightly higher than the 133 HU reported for the green aphid, Schizaphis graminum (Rondani), on barley in Iran (Tofangsazi et al., 2010). Global warming also influences the thermal growing limits; generally, the aphids do not survive at 40ºC. The low-temperature limits are still unknown for many species but are typically recorded at 4 °C (Hullé et al., 2010). Still, the theoretical threshold estimated for M. sorghi (2.0 °C) exceeded the preliminary known values for this species (8.7 and 3.5 °C, Quijano-Carranza et al., 2016; Peña-Martínez et al., 2018a, respectively) obtained from Guanajuato populations. As complementary information, the M. sorghi freezing point (super-cooling) limit was estimated between -22 and -25 °C in OKL populations (De Souza et al., 2018).
In reference to the population parameters, Rm integrates the development and fertility values, so it is a relevant element in estimating the biotic potential of a pest species (Anjali et al., 2017). The Rm values estimated in this study are like those from Rhopalosiphum maidis at 27 °C in sorghum and those estimated by De Souza et al. (2018) on M. sorghi at 30 °C; but higher than species such as S. graminum at 27 °C in Barley (Tofangsazi et al., 2010) and Aphis (Toxoptera) citricida at 25 °C in citrus (Tang et al., 1999). On the other hand, these values are lower than those estimated for Aphis glycines Matsumura in soybeans at 25 °C in Minnesota, USA (McCornack et al., 2004), and for A. gossypii Glover in Cucurbita pepo L. (Aldyhim & Khalil, 1993).
Other works used different temperatures and hosts for M. sorghi growth, making it difficult to compare the values of the population parameters estimated in this work. For example, Lopes-da-Silva et al. (2014) in Brazil, studied M. sorghi in sorghum and sugarcane at 24 °C ± 1 °C, 70% RH and a 14:10 photophase period. In both hosts, the Ro value was lower than those estimated for M. sorghi in GTO, except for the generation time (T), which was slightly higher in sorghum, as compared to the results obtained at 25 °C in our study. Likewise, Du et al. (2018), in Fengyang, China, used petri dish foliar disc from seedlings of four varieties of sorghum and three of S. bicolor (L.) Moench. x S. sudanense (P.) Staph., estimating a higher Ro value at 24 °C than our estimate (79.06 at 25 °C). Authors attribute these results to different nutritional quality or secondary metabolites in plants. Still, some data estimated in this work have similarities with other aphid specie, such as R. maidis fed on sorghum (Anjali et al., 2017), S. graminum fed on barley (Tofangsazi et al., 2010), oats (Vasicek et al., 2010), wheat (La Rossa et al., 2014) and different biotypes of wheat and sorghum (Royer et al., 2015).
In general, results of the present work, as compared with previously published results, show differences that could be explained using different methodologies, host plants (Vasicek et al., 2010), varieties (Du et al., 2018), photoperiod (Wu et al., 2018), and clones or geographic biotypes (De Souza et al., 2018; Nibouche et al., 2014), among other factors.
The review by Royer et al. (2015) mentions that of mortality and migration influenced by natural enemies, host conditions, and climate conditions produce fluctuation in the abundance of aphid populations. Results obtained in the present study indicate that M. sorghi is probably better adapted to grow in a wider range of high temperatures in warm regions, which also occurs in R. maidis (Anjali et al., 2017).
Interactions of aphids with biotic and abiotic factors are multiple and generate diverse biological responses that may vary depending on the influence of each factor. The importance of experimental monitoring of the population parameters of local aphid populations has been highlighted (Nibouche et al., 2014, 2015; Simon & Peccoud, 2018; Vorburger, 2006). Although it is considered that there is a predominant clone in the world (Nibouche et al., 2014), there are records that detect probable differences in genomic sequences in M. sorghi populations in Mexico, which suggest that the clones from the Mexican states of Querétaro and Guanajuato could be a particular biotype (Ibarra et al., 2016). Therefore, a more detailed molecular study is required including samples ese regions on global tests on this species. This considering that various authors mention that evolutionary changes can arise in populations even without genetic recombination (Nibouche et al., 2015; Simon & Peccoud, 2018; Vorburger, 2006).
In conclusion, temperature is a factor that directly affects the development and reproductive process of M. sorghi. The optimal temperatures for development and reproduction comprise from 20 to 30 °C, which means this species has a high developmental potential in spring–summer, the crop growth period in this region. Additionally, the remarkably high longevity of M. sorghi at low temperatures represents an excellent capacity for survival not previously recognized for this species. This information is expected to provide a scientific basis for developing population models applicable in local, regional, and national climatic conditions to optimize integrated management actions.
Data availability
Not applicable.
References
Aldyhim, Y. N., & Khalil, A. F. (1993). Influence of temperature and daylength on population development of Aphis gossypii on Cucurbita pepo. Entomololgy Experimentia Et Applicata., 67, 167–172. https://doi.org/10.1111/j.1570-7458.1993.tb01665.x
Anjali, S. C. A., Sridevi, G., Prabhakar, M., Kalpana, M., & Pushpavathi, B. (2017). Life table- parameters and morphometrics of the corn leaf aphid, Rhopalosiphum maidis (Fitch) (Hemiptera: Aphididae), reared on sorghum host plant. Journal of Entomology and Zoology Studies, 5(5), 558–563.
Ballou, J., Tsai, J., & Center, T. (1986). Effects of temperature on the development, natality, and longevity of Rhopalosiphum nymphaeae L. (Homoptera: Aphididae). Environmental Entomology, 15(5):1096–1099. https://doi.org/10.1093/ee/15.5.1096
Blackman, R. L., & Eastop, V. F. (2018). Aphids on the world’s plants. An online identification and information guide. Retrieved January 20, 2023, from http://www.aphidsonworldsplants.info
Bournoville, R., Simon, J. C., Badenhausser, I., Girousse, C., Guilloux, T., & Andre, S. (2000). Clones of pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae) distinguished using genetic markers, differ in their damaging effect on a resistant alfalfa cultivar. Bulletin of Entomological Research, 90(1), 33–39. https://doi.org/10.1017/S0007485300000055
Bowling, R. D., Brewer, M. J., Kerns, D. L., Gordy, J., Seiter, N., Elliott, N. E., Buntin, G. D., Way, M. O., Royer, T. A., Biles, S., & Maxson, E. (2016). Sugarcane Aphid (Hemiptera: Aphididae): A New Pest on Sorghum in North America. Journal of Integrated Pest Management, 7(1), 1–13. https://doi.org/10.1093/jipm/pmw011
De Souza M., Armstrong, J. S, Hoback, W., Mulder P., & Foster J. E. (2018). The effects of temperature on sugarcane aphid, Melanaphis sacchari life history, on three different host plants. Chapter II pp 15–40. In. M. De Souza M.S. Thesis, Oklahoma State University, Oklahoma.
Du, J., Zhan, Q., Huang, B., Liu, Y., Li, J., Wu, D., Tang, Y., & Li, J. (2018). Biological characteristics and life table parameters of the sorghum aphid (Melanaphis sacchari) on different sorghum and Sorghum bicolor × Sorghum sudanense cultivars. Journal of Yunnan Agricultural University (Natural Science), 33, 191–197.
Hinson, P. O. (2017). Effect of temperature on the development of sugarcane aphid, Melanaphis sacchari, on sorghum. M.S. Thesis A & M University, Texas.
Hullé, M., Acier, A. C. D., Bankhead-Dronnet, S., & Harrington, R. (2010). Aphids in the face of global changes. Comptes Rendus Biologies, 333(6–7), 497–503. https://doi.org/10.1016/j.crvi.2010.03.005
Hullé M., Chaubet, B., Turpeau, E., & Simon, J.C. (2020, March 21). Encyclop’Aphid: a website on aphids and their natural enemies. Entomologia generalis: Retrieved March 21, 2020, from https://www6.inrae.fr/encyclopedie-pucerons_eng/Species/Aphids/Melanaphis/M.-pyrariahttps://doi.org/10.1127/entomologia/2019/0867
Ibarra, J. E., García-Suárez, R., Luévano-Borroel, J., & Peña-Martínez, R. (2016). Identificación molecular de biotipos del pulgón amarillo del sorgo, Melanaphis sacchari, en el estado de Guanajuato. In Yañez-López (Ed.), Proceedings, Symposium: Avances en la Investigación del Manejo Integrado del Pulgón Amarillo del Sorgo en Guanajuato (pp. 11–24). Guanajuato, Mexico.
La Rossa, F., Vasicek, A., López, M., Bosco, N., Imperiale, P., & Bainotti, C. (2014). Respuesta biológica y poblacional de Schizaphis graminum (Rond.) (Hemiptera: Aphididae) sobre ocho cultivares de trigo (Triticum aestivum L.) en condiciones de laboratorio. Revista de Investigaciones Agropecuarias, 40(3), 281–289.
Li, Y., & Akimoto, S. I. (2018). Evaluation of an aphid-rearing method using excised leaves and agar medium. Entomological Science, 21(2), 210–215. https://doi.org/10.1111/ens.12296
Lopes-da-Silva, M., Rocha, D. A., & Da Silva, K. T. B. (2014). Potential population growth of Melanaphis sacchari (Zethner) reared on sugarcane and sweet sorghum. Current Agricultural Science and Technology, 20, 21–25.
Maia, A. de H. N., Luiz, A. J., & Campanhola, C. (2000). Statistical inference on associated fertility life table parameters using jackknife technique: computational aspects. Journal of Economic Entomology, 93, 511–518.https://doi.org/10.1603/0022-0493-93.2.51
McCornack, B. P., Ragsdale, D. W., & Venette, R. C. (2004). Demography of soybean aphid (Homoptera: Aphididae) at summer temperatures. Journal of Economic Entomology, 97(3), 854–861. https://doi.org/10.1093/jee/97.3.854
Nibouche, S., Fartek, B., Mississippi, S., Delatte, H., & Reynaud, B. (2014). Low genetic diversity in Melanaphis sacchari aphid populations at the worldwide scale. PLoS ONE, 9(8), e106067. https://doi.org/10.1371/journal.pone.0106067
Nibouche, S., Mississippi, S., Fartek, B., Delatte, H., Reynaud, B., & Costet, L. (2015). Host plant specialization in the Sugarcane Aphid Melanaphis sacchari. PLoS ONE, 10, e0143704. https://doi.org/10.1371/journal.pone.0143704
Nibouche, S., Costet, L., Holt, J. R., Jacobson, A., Pekarcik, A., Sadeyen, J., Armstrong, S., Peterson, G. C., McLaren, N., & Medina, R. F. (2018). Invasion of sorghum in the Americas by a new sugarcane aphid (Melanaphis sacchari) superclone. PLoS ONE, 13(4), e0196124. https://doi.org/10.1371/journal.pone.0196124
Nibouche, S., Costet, L., Medina, R. F., Holt, J. R., Sadeyen, J., Zoogones, A. S., Brown, P., & Blackman, R. L. (2021). Morphometric and molecular discrimination of the sugarcane aphid, Melanaphis sacchari (Zehntner, 1897) and the sorghum aphid Melanaphis sorghi (Theobald, 1904). PLoS ONE, 16, e0241881. https://doi.org/10.1371/journal.pone.0241881
Nowierski, R. M., Zheng, Z., & Scharen, A. L. (1995). Age-specific life table modeling of the Russian wheat aphid (Homoptera: Aphididae) on barley grown in benzimidazole agar. Enviromental Entomology, 24(5), 1284–1290. https://doi.org/10.1093/ee/24.5.1284
Peña-Martínez, R., Muñoz-Viveros, A. L., Bujanos-Muñiz, R., Luévano-Borroel, J., Tamayo-Mejía, F., & Cortez-Mondaca, E. (2016). Formas sexuales del complejo Pulgón Amarillo del Sorgo (Hemiptera: Aphididae) en México. Southwestern Entomologist, 41(1), 127–131. https://doi.org/10.3958/059.041.0114
Peña-Martínez, R., Lomeli-Flores, J. R., Bujanos-Muñiz, R., Muñoz-Viveros, A. L., Vanegas-Rico, J. M., Salas-Monzón, R., Hernández-Torres, O. E., Marín-Jarillo, A., & Ibarra, J. E., (2018a). Pulgón amarillo del sorgo, (SCA), Melanaphis sacchari (Zehntner, 1897), interrogantes biológicas y tablas de vida. Fundación Guanajuato Produce, AC.
Peña-Martínez R., Muñoz-Viveros, A. L., Marín-Jarillo, A., Bujanos-Muñiz, R., Luévano-Borroel, J., Sánchez-Segura, L., & Ibarra J. E., (2018b). Spontaneously aborted embryos in the Sugarcane Aphid (Hemiptera: Aphididae) Annals of the Entomological Society of America, 111(6), 312–318 https://doi.org/10.1093/aesa/say020
Quijano-Carranza, J. A., Vázquez-Ortega, A., Juan Diego-García, L. B., Cuéllar-Zambrano, C., Cerrito-Arellano, R., & Yáñez-López, R. (2016). Sistema de monitoreo del pulgón amarillo del sorgo, Melanaphis sacchari (Zehntner). In R. Yáñez-López (Ed.), Proceedings, Symposium: Avances en la investigación del Manejo integrado del Pulgón amarillo del Sorgo en Guanajuato (pp.140–148). Guanajuato, Mexico.
Quijano-Carranza, J. A., Pecina-Quintero, V., Bujanos-Muñiz, R., Marín-Jarillo, A. & Yañez-López, R. (2017). Folleto para productores No. 1: Guía para el manejo del pulgón amarillo del sorgo. Fundación Guanajuato Produce A.C. Editorial.
Rodríguez-del-Bosque, L. A., & Terán, A. P. (2015). Melanaphis sacchari (Hemiptera: Aphididae): A new sorghum insect pest in Mexico. Southwestern Entomologist, 40(2), 433–434.
Royer, T. A., Pendleton, B. B., Elliott N. C., & K. L. Giles. (2015). Greenbug (Hemiptera: Aphididae) biology, ecology, and management in wheat and sorghum. Journal of Integrated Pest Management, 6(1), 1–10; https://doi.org/10.1093/jipm/pmv018
Salas-Monzón, R., Hernández-Torres, O. E., Lomeli-Flores, J. R., Peña-Martínez, R., Muñoz-Viveros, A.L. & Vanegas-Rico, J. M. (2018). Metodología para el estudio del desarrollo de Melanaphis sacchari (Zehntner) (Hemiptera: Aphididae) bajo condiciones de laboratorio. Folia Entomológica Mexicana. (n.s), 4(3), 85−90, 2018.
SAS Institute Inc. (n.d.). SAS 9.1.3 help and documentation (pp. 2002−2004). SAS Institute Inc.
Setokuchi, O. (1973). Ecology of Longiunguis sacchari (Zehntner) (Aphididae) infesting sorghum SCA I. Nymphal period and fecundity of apterous viviparous female. Proccedings of Association of Plant Protection of Kyushu, 19, 95–97.
Setokuchi, O. (1974). Ecology of Longiunguis sacchari (Zehntner) (Aphididae) infesting sorghum SCA. II. Observations on the oviposition. Proccedings of Association of Plant Protection of Kyushu, 20, 26–27.
Setokuchi, O. (1975). The hibernation of Longiunguis sacchari (Zehntner) on sorghum SCA. Kagoshima Agricultural Experimerntal Station, Kushira, Kagoshima, 893(16), 296–297.
Simon, J. C., & Peccoud, J. (2018). Rapid evolution of aphid pests in agricultural environments Current Opinion in Insect. Science, 26, 1–8. https://doi.org/10.1016/j.cois.2017.12.009
Tang, Y. Q., Lapointe, S. L., Brown, L. G., & Hunter, W. B. (1999). Effects of host plant and temperature on the biology of Toxoptera citricida Homoptera: Aphididae). Environmental Entomology, 28(5), 895–900. https://doi.org/10.1093/ee/28.5.895
Tofangsazi, N., Kheradmand, K., Shahrokhi, S., & Tayebi, A. A. (2010). Temperature-dependent life history of Schizaphis graminum on barley. Bulletin of Insectology, 63(1), 79–84.
Vasicek, A., La Rossa, F., Paglioni, A., & López, M. C. (2010). Estadísticos biológicos y demográficos de Diuraphis noxia (Mordv.), Metopolophium dirhodum (Wlk.), Rhopalosiphum padi (L.), Schizaphis graminum (Rond.) y Sipha maydis (SCAs.) (Hemiptera: Aphididae) sobre diferentes cultivares de Avena sativa L. en condiciones controladas. Boletín De La Sociedad Entomológica Aragonesa, 6, 591–596.
Vorburger, C. (2006). Temporal dynamics of genotypic diversity reveal strong clonal selection in the aphid Myzus persicae. Journal of Evolutionary Biology, 19(1), 97–107. https://doi.org/10.1111/j.1420-01.2005.00985
Wu D. G., Zhan, Q. W., Huang, B. H., Wang, Z. X., Huang, W. D., Bi., Y. L., Liu C. Z., & Du, J. L. (2018). Effects of photoperiod on the population parameters of the sugarcane aphid, Melanaphis sacchari (Hemiptera: Aphididae) Acta Entomologica Sinica, 61(4), 511–518. https://doi.org/10.16380/j.kcxb.2018.04.014
Zapata, S. D., Dudensing, R., Sekula, D., Esparza-Díaz, G., & Villanueva, R. (2018). Economic impact of the sugarcane aphid outbreak in South Texas. Journal of Agricultural and Applied Economics., 50(1), 104–128. https://doi.org/10.1017/aae.2017.24
Acknowledgements
The authors appreciate all the logistical support from the technical staff of Guanajuato’s State Committee for Plant Health (CESAVEG).
Funding
This work was supported by Fundación Guanajuato Produce.
Author information
Authors and Affiliations
Contributions
All authors conceived the research. R.P.M., research project leader; J.R.L.F., laboratory experiment supervisor; R.B.M. field work; A.L.M.V., manuscript concept; R.S.M., laboratory work; O.E.H.T., laboratory work; A.M.J. field work; J.E.I., manuscript review and funding representative; J.M.V.R, data analysis, Figures and Tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The authors declare compliance with the ethical and scientific standards. The present study does not include results of studies involving animals or humans.
Consent for publication
All the authors give their consent to publish the manuscript.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peña-Martínez, R., Lomeli-Flores, J.R., Bujanos-Muñiz, R. et al. Comparative biology and life tables of sorghum aphid Melanaphis sorghi (Theobald) (Hemiptera: Aphididae) from Mexico, at different temperatures. Phytoparasitica 52, 33 (2024). https://doi.org/10.1007/s12600-024-01152-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12600-024-01152-8