Abstract
Wheat (Triticum aestivum L.) is the major global staple food crop that meets the food security demands of various nations across the continents. The recent reduction in wheat production is attributed to several biotic and abiotic factors especially, temperature and rainfall patterns, and pest occurrence. Among insect pests, aphid species are emerging as new pests of economic importance in India and elsewhere. The present investigation identified a new association of Macrosiphum euphorbiae Thomas with the wheat crop. Life table parameters were studied for M. euphorbiae and Rhopalosiphum padi fed on wheat foliage. The total nymphal duration and life cycle duration, respectively, of R. padi (4.76 ± 0.54 and 9.71 ± 1.38 days) and M. euphorbiae (5.84 ± 0.69 and 9.96 ± 1.31 days) were significantly different for these species. The fecundity of the two aphid species was 23.95 ± 8.67 and 11.6 ± 4.10 progeny/female, respectively. Age-specific survival rate (lx), age-specific fecundity (fx), and population age-specific fecundity (mx) were higher in R. Padi compared to M. euphorbiae. Reproductive value (Vxj) was high in R. padi and the duration of reproduction was less, while these parameters showed an opposite trend in M. euphorbiae. The gross reproduction rate (GRR) was found higher in R. Padi (29.17 offspring/adult lifetime) compared to M. euphorbiae (19.58 offspring/adult lifetime). The M. euphorbiae being a pest of solanaceous crops seems to have shifted to a new host, i.e., wheat. This new adaptation strategy to survive for long periods on a wheat crop might pose a serious threat to wheat crop cultivation in near future.
Similar content being viewed by others
Introduction
Of late, agricultural production is facing continuous challenges by ever-increasing adverse environmental instability and abiotic and biotic stresses. Wheat (Triticum aestivum L.) domestication is closely associated with the efforts of humans to protect themselves from hunger and to gain control over their food supply. Wheat is cultivated worldwide, and it is the second most important cereal source of food and essential nutrients. Wheat has the advantage of requiring less water for cultivation than other similar crops. Presently, China and India are the top two wheat-producing countries, where it serves as a staple food along with rice (Igrejas and Branlard 2020). In India, despite unfavorable weather conditions and limited availability of farm workers due to COVID-19 restrictions, 32.0 million hectares (mha) of wheat was harvested in 2021 with a yield average of 3.38 tons per hectare (t/ha), and the production is estimated at a record 108 million metric tons. However, the wheat yield is almost 2 percent lower than the previous year because of warmer-than-average temperatures, dry conditions, and biotic stress factors across the Indian wheat belt (Commodity Intelligence Report, USDA 2021). An increase in temperature may affect the grain filling duration and sterility of grains and ultimately reduce the productivity of yield (Hatfield et al. 2011). Several insect pests, viz. aphids; termites, Odontotermes obesus (Ramb); shoot fly, Atherigona naqvii (Steyskal); brown wheat mite, Petrobia latens (Muller); armyworm, Mythimna separata Walker and cutworms, Agrotis ipsilon (Hufnagel), are important pest attacking the wheat crop. Among them, aphid species (Wains et al. 2010) heavily infect the wheat crop. In India, aphids pose a serious threat to wheat cultivation due to the advent of the rice–wheat cropping system (Aggarwal et al. 2000). Aphids are phloem-feeding insect pests that use their piercing and sucking mouthparts to feed on plant sap. Also, aphids are "r-strategists," indicating that their simple bodies enable them to consume and reproduce quickly, with the majority of the energy going toward the production of nymphs (Rabasse and Steenis 1999). They can cause 35–40% yield loss directly by sucking sap and 20–80% indirectly by the transmission of viral diseases like barley yellow dwarf virus (BYDV) and cereal yellow dwarf virus (CYDV) (Rossing et al. 1994; Bukvayova et al. 2006). The climatic variability became an advantage for the aphids which are emerging as an important pest of wheat in the recent past, and more than 11 aphid species have been reported on wheat. The bird cherry-oat aphid, Rhopalosiphum padi (L.) and English grain aphid, Sitobion avenae (F.) are more dominant, destructive, and commonly occurring pest species responsible for significant yield and quality losses in the wheat crop in many parts of the world and India (Blackman and Eastop 1984; Oerke et al. 1996; Liu et al. 2012; Dhadwal et al. 2014; Alawi et al. 2021).
In the present investigation during 2021–2022, for the first time potato aphid, Macrosiphum euphorbiae, infestation was observed on wheat crop in New Delhi, India, along with the bird cherry-oat aphid, Rhopalosiphum padi (L.). M. euphorbiae feeds on more than 200 plant species from 20 distinct families, with a wide host range, including herbaceous crops, solanaceous plants, and ornamental crops. M. euphorbiae can also infest tomato globally, occasionally reported as a major pest in California, which resulted in crop losses of one ton per acre approx. (Zalom 2007). M. euphorbiae is a potential virus vector and spreads plant viruses such as potato leafroll virus (PLRV) and potato virus Y (PVY) (Moeller 1973; Singh and Boiteau 1986; Millar 1990; Chan et al. 1991; Radcliffe and Ragsdale 2002; Blackman and Eastop 2007). M. euphorbiae is significant in viral epidemiology studies due to its abundance and tendency to generate alatae forms. It is a concern that M. euphorbiae can feed on wheat, and this sends a warning signal to all wheat-growing areas as this aphid species was originally reported from North America and has now spread throughout the globe (Starý et al. 1993; Finlayson et al. 2009; Valenzuela et al. 2009; Raboudi et al. 2011; Le Guigo et al. 2012). In the course of biological evolution, the environment acts as a selective force on any species, influencing its genetic potential to respond to its survival (Harrington et al. 2001). Therefore, this research aims to understand the age-specific mortality and survival rate in each developmental stage or age interval and the measurement of population density at several points in the life cycle during each generation of R. padi and M. euphorbiae infesting on wheat crop. This information forms a basis and is highly needed for further studies of aphid pest control in wheat crop.
Materials and methods
The study was conducted at ICAR-Indian Agricultural Research Institute (IARI), Pusa, New Delhi, India (28°38'N latitude, 77°09'E longitude, 228.61 m altitude), during 2021–2022.
Aphid culture
The initial cultures of R. Padi and M. euphorbiae aphids were collected from a wheat (HD-3086) field in ICAR-IARI, Pusa, New Delhi, India. The field-collected aphids were transferred individually to Petri plates (90 mm dia.) for nymphoposition. Each plate was facilitated with air holes on the lid and sterile filter paper at the bottom of the plate for holding moisture on which a wheat leaf was placed to keep the leaves turgid and fresh. Every day, leaves were replaced with fresh ones from each Petri plate. The Petri plates were placed at a normal room temperature of 25° ± 1 °C and 65–70% RH with 12 h each of a dark and light phase. Both the aphid species were allowed to lay nymphs for 24 h, and after 24 h offspring were separated and kept individually in separate Petri plates to record daily observations, viz. instar-wise developmental duration, reproductive period, reproductive rate, adult mortality, and longevity. The offspring were removed daily to record the fecundity of each adult aphid until its death. The mortality and survival of the aphids and nymphs were recorded at 24 h of intervals at approximately the same time each day and the longevity and percentage of survival were further calculated. A total of 40 (n = 40) replicates were maintained for this study.
Morphological and molecular identification of the aphid species
Aphids collected from wheat fields were stored in 70% ethanol and used for morphological and molecular identification. The morphological identification was undertaken at the Division of Germplasm Collection and Characterization, ICAR-National Bureau of Agricultural Insect Resources, Bengaluru, India. The aphids were identified based on the keys developed by Blackman and Eastop (2000). For molecular identification, total genomic DNA was extracted from single adult aphids using a DNeasy Blood and Tissue kit (Qiagen™). The extracted DNA was used as a template in PCR-based detection following quality and quantity checks using nanodrop 2000 (Thermoscientific™). The mitochondrial cytochrome oxidase I (MtCO1) gene was targeted to amplify with the forward LCO(F) 5'-GGTCAACAAATCATAAAGATATTGG-3' and reverse HCO(R) 5'-TAACTTCAGGGTGACCAAAAAATCA-3' primers. The PCR comprised an initial denaturing step at 94 °C for 4 min and 35 amplification cycles (94 °C for 1 min., 48 °C optimal annealing temperature for 1 min, 72 °C for 1 min), followed by a final extension step at 72 °C for 5 min. The amplified products were resolved on 1% gel electrophoresis and further used for Sanger sequencing. The MtCO1 gene sequences obtained were curated and aligned with the help of reference sequences obtained from the Basic Local Alignment Search Tool (nBLAST) of the National Centre for Biotechnology Information (NCBI) database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) using BioEdit v 72 program. The final sequences with identity checks were submitted to NCBI to get the accession number and aligned.
Life table construction
The life table studies were carried out by rearing aphids separately on wheat leaves. According to the age-stage, two-sex life table principle and method (Tuan et al. 2014), the following parameters were calculated and the age-stage, two-sex life tables of R. padi and M. euphorbiae on the wheat were established.
-
1.
Age-stage-specific survival rates (Sxj): It is the probability that a newly laid nymph will survive to age x and stage j.
$${S}_{XJ}= \frac{{n}_{xj}}{{n}_{01}}$$where n01 is the number of nymphs used at the beginning of the life table study and nxj is the number of individuals surviving to age x and stage j (Chang et al. 2016).
-
2.
Age-specific survival rate (lx): It is the probability that a newly laid nymph will survive to age
$${l}_{x}=\sum_{j=1}^{m}{S}_{xj}$$where, m is the number of stages.
-
3.
Age-stage-specific fecundity (fxj): It is the number of hatched nymphs produced by the adult female at age x and j is the life stage number.
-
4.
Age-specific fecundity (mx): The number of nymphs per individual at age x
$${m}_{x}=\frac{{\sum }_{j=1}^{m}{S}_{xj }{f}_{xj}}{{\sum }_{j=1}^{m}{S}_{xj}}$$ -
5.
Age-specific maternity (lx*mx): The product of lx and mx.
-
6.
Age-stage-specific life expectancy (exj): It is the time that an individual of age x and stage y is expected to live
$${e}_{xj}=\sum_{j=i}^{m}\sum_{j=1}^{m}{S}_{ij}$$where Sij is the probability that an individual of age x and stage y will survive to age and stage j.
-
7.
Age-stage-specific reproductive value (Vxj): It is the contribution of individuals of age x and stage y to the future population
$${v}_{xj}=\frac{{e}^{-r(x+1)}}{{S}_{xj}}\sum_{i=x}^{n}{e}^{-r(i+1)}\sum_{j=y}^{m}{S}_{ij}{f}_{ij}$$ -
8.
Intrinsic rate of increase (r): It is the population growth rate as the time approaches infinity and the population reaches the stable age-stage distribution. The population size will increase at the rate of er per time unit.
$$\sum_{x=0}^{\infty }{e}^{-r(x+1)}{l}_{x}{m}_{x}=1.$$ -
9.
Finite rate of increase (λ): The finite rate is the population growth rate as the time approaches infinity and the population reaches the stable age-stage distribution. The population size will increase at the rate of λ per time unit.
Simply the proportional change in population size from one time period to the next.
$${\varvec{\lambda}}={{\varvec{e}}}^{{\varvec{r}}}$$ -
10.
Net reproductive rate (R0): It is referred to as the "carrying capacity" of the average insect under defined environmental conditions. It is the mean number of female offspring produced by a female during her entire lifetime (i.e., the replacement rate). It is also Ro < 1 indicates the members of the population are not replacing themselves (i.e., the population is declining): Ro > 1 denotes an increasing population and Ro = 1 indicates a stationary population. Its unit is females/female/generation.
$${R}_{o}=\sum_{x=0}^{\infty }{l}_{x}{m}_{x}$$ -
11.
Mean generation time (T): It is the period that a population requires to increase to R0-fold of its size as the time approaches infinity and the population settles down to a stable age-stage distribution (SASD), i.e., stable age distribution SAD, too. A shorter generation time does not mean a faster growth rate.
Simply, the average interval between the birth of an individual and the birth of its offspring.
$$T=\frac{{1}_{n}{ R}_{o}}{r}$$
Statistical analysis
The life-history data of R. padi and M. euphorbiae were used separately to calculate each population parameter and analyzed using TWOSEX-MSChart software (http://140.120.197.173/Ecology/prod02.htm) (Chi 1988; Huang and Chi 2012). The means, standard errors and variances of the population parameters were arrived at by the bootstrap technique (100,000 repetitions) (Efron and Tibshirani 1993). Sigma plot 14.5 (https://systatsoftware.com/sigmaplot/) was used to create graphs.
Results
Morphological and molecular identification of the aphid species
Morphological characteristics of the two different aphid morphotypes revealed the features of R. padi as apterous pale green to dark green adults which had a rust-red shade around the base of each cornicle and abdominal end. The body length of the apterae ranged between 1.2 and 2.4 mm. The terminal process of the sixth antennal segment of the apterae was 4–5 times longer than its base. The apical ends of the cornicles were slightly swollen and ended with a strong flange preceded by a distinct construction, and the cauda was shorter than the cornicles. Similarly, morphological characteristics of M. euphorbiae are; apterae either green with a darker green longitudinal stripe, their eyes noticeably red, and the antennae darker toward their tips.
Molecular identification of the two different aphid morphotypes collected from wheat was performed by targeting the mitochondrial cytochrome oxidase I (MtCO1) gene. In nucleotide BLAST analysis, the bird cherry morphotype RP-IARI-DL-1 showed 100% similarity with other aphid species of R. padi (bird cherry-oat aphid), and interestingly the green morphotype, ME-IARI-DL-1 showed 99% similarity with M. euphorbiae (potato aphid). This has confirmed the infestation and damage caused by two aphid species R. padi RP-IARI-DL-1 and M. euphorbiae ME-IARI-DL-1 on wheat crops. The two sequences were submitted to the NCBI database using GenBank, and accession numbers have been received for R. Padi, RP-IARI-DL-1 (OP380251) and M. euphorbiae, ME-IARI-DL-1 (OP373188). However, to further analyze their phylogenetic relationship with other aphid species, a dendrogram was constructed using the neighborhood joining method. In the phylogenetic analysis, R. padi (RP-IARI-DL-1) closely clustered with other strains of R. Padi (MF958485, MF958483, MN320354, MN320341) with 100% bootstrap value and 100% nucleotide identity (Fig. 1). Similarly, M. euphorbiae (ME-IARI-DL-1) showed close clustering with other strains of M. euphorbiae infesting potato crop (EU701726, KC008068, KY613938, ON929001) with 100% bootstrap value and 99.85% nucleotide identity. Lastly, both the aphid species were distantly clustered with Rhalosiphum maidis (Fitch), Rhalosiphum rufiabdominalis (Sasaki), Schizaphis graminum (Rondani), Hyperomyzus carduellinus (Theobald), Hysteroneura setariae (Thomas), Macrosiphum parvifolii (Richards), Macrosiphum occidentalis (Essig), Macrosiphum insularis (Pergande), Sitobion miscanthi (Takahashi), Diuraphis noxia (Kurdjumov), Aphis craccivora Koch, Aphis gossypii Glover and Thrips tabaci Lindeman (Fig. 1).
Biological aspects of R. padi and M. euphorbiae reared on wheat
The total nymphal duration of 4.76 ± 0.54 days for R. padi and of 5.84 ± 0.69 days for M. euphorbiae were significantly different. The first nymphal instar duration for both R. padi and M. euphorbiae was recorded as 1 ± 0.00 days, while the respective second nymphal instar duration was 1.33 ± 0.48 and 1.52 ± 0.51 days, which, however, did not differ significantly. The third and fourth nymphal instar durations, respectively, were 1.19 ± 0.40 and 1.24 ± 0.44 days for R. padi and 1.64 ± 0.49 and 1.68 ± 0.48 days for M. euphorbiae differed significantly. The adult aphid duration was recorded as 4.95 ± 1.50 days for R. padi and 4.12 ± 1.17 days for M. euphorbiae. The total life cycle duration was recorded as 9.71 ± 1.38 and 9.96 ± 1.31 days for R. padi and M. euphorbiae, respectively. The fecundity was 23.95 ± 8.67 and 11.6 ± 4.10 (progeny/female), respectively, in R. padi and M. euphorbiae and there was no pre-viviposition period in both the species. However, the post-viviposition period was 0.43 ± 0.51 and 0.16 ± 0.37, and the viviposition period was 4.52 ± 1.47 and 3.96 ± 1.10 days, respectively, for R. padi and M. euphorbiae (Table 1).
Life table parameters of wheat aphids R. padi and M. euphorbiae
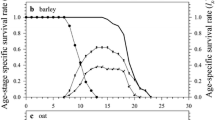
The relation of Age-stage survival rate (Sxj), survival rate (lx), life expectancy (Exj), and reproductive value (Vxj) between R. Padi and M. euphorbiae on wheat was studied and results showed that the age-stage survival rate (Sxj) duration was higher in M. euphorbiae as compared to R. padi during all the stages of the life cycle (Fig. 2). Age-specific survival rate (lx), age-specific fecundity (fx), and population age-specific fecundity (mx) were higher in R. padi compared to M. euphorbiae (Fig. 3). Further, life expectancy (Exj) was higher in M. euphorbiae as compared to R. padi (Fig. 4). Reproductive value (Vxj) was higher in R. padi but the duration of reproduction was lower, while in M. euphorbiae the opposite trend was observed (Fig. 5). Finally, the comparison between the reproductive value v(x) and life expectancy (ex) of both the aphids showed that reproductive capacity was higher in R. padi with less duration but reproducing capacity was lower with higher life expectancy in M. euphorbiae (Fig. 6).
In the present study, the intrinsic rate of population increase (rm) was recorded as 0.37 (R. padi) and 0.24 (M. euphorbiae). Similarly, the information about population multiplication in a unit of time represented as the finite rate of increase (λ) was 1.45 in R. padi and slightly lower at 1.28 in M. euphorbiae. Further, the net reproductive rate (R0) that signifies the total number of offspring that an average individual can produce during their lifetime was 14.37 (R. padi) and 7.25 (M. euphorbiae) females/adult. The mean generation time (T) which is the time lapse between the birth of the parents and the birth of the offspring was 7.15 days (R. padi) and 7.99 days (M. euphorbiae). Further, the time required for the aphid population to double (DT) was 1.86 days (R. padi) and 2.79 days (M. euphorbiae). The gross reproduction rate (GRR) that characterizes the average number of offspring/adult/generations was found to be higher in R. Padi (29.17) compared to M. euphorbiae (19.58). Also, all the population parameters were found significantly different for the two aphid species in the present study (Table 2).
Discussion
Wheat, a staple food for millions, is now extremely vulnerable to climate change, especially to temperature, rainfall, humidity, and other weather vagaries. Minor pests and diseases are becoming major and unpredicted genetic mutations are expected in already known major biotic agents. In this scenario, many insect species are forced to shift toward unconventional host plants for their survival. In the present study, we observed two different aphid morphotypes (bird cherry and green aphid) infesting wheat during 2021–2022 in the fields of ICAR-IARI, Pusa, New Delhi, India. Morphological and molecular characterization based on the mitochondrial cytochrome oxidase (MtCO1) gene sequences showed that the bird cherry morphotype of R. padi showed greater similarity to other strains of R. padi infecting wheat, maize, and other cereals. R. padi is known for its broad host range comprising 20 plant families, particularly grasses, and cereals like rice, wheat, and rosaceous, aquatic, and semi-aquatic plants (Blackman and Eastop 2006; Holman 2009). The green morphotype was identified as M. euphorbiae and showed a close clustering with other potato infecting aphid morphotypes of M. euphorbiae, thus revealing wheat as its new host plant. This demonstrates that monocot wheat is a new host plant species for M. euphorbiae ME-IARI-DL-1 which is otherwise well known for infesting dicot plant species including rose, potato, and verveine. However, other Macrosiphum species such as M. pachysiphon, M. dirhodum, M. scabripes, M. longicaudatum, and M. miscanthi have been already reported as pests in the Triticum genus (Singh and Singh 2017).
A life table is a collection of extensive datasets on a population's survival and development and fertility of a population. Understanding the different parameters of a pest's population growth would make it much easier to design a holistic pest management program for its host crop. For the development of effective pest management measures, a better understanding of the biology and life cycle features of aphids on the wheat crop is required. The population growth rate of aphids in the given generation can be predicted by these parameters. Hence, regional studies on aphid biology and life table in the wheat crop are necessary to provide knowledge to develop aphid management measures related to particular environmental conditions. The mean duration of the R. padi nymphal instars reared on wheat leaves at 25 to 26 °C in the present study was 1.00 ± 0, 1.33 ± 0.48, 1.19 ± 0.40, and 1.24 ± 0.44 days for 1st, the 2nd, 3rd, and 4th instars, respectively, and the total life cycle was 9.71 ± 1.38 days, which is in accordance with the earlier studies (Auad et al. 2009) on Signal grass, Brachiaria ruziziensis (Prosea) at 28 °C with a life cycle duration of 7.30 ± 0.44 days and on barley (Park et al. 2017) The pre-viviposition was absent in R. padi, and viviposition and post-viviposition periods in the present study were found to be 4.52 ± 1.47 and 0.43 ± 0.51, respectively. However, Park et al. (2017) found the pre-oviposition period (0.40 ± 0.06), oviposition period (9.35 ± 0.37), and post-oviposition period (4.07 ± 0.51) days with 12.92 ± 0.86 days longevity and 70.32 ± 2.1 nymphs/female. The net reproductive rate shows the carrying capacity of the mean insect population under different environmental conditions. The net reproductive rate (R0) of R. padi was 14.37 in our study, while the R0 value in other studies varied from 9.85 to 66.67 (Sengonca et al. 1994), Auad et al. 2009, Park et al. 2017). The differences in oviposition periods and net reproductive rates might be due to the variation in host plant species and temperatures that affect the developmental duration of R. padi under different environmental conditions.
The nymphal duration of M. euphorbiae was observed as 5.84 ± 0.69 days, with a total life cycle duration of 9.96 ± 1.31 days. However, the fecundity of M. euphorbiae (11.6 ± 4.10 progeny/female) was lower compared to R. padi in a wheat crop. Previously, Beetge and Krüger (2019) studied the life-history traits of M. euphorbiae at different day-night temperatures of 25–15 °C and well-watered on potato leaves and reported that total nymphal duration was 7.9 ± 0.3 days. The pre-reproductive period was 2.5 ± 0.2 days, the reproductive period was 18.0 ± 1.6 days, the post-reproductive period was 1.0 ± 0.0 days, the total life cycle duration was 21.3 ± 1.6 days, and the fecundity was 47.5 ± 4.5 (progeny/female). These variations in life history and developmental days might be due to host specificity and the prevailing environment. The life history and development of M. euphorbiae were also previously studied by various workers (Smith 1919; Patch 1925; MacGillivray and Anderson 1958), and most of them showed temperature-based variation in developmental phases. Life table parameters including the intrinsic rate of increase (rm) (0.24), finite rate of increase (λ) (1.28), net reproductive rate (R0) (7.25), mean generation time (T) (7.99), doubling time (DT) (2.79), and gross reproductive rate (GRR) (19.58) of M. euphorbiae were significantly different from R. padi when fed on wheat leaves. The earlier study on M. euphorbiae infesting potato revealed a gross reproductive rate (GRR) of 76.45 ± 9.81, net reproductive rate (R0) of 48.82 ± 6.23, mean generation time (T) of 20.90 ± 1.41, intrinsic rate of increase (rm) of 0.17 ± 0.01, finite rate of increase (λ) of 1.21 ± 0.01, and doubling time (DT) as 3.78 ± 0.20 (Beetge and Krüger 2019).
The pre-reproductive period was not observed for M. euphorbiae under normal room temperature on the wheat crop; however, Conti et al. 2010 recorded a pre-reproductive period of 2.8 days at 16 °C on lettuce. However, the reproductive period was 3.96 ± 1.10 days under room temperature for M. euphorbiae. Similarly, in our study fecundity of M. euphorbiae was 11.6 ± 4.10 progeny/female. However, at 25 °C Barlow 1962 reported a slightly higher fecundity of 13.8 ± 1.95 progeny/female on potato. On wheat, the net reproductive rate (R0), intrinsic rate of increase (rm), finite rate of increase (λ), and mean generation time (T) of M. euphorbiae was 7.25 ± 0.094, 0.28 ± 0.002, 1.28 ± 0.002, and 7.99 ± 0.020, respectively. The life table parameters were reported to be different in previous studies (Cooper and Goggin 2005; Ribeiro et al. 2006; Conti et al. 2010; Jeon et al. 2019) where the net reproductive rate (R0), intrinsic rate of increase (rm), finite rate of increase (λ), and mean generation time (T) of M. euphorbiae were 6.1 ± 1.92, 0.08 ± 0.019, 1.092 ± 0.021, and 16.60 ± 1.00, respectively.
The host plant shift of the aphid species M. euphorbiae was found to produce significant differences in its life table, and these can be attributed to the food source, microclimatic conditions, and environmental effects.
Conclusion
In the present investigation, two different aphid species, R. padi and M. euphorbiae, were characterized based on morpho-molecular studies. The potato aphid M. euphorbiae has been reported for the first time on wheat crop. The life table studies highlighted the significant differences between R. padi and M. euphorbiae which completed the total life cycle on the wheat crop. The fecundity (progeny/female), net reproductive rate (R0), and gross reproduction rate (GRR) of R. padi were observed to be higher in wheat as compared to potato aphid M. euphorbiae. However, the potato aphid showed high fecundity and reproduction rates on the potato crop and this shift to new hosts could lead to several significant changes in growth and development and future generations according to the feeding habits. Interestingly, though the reproduction rate was low, the other survival parameters such as age-stage survival rate (Sxj), survival rate l(x), and life expectancy (Exj) were higher in M. euphorbiaeas compared to R. padi. Hence, we concluded that the changing climatic conditions may have a great effect on aphid species occurrence in wheat crop, their host adoption, and growth and reproduction.
References
Aggarwal PK, Bandyopadhyay SK, Pathak H, Kalra N, Chander S, Kumar S (2000) Analysis of yield trends of the rice-wheat system in north-western India. Outlook Agric 29(4):259–268
Alawi SH, Suroshe SS, Chander S, Nebapure SM, Poddar N (2021) Spatial distribution and sequential sampling of aphid and their natural enemies on wheat. Indian J Exp Biol 59(4):246–253
Auad AM, Alves SO, Carvalho CA, Silva DM, Resende TT, Veríssimo BA (2009) The impact of temperature on biological aspects and life table of Rhopalosiphum padi (Hemiptera: Aphididae) fed with signal grass. Florida Entomol 92(4):569–577
Barlow CA (1962) Development, Survival, and Fecundity of the Potato Aphid, Macrosiphum euphorbiae (Thomas), at Constant Temperatures1. Can Entomol 94(6):667–671
Beetge L, Krüger K (2019) Drought and heat waves associated with climate change affect performance of the potato aphid Macrosiphum euphorbiae. Sci Rep 9(1):1–9
Blackman RL, Eastop VF (2006) Aphids on the world’s herbaceous plants and shrubs. Vol 8(2). Natural History Museum, London, pp 1025–1439
Blackman RL, Eastop VF (2007) Taxonimic issues in Aphids as crop Pest (ed): Helmut F. van Emden and Richard Harrington.
Blackman RL, Eastop VF (1984) Aphids on the world’s crops: an identification and information guide. Wiley, Hoboken
Blackman RL, Eastop VF (2000) Aphids on the world’s crops (2nd edn). Wiley, Chichester, p 466
Bukvayova N, Henselova M, Vajcikova V, Kormanova T (2006) Occurrence of dwarf virus of winter wheat and barley in several regions of Slovakia during the growing seasons 2001–2004. Plant Soil Environ 52(9):392–401
Chan CK, Forbes AR, Raworth DA (1991) Aphid-transmitted viruses and their vectors of the world. Agric Can Tech Bull 3E:1–216
Chang C, Huang CY, Dai SM, Atlihan R, Chi H (2016) Genetically engineered ricin suppresses Bactrocera dorsalis (Diptera: Tephritidae) based on demographic analysis of group-reared life table. J Econ Entomol 109(3):987–992
Chi H (1988) Life-table analysis incorporating both sexes and variable development rates among individuals. Environ Entomol 17(1):26–34
Commodity Intelligence Report, Foreign Agricultural Service, USDA (2021).
Conti BFD, Bueno VHP, Sampaio MV, Sidney LA (2010) Reproduction and fertility life table of three aphid species (Macrosiphini) at different temperatures. Rev Bras Entomol 54:654–660
Cooper WR, Goggin FL (2005) Effects of jasmonate-induced defenses in tomato on the potato aphid Macrosiphum euphorbiae. Entomol Exp Appl 115(1):107–115
Dhadwal R, Sharma PK, Vashisth S, Kumar S, Verma KS (2014) Insect pest complex of wheat (Triticum aestivum L.) in Himachal Pradesh. J Entomol Res 38(2):147–152
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman & Hall, New York
Finlayson CJ, Alyokhin AV, Porter EW (2009) Interactions of native and non-native lady beetle species (Coleoptera: Coccinellidae) with aphid-tending ants in laboratory arenas. Environ Entomol 38(3):846–855
Harrington R, Fleming RA, Woiwod IP (2001) Climate change impacts on insect management and conservation in temperate regions: can they be predicted? Agric Entomol 3:233–240
Hatfield JL, Boote KJ, Kimball BA, Ziska LH, Izaurralde RC, Ort DR, Thomson AM, Wolfe D (2011) Climate impacts on agriculture: implications for crop production. Agron J 103:351–370
Holman J (2009) Host plant catalog of aphids: Palaearctic region, vol 1216. Springer, Berlin
Huang YB, Chi H (2012) Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera:Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci 19:263–273
Igrejas G, Branlard G (2020) The importance of wheat. In: Igrejas G, Ikeda T, Guzmán C (eds) Wheat quality for improving processing and human health. Springer, Cham. https://doi.org/10.1007/978-3-030-34163-3_1
Jeon SW, Kim KH, Lee SG, Lee YH, Park SK, Kang WS, Park B, Kim KH (2019) Temperature-dependent developmental models and fertility life table of the potato aphid Macrosiphum euphorbiae Thomas on eggplant. Korean J Environ Biol 37(4):568–578
Le Guigo PA, Rolier J, Corff Le (2012) Plant neighborhood influences colonization of Brassicaceae by specialist and generalist aphids. Oecologia 169(3):753–761
Liu XL, Yang XF, Wang CY, Wang YJ, Zhang H, Ji WQ (2012) Molecular mapping of resistance gene to English grain aphid (Sitobion avenae F.) in Triticum durum wheat line C273. Theor Appl Genet 124:287–293
MacGillivray ME, Anderson GB (1958) Development of four species of aphids (Homoptera) on potato1. Can Entomol 90(3):148–155
Millar IM (1990) The aphids (Homoptera: Aphidoidea) of South Africa. Entomology Memoir-Republic of South Africa, Department of Agricultural Development (78).
Moeller FW (1973) The host plants of the potato aphid Macrosiphum euphorbiae and of closely related species. Wiss Z Univ Rostock Math Naturwiss Reihe 22:1179–1184
Oerke EC, Dehne HW, Schönbeck F, Weber A (1996) Crop production and crop protection: estimated losses in major food and cash crops. Agric Syst 51:493–495
Park CG, Choi BR, Cho JR, Kim JH, Ahn JJ (2017) Thermal effects on the development, fecundity and life table parameters of Rhopalosiphum padi (Linnaeus)(Hemiptera: Aphididae) on barley. J Asia-Pac Entomol 20(3):767–775
Patch EM (1925) Potato aphids. Univ., Maine Agricultural Experiment Station, vol 323
Rabasse JM, Steenis MJV (1999) Biological control of aphids. Integrated pest and disease management in greenhouse crops. Springer, Dordrecht, pp 235–243
Raboudi F, Makni H, Makni M (2011) Genetic diversity of potato aphid, Macrosiphum euphorbiae, populations in Tunisia detected by RAPD. Afr Entomol 19(1):133–140
Radcliffe EB, Ragsdale DW (2002) Aphid-transmitted potato vi-ruses: the Importance of understanding vector biology. Am J Potato Res 79:353–386
Ribeiro ADO, Pereira EJG, Galvan TL, Picanco MC, Picoli EDT, Da Silva DJH, FariMGOtoni WC (2006) Effect of eggplant transformed with Oryza cystatin gene on Myzus persicae and Macrosiphum euphorbiae. J Appl Entomol 130(2):84–90
Rossing WAH, Daamen RA, Jansen MJW (1994) Uncertainty analysis applied to supervised control of aphids and brown rust in winter wheat. Part 11. Relative importance of different components of uncertainty. Agric Syst 44:449–460
Sengonca C, Uygun N, Ulusoy MR, Kersting U (1994) Laboratory studies on biology and ecology of Eretmocerus debachi Rose and Rosen (Hym., Aphelinidae) the parasitoid of Parabemisia myricae (Kuwana)(Hom., Aleyrodidae). J Appl Entomol 118(4):407–412
Singh RP, Boiteau G (1986) Reevaluation of the potato aphid, Macrosiphum euphorbiae (Thomas), as vector of potato virus Y. Am Potato J 63:335–340
Singh G, Singh R (2017) Updated checklist of host plants of Calaphidinae (Aphididae: Hemiptera) in India. Int J Contemp Res Rev 8(2):20171–20190
Smith LB (1919) The life history and biology of the pink and green aphid (Macrosiphum euphorbiae Ashmead). Virginia Truck Expt. Sta. Bull27.
Starý P, Gerding IM, Norambuena IH, Remaudiere G (1993) Environmental research on aphid parasitoid biocontrol agents in Chile (Hym., Aphidiidae; Hom., Aphidoidea). J Appl Entomol 115(1–5):292–306
Tuan SJ, Lee CC, Chi H (2014) Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag Sci 70(5):805–813
Valenzuela I, Carver M, Malipatil MB, Ridland PM (2009) Occurrence of Macrosiphum hellebori Theobald & Walton (Hemiptera: Aphididae) in Australia. Aust J Entomol 48(2):25–129
Wains MS, Ali MA, Waris IH, Anwar J, Zulkiffal M, Sabir W, Rehman A, Hussain M, Miraj K (2010) Rigorous, a homeopathic medicine, effective for controlling aphids in bread wheat. J Anim Plant Sci 20:248–252
Zalom FG (2007) UC IPM Pest Management Guidelines--tomato. University of California, ANR/Communication Services 3470
Acknowledgements
The authors are highly grateful to Director, ICAR-IARI, New Delhi, for providing financial support for this study, and also the authors would like to thank Prof. Hsin Chi from National Chung Hsing University in Taiwan for providing the “age-stage, two-sex life table programme” software.
Funding
The funding was provided by Indian Agricultural Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Mike Taylor.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Venkatesh, Y.N., Rajna, S., Suroshe, S.S. et al. Wheat as a new host for potato aphid Macrosiphum euphorbiae Thomas (Hemiptera: Aphididae) and construction of its age-stage two-sex life tables. CEREAL RESEARCH COMMUNICATIONS 52, 177–187 (2024). https://doi.org/10.1007/s42976-023-00370-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-023-00370-w