Abstract
The highly reversible insertion/extraction of large-radius K+ into electrode materials remains a tough goal, especially for conversion-type materials. Herein, we design a current collector-integrated electrode (N–CoSe/CoSe2–C@Cu) as an advanced anode for potassium-ion battery (PIBs). The conductive CoSe/CoSe2 heterojunction with rich Se vacancy defects, conductive sp2 N-doped carbon layer, and the elastic copper foil matrix can greatly accelerate the electron transfer and enhance the structural stability. Consequently, the well-designed N–CoSe/CoSe2–C@Cu current collector-integrated electrode displays enhanced potassium storage performance with regard to a high capacity (325.1 mAh·g−1 at 0.1 A·g−1 after 200 cycles), an exceptional rate capability (223.5 mAh·g−1 at 2000 mA·g−1), and an extraordinary long-term cycle stability (a capacity fading of only 0.019% per cycle over 1200 cycles at 2000 mA·g−1). Impressively, ex situ scanning electron microscopy (SEM) characterizations prove that the elastic structure of copper foil is merged into the cleverly designed N–CoSe/CoSe2–C@Cu heterostructure, which buffers the deformation of structure and volume and greatly promotes the cycle life during the potassium/depotassium process.
Graphical abstract

摘要
大半径K+ 在电极材料中高度可逆地嵌入/脱出仍然是一个艰巨的目标,特别是对于转换型材料。在此,我们设计了一种电流集集成电极N-CoSe/CoSe2-C@Cu作为钾离子电池的先进负极。具有丰富Se空位缺陷的导电CoSe/CoSe2异质结、导电sp2-N掺杂碳层和弹性铜箔基体可以极大地促进电子转移,提高结构稳定性。因此,精心设计的N-CoSe/CoSe2-C@Cu电流集集成电极实现了增强的储钾性能,表现为高比容量(在0.1A·g-1下循环200 圈后容量为325.1mAh·g-1)、优异的倍率性能(在2000mA·g-1下容量为223.5mAh·g-1)和卓越的长循环稳定性(在2000mA·g-1下循环1200次圈,每圈的容量衰减仅0.019%)。令人印象深刻的,非原位扫描电子显微镜(SEM)表征证明了铜箔的弹性结构融合巧妙设计的N-CoSe/CoSe2-C@Cu异质结构,协同缓冲了结构和体积的形变,极大地提高了嵌钾/脱钾过程的循环寿命。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is well-known that the deficiency and unequal distribution of lithium ore hinder the further widespread application of lithium-ion batteries (LIBs) in the next-generation energy storage systems, which has been confirmed by the sharp increase in the price of lithium salt in recent years [1,2,3,4,5]. In addition, the safety of LIBs is also a major problem caused by lithium dendrites [6]. Therefore, it is crucial to develop new, low-cost, and extremely secure energy storage technologies to take the position of LIBs. Potassium-ion batteries (PIBs) have received a lot of attention due to the significant amounts of potassium in the Earth's crust (1.5 wt%) and their low redox potential (K+/K, – 2.93 V versus SHE) [7, 8]. Nevertheless, the absence of appropriate anode materials has severely hindered the development of PIBs resulting in a grand challenge that the anode materials are designed to be high-performance with stable construction for enhancing cycle life and high-rate capability [9].
Recently, anode electrode materials for PIBs have frequently used carbon-based compounds [10,11,12,13]. Nevertheless, the 200–400 mAh·g−1 capacity is somewhat low, and the rate capability is much worse than that in LIBs [13,14,15,16]. Similarly, transition metal selenides (TMSDs) have received a lot of attention as potential PIBs anode materials because of their high specific theoretical capacity. The large volume expansion and poor electronic conductivity, however, are two significant challenges to overcome [17, 18]. Therefore, in order to further boost the K+ storage property, it is imperative to look for suitable anode materials.
Many researchers have suggested various methods, such as creating special nanostructured materials and integrating them with conductive carbon substrate, to increase the performance of transition metal selenides in storing potassium [19, 20]. Metal selenides differ from their sulfide counterparts in that they have improved metallic properties, a higher covalency level in the metal–anion bonding, and bigger lattice parameters since Se has a higher atomic number [21]. On the one hand, the possibility of CoSe as the anode electrode for PIBs has been investigated due to the remarkable metallic properties of Co [22]. But on the other hand, novel variable valence-alloying anodes materials received wide attention in recent years. These anodes are able to demonstrate lower redox voltages as a result of the alloying process, in addition to delivering increased specific capacity owing to the contribution of cationic metal conversion and metal alloying reactions [23,24,25].
However, the volume change is severe in the process of potassiation/depotassiation, resulting in poor structural stability and fast capacity decay, which seriously hinders its practical application. An effective method is to optimize CoSe materials into nanostructures for the purpose of overcoming these problems. By shrinking the CoSe material to the nanoscale, the volume change can be successfully mitigated [24]. Besides, building CoSe composites that are combined with conductive substrates like graphene, amorphous carbon, or carbon nanotubes can efficiently enhance the electronic conductivity and mitigate the structure change [26,27,28,29]. The carbon materials not only provide a highly conductive pathway for rapid electronic transfer but also have a structural buffer effect to accommodate the inner strain caused by a change in volume of CoSe materials during potassiation/depotassiation process.
The two most popular approaches to solving the aforementioned issues are carbon coating and nanostructural building, both of which have been extensively researched [30,31,32]. Furthermore, adding heteroatoms to carbon is a useful strategy for creating a strong binding between the active material and the carbon substrate. N is an ideal choice for this since sp3 and sp2 N, which have lone pair of electrons, bond well with metals that have vacant orbits [33]. On the other hand, N-doping has the advantages of simple experiment, easy availability of raw materials, and low cost. More precisely, precursors rich in nitrogen, such as melamine, MOF, and dopamine, can be carbonized to introduce N atoms [34]. The two hybrid models of N typically coexist in a carbon substrate. sp2 N, also known as pyridine-N and pyrrole-N, distributes at the vacancies and edges with a lone pair of electrons on nitrogen, meaning that sp2 N typically has higher electrochemical activity. The delocalized bond is formed by the electrons of sp3 N, sometimes referred to as graphene-N, which is present in the graphene plane [35, 36]. More critically, sp2 N has excellent alkali ion adsorption capabilities that improve electrochemical characteristics, and the lone pair electrons are essential for creating variable valence selenides [29].
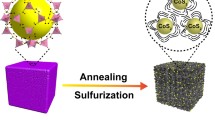
Herein, a novel heterostructure is proposed via anchoring N-doped CoSe/CoSe2–C microsphere onto copper foil for enhanced potassium storage. N-doped carbon etches defects on the surface and perforates hollow structure on the interior of the microsphere. It is worth noting that the method of selenization of heating can not only change the macrostructure but also punch holes and leave zigzag void space among particles. As a result, an N-doped, multistage pore structure, and varivalent cobaltous selenides nanospheres heterostructure (N–CoSe/CoSe2–C@Cu) is synthesized. The reversible multistep evolutions of intercalation/deintercalation and conversion processes in different Co valence states are carried out by CoSe/CoSe2 in this heterostructure. In short, the optimized N–CoSe/CoSe2–C@Cu current collector-integrated electrode realizes a stable capacity of 325.1 mAh·g−1 at 100 mA·g−1. Additionally, the cycle stability is prominent, with over 1200 cycles at 2000 mA·g−1, exceeding monovalent selenides for PIBs in terms of gravimetric capacity.
2 Experimental
2.1 Materials
Cobaltous nitrate hexahydrate (Co(NO3)2·6H2O, 99%), ammonium fluoride (NH4F, ≥ 99.99%), urea (CH4N2O, ≥ 99.5%), and ethanol (C2H5OH, 99.5%) were purchased from Aladdin. Dopamine hydrochloride (C8H12ClNO2, 98%) and selenium powder (Se, ≥ 97%) were purchased from Sigma-Aldrich. All reagents were used as received without further purification. Deionized (DI) water and ethanol were used throughout all the experiments.
2.2 Preparation of N–Co3O4–C@Cu needle-like microspheres
A straightforward one-step solvothermal technique was used to create the Co3O4 nanowire microspheres. Typically, 30 ml of deionized water was stirred for 30 min to dissolve 1.5 mmol of cobaltous nitrate hexahydrate (Co(NO3)2·6H2O), 3.0 mmol of ammonium fluoride (NH4F), and 7.5 mmol of urea (CO(NH2)2). To produce Co3O4 nanowire development, a 3 cm × 6 cm rectangle of copper foil was submerged in the aforementioned mixed solution and moved to a 50-ml Teflon-lined stainless steel autoclave for 5 h at 110 °C. Following the completion of the reaction, the product was collected, cleaned three times with distilled water or alcohol, and then dried for 12 h at 60 °C in a vacuum oven; 85 mg of the gathered Co3O4 nanowire microspheres was ultrasonically mixed for one hour into 50 ml of boiling deionized water (pH was adjusted to 8.5). The solution was then supplemented with 25 mg of dopamine hydrochloride and rapidly agitated for 4 h. The thick, dark-brown precipitate was centrifuged, completely rinsed with boiling deionized water and ethanol, and then dried overnight at 60 °C under vacuum. The aforementioned samples were put in the middle of the tube furnace, and N2 was continuously pumped at a flow rate of 20 sccm during the annealing process. The tube furnace was heated to 350 °C at a rate of 3 °C·min−1, and it remained at that temperature for 2 h. It was only after cooling to room temperature that the Co3O4 nanowire microspheres could be produced.
2.3 Preparation of N–CoSe/CoSe2–C@Cu necklace-like microspheres
The N–CoSe/CoSe2–C@Cu particle nanowire microspheres were prepared via a simple one-step solvothermal method. Typically, 1.5 mmol of cobaltous nitrate hexahydrate (Co(NO3)2·6H2O), 3.0 mmol of ammonium fluoride (NH4F), and 7.5 mmol of urea (CO(NH2)2) were dissolved in 30 ml of deionized water to form a homogeneous solution after stirring for 30 min. A 3 cm × 6 cm sized rectangle of copper foil was immersed in the above mixed solution and transported to a 50-ml Teflon-lined stainless steel autoclave, where they were kept at 110 °C for 5 h to achieve Co3O4 nanowire growth. Following the completion of the reaction, the product was collected, cleaned three times with distilled water or alcohol, and then dried for 12 h at 60 °C in a vacuum oven. After being sonicated for 1 h to disperse 85 mg of collected Co3O4 nanowire microspheres into 50 ml of boiling deionized water (pH was adjusted to 8.5), 25 mg of dopamine hydrochloride was added, and the mixture was vigorously agitated for 4 h. The thick, dark-brown precipitate was centrifuged, completely rinsed with boiling deionized water and ethanol, and then dried overnight at 60 °C under vacuum.
The aforementioned samples were put in the middle of the tube furnace, and N2 was continuously pumped at a flow rate of 20 sccm during the annealing process. The tube furnace was heated to 350 °C at a rate of 3 °C·min−1, and it remained at that temperature for 2 h. Spread 60 mg of the collected sample uniformly in the center of the tube furnace. Place 480 mg (1:8) of selenium powder upstream of the vent, and maintain a continuous flow of Ar/H2 at a rate of 20 sccm during the annealing process. The tube furnace was heated to 300 °C at a rate of 3 °C·min−1, and it was maintained at this temperature for 6 h.
The CoSe/CoSe2@C particle nanowire microspheres were ultimately obtained after cooling to room temperature.
2.4 Material characterizations
Using Cu Kα radiation (λ = 0.15406 nm), the samples' crystalline structures were obtained using an X-ray diffractometer (XRD, Rigkau MiniFlex Bruker D). T20 transmission electron microscopy (TEM, Tecnai T20) and scanning electron microscopy (SEM, SU8000) were used to assess the morphology of the materials. The Raman study was conducted using a modified LabRAM HR800 confocal Raman microscope. An analysis of the composite's elemental composition was conducted using X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB Xi +). At 77 K, nitrogen sorption was used to measure the Brunauer–Emmett–Teller (BET) using ASAP 2010. The analysis was conducted utilizing Brunauer–Emmett–Teller (BET) theory. Using the TG/DTA 6300 equipment, the thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC, TG/DTA 6300) were calculated at a heating rate of 3 °C·min−1.
2.5 Electrochemical measurements
Using a LAND CT 2001A analyzer, the electrochemical tests for potassium-ion half batteries were carried out at various current densities in the voltage range of 0.01–2.6 V versus K+/K. Under the protection of an Ar glove box (H2O and O2 < 0.01 × 10–6, Mbraun, Unilab), CR2032-type coin cells with a current collector-integrated electrode were put together. The counter electrode was made of potassium metal. The LAND CT2001A battery-testing equipment was used to conduct galvanostatic charge/discharge tests. All samples were cut into circular pieces with a mass loading of ca. 1.4 mg·cm−2.
Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were carried out on an electrochemical workstation (IVIUM Technology, nSTAT). The specific capacity of samples is calculated using the active substance mass of the N–CoSe/CoSe2–C at room temperature.
3 Results and discussion
The synthesis procedure for N–CoSe/CoSe2–C@Cu is schematically illustrated in Fig. 1. First, the Co3O4@Cu precursor was prepared via a simple one-step hydrothermal reaction of cobaltous nitrate hexahydrate (Co(NO3)2·6H2O), urea (CO(NH2)2), and ammonium fluoride (NH4F) in the presence of copper foil. Furthermore, the CoSe/CoSe2@Cu was synthesized by selenylation of the Co3O4@Cu precursor using selenium (Se) powder as a Se source in N2 flow at 350 °C. Then, after carbon coating with dopamine, the N–CoSe/CoSe2–C@Cu precursor was obtained. Finally, the tube furnace was heated to 300 °C under Ar/H2 atmosphere, leading to the formation of hierarchical pore structure N–CoSe/CoSe2–C@Cu heterojunction. As shown in the digital photograph (Fig. S1), the current collector-integrated electrode of N–CoSe/CoSe2–C@Cu was prepared by one-step hydrothermal process and selenylation in tube furnace.
TEM and SEM were used to analyze the materials' intricate morphologies and microstructures. The large-sized scale morphology displays a tight layer structure that the N–CoSe/CoS2 –C is closely anchored onto the surface of copper foil (Fig. 2a). The N–CoSe/CoSe2–C necklace-like nanowires are uniformly self-polymerized into microspheres, leading to the formation of a loose network structure under the action of N-doped carbon on the surface (Fig. 2b, c). As shown in (Fig. S2a, S2b), the morphology is modified into a hierarchical structure by the N-doped carbon. In addition, the elemental mapping image of the CoSe/CoSe2@Cu reveals the uniform distribution of Co, Se and Cu elements (Fig. S2a). After N-doping, the N–CoSe/CoSe2–C necklace-like nanowires show obvious branched morphology (Fig. 2d). The lattice fringes of 0.26 and 0.21 nm, indexed to the (101) plane and (102) plane of CoSe, respectively, are visible in the high-resolution TEM (HRTEM) image of N–CoSe/CoSe2–C (Fig. 2e) [37]. Furthermore, the lattice fringes of CuSe2 (110) and CoSe (102) are visible in HRTEM picture of (Fig. 2f), and a tiny lattice mismatch is seen as a result of heterojunction formation [38, 39]. The N–CoSe–C composite's nanocrystals are made up primarily of cubic CoSe and minor orthorhombic CoSe2 crystalline phases, according to the SAED patterns (Fig. 2g). Furthermore, the homogeneous distribution of Co, Se, and C elements within the heterostructure is further demonstrated by the elemental mapping study of the N–CoSe/CoSe2–C (Fig. 2h, i). On the one hand, the Co3O4@Cu electrode exhibits uniform needle-like nanowire array on the other hand, needle-like microsphere by self-assembly after cobalt excess (Fig. S3a, S3b). The elemental mapping image (Fig. S3c) of Co3O4@Cu reveals the distribution of Co, O and Cu elements. The atomic ratio of Co, O and Cu in the Co3O4@Cu was determined by the energy-dispersive spectrometer (Table S1) [38, 39]. Notably, the weight of N–Co3O4–C@Cu and N–CoSe/CoSe2–C@Cu sharply decreases by 19 and 16% below 250 °C, which is due to the oxidation of surface carbon and the loss of a tiny amount of water. Therefore, the mass loading of active materials in N–Co3O4–C@Cu and N–CoSe/CoSe2–C@Cu can be calculated to be 81% (1%–19%) and 84% (1%–16%) (Fig. S4) [40, 41].
Using XRD and BET, the materials' crystalline structures, pore size distribution, phase components, and elemental characteristics were further investigated. The diffraction peaks of N–CoSe/CoSe2–C@Cu can be seen in the XRD pattern, which are situated at 36.8°, 43.6°, 50.7°, 90.1° and 31.0° and can be indexed to the (101), (102), (110) and (212) planes of CoSe and (110) planes of CoSe2, respectively (PDF No. 15–0464) (Fig. 3a). Besides, the peak at 74.3° can be indexed to the copper foil. Before selenization, the new peaks at 36.5° and 44.2° belong to the characteristic peak of N–Co3O4–C@Cu, corresponding to the (311) and (400) planes of Co3O4, respectively (Fig. 3b) [42]. In addition, three peaks at 75.9° corresponding to the characteristic reflections of Cu/CuOx are also observed. The specific surface areas of Co3O4, N–Co3O4–C@Cu, CoSe/CoSe2, and N–CoSe/CoSe2–C@Cu are calculated to be 48.2, 53.1, 66.5 and 124.4 m2·g−1, respectively (Fig. 3c). The hierarchical pore structure with coexisting micropores and mesoporous material is evident in the pore size distribution of N–CoSe/CoSe2–C@Cu, as determined by the BET test of the as-prepared powders (Fig. S5). After selenization using a high-temperature method, the robust chemical interaction, an abundance of Se vacancy defects, and large specific surface area for N–CoSe/CoSe2–C can increase structural stability, speed up electron transfer, supply an abundance of active sites, shorten the ion diffusion channel, and promote the electrolyte infiltration [43, 44].
The surface electronic state of the N–CoSe/CoSe2–C and the presence of the components Co, Se, C and N were examined using XPS. The Co–Se bonds are responsible for the two split peaks in the high-resolution Co 2p spectra of N–CoSe/CoSe2–C@Cu, which are located at 794.5 and 779.6 eV and correspond to Co 2p1/2 and Co 2p3/2, respectively (Fig. 3d) [20]. As a contrast, for the high-resolution Co 2p XPS spectrum of N–Co3O4–C@Cu, 793.9 and 778.5 eV correspond to Co2+/Co3+. Co vacancies will produce more Co2+/Co3+ induced by O vacancies, and therefore, the appearance of the peaks due to Co2+/Co3+ is led by the oxidation effect [45]. For the high-resolution Se 3d spectra of N–CoSe/CoSe2–C@Cu, the peaks located at 54.8 eV can be assigned to the Co–Se bond, and the peak corresponding to binding energies of 60.3 eV can be ascribed to the SeOx peak (Fig. S6a). For the spectrum of N–CoSe/CoSe2–C@Cu high-resolution C 1s (Fig. 3e), three distinct peaks are observed at 284.8, 285.5 and 288.7 eV, which are attributed to C–C/C=N bond, C–O/C=O bond and O–C=O bon
d, respectively [46]. Notably, the high-resolution N 1s spectra of N–CoSe/CoSe2–C@Cu show three peaks located at 398.4 eV (pyridinic-N), 400.8 eV (graphitic-N), and 405.4 eV (oxidized-N) (Fig. 3f) [47, 48]. These peaks further suggest that CoSe/CoSe2 has efficient N-doping on its surface. The chemical compositions and forms of the samples are shown in Fig. S6b, and the Co/Se ratio in N–CoSe/CoSe2–C@Cu is fairly similar to the EDS results. Raman spectroscopy was employed to further investigate the N–Co3O4–C@Cu and N–CoSe/CoSe2–C@Cu current collector-integrated electrode. There are three obvious peaks at 478, 523 and 687 cm−1 that correspond to the Eg, F2g and A1g modes in Co phase, respectively. Additionally, a new CuOx peak at 481 cm−1 was discovered, and the peak at 677 cm−1 corresponds to A1g modes of Co3O4. Similarly, in the two composites of N–Co3O4–C@Cu and N–CoSe/CoSe2–C@Cu, the typical peaks of carbon in Raman spectra were 1525 and 1463 cm−1, corresponding to G and D bands of the carbon backbone, respectively (Fig. S7). To sum up, the N–CoSe/CoSe2–C@Cu current collector-integrated electrode was successfully synthesized, featuring the heterostructure of CoSe/CoSe2 and hierarchical pore structure.
To clarify the merits of heterojunction, the N–CoSe/CoSe2–C@Cu was evaluated as the current collector-integrated anode for PIBs. Figure 4a shows the CV curves of N–CoSe/CoSe2–C@Cu in the voltage range of 0.01–2.6 V with a scan rate of 0.1 mV·s−1 in the first, second, and fifth cycles. During the initial negative sweep of CV curves for N–CoSe/CoSe2–C@Cu, two reduction peaks at 1.2 and 0.4 V are observed, which can be assigned to the multistep conversion reaction of CoSe/CoSe2 to KxSe and Co, indicating that K+ was inserted into the pore structure (Fig. 3a) [49, 50]. In the successive positive sweep, a clear peak at 1.96 V and a broad peak from 1.0 to 1.4 V are attributed to the reversed conversion reactions and the continuous formation of solid electrolyte interphase (SEI) film. The cathodic peak at 0.4 V in the first negative sweep is observed and then disappears in the following scans, mainly ascribed to the formation of a stable SEI [51, 52]. The CV curves practically overlap quite well in the subsequent scans, indicating the highly reversible kinetics in the progress of charge–discharge behavior [53, 54]. For comparison, Fig. S8 shows the cyclic voltammetry curve of N–Co3O4–C@Cu and illustrates the Co3O4 conversion reaction mode of alloying. Furthermore, Fig. 4b presents the galvanostatic charge/discharge curves of the N–CoSe/CoSe2–C@Cu current collector-integrated electrode at a current density of 100 mA·g−1. The discharge capacities of N–CoSe/CoSe2–C@Cu are 547.2, 318.5, 299.4, 286.6 and 272.5 mAh.g−1 at the 1st, 2nd, 10th, 50th and 200th cycle, respectively.
a CV curves of N–CoSe/CoSe2–C@Cu at a scan rate of 0.1 mV·s−1; b discharge–charge profiles of N–CoSe/CoSe2–C@Cu at 0.1 A·g−1 at different cycles; c EIS of N–CoSe/CoSe2–C@Cu, CoSe/CoSe2@Cu, and N–Co3O4–C@Cu and inset equivalent circuit; d cycling performance of N–CoSe/CoSe2–C@Cu and N–Co3O4–C@Cu and (inset) SEM image of N–CoSe/CoSe2–C@Cu; e cycling performance of N–CoSe/CoSe2–C@Cu and CoSe/CoSe2@Cu; and f rate capability of N–CoSe/CoSe2–C@Cu and CoSe/CoSe2@Cu and inset SEM image of CoSe/CoSe2@Cu
EIS test is carried out in the frequency range of 100 kHz–0.01 Hz. The data were subsequently fitted using an equivalent circuit model (Fig. 4c). Each Nyquist plot shows the charge transfer resistance (Rct) and Warburg impedance (W) as a depressed semicircle in the high-to-medium-frequency areas and a straight line in the low-frequency regions, respectively [55]. As expected, the N–CoSe/CoSe2–C@Cu anode gives a lower Rct of 1156 Ω than those of CoSe/CoSe2@Cu (2133 Ω) and N–Co3O4–C@Cu (2996 Ω), demonstrating the N–CoSe/CoSe2–C@Cu electrode offers the lowest charge transfer impedance bringing about the fastest electron transport.
After 200 cycles, the N–CoSe/CoSe2–C@Cu electrode maintains a higher reversible capacity of 313.7 mAh·g−1 than those of N–Co3O4–C@Cu (Fig. 4d). Meanwhile, the initial morphology of the above-mentioned two materials was also compared (Fig. 4d). In particular, the facilitation of nitrogen-doped carbon on the properties of the material was confirmed, by comparing the cyclic properties of CoSe/CoSe2@Cu and N–CoSe/CoSe2–C@Cu. As shown in Fig. 4e, the cyclic performance of the CoSe/CoSe2@Cu and N–CoSe/CoSe2–C@Cu was evaluated at a current density of 100 mA·g−1. The N–CoSe/CoSe2–C@Cu delivers a high reversible capacity of 325.1 mAh·g−1 after 200 cycles, which obviously exceeds the CoSe/CoSe2@Cu in the field of both capacity and cyclability. It is worth noting that an excessively high or low mass loading would much affect the battery performance. The mass loading of the active material was determined to be ~ 1.4 mg·cm−2 through selecting the cut electrode pieces.
The rate capability of the prepared samples was evaluated under varied current densities ranging from 0.1 to 2 A·g−1 (Fig. 4f). The N–CoSe/CoSe2–C@Cu achieves the reversible capacities of 420.8, 368.3, 347.2, 279.7, 223.5 and 222.5 mAh·g−1 at 0.1, 0.2, 0.5, 1.0 and 2.0 A·g−1, respectively, much superior to those of N–Co3O4–C@Cu anodes. On the contrary, when CoSe/CoSe2@Cu is charged to a high current density, its capacity declines significantly, and it is difficult to recover reversibly. The illustration visually shows the serious agglomeration of CoSe/CoSe2@Cu after cycling, demonstrating the superiority of N-doped carbon which provides structural stability and faster reaction kinetics for N–Co3O4–C@Cu (Fig. 4f).
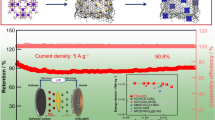
As shown in Fig. 5a, the N–CoSe/CoSe2–C@Cu current collector-integrated anode presents the superiority of long-term cyclability at a high current density of 2000 mA·g−1. Among them, it can be clearly seen that N–Co3O4–C@Cu exhibits poor long-cycle performance. Impressively, the N–CoSe/CoSe2–C@Cu realizes a capacity of 334.6 mAh·g−1 for the first cycle and still maintains a capacity of 175.3 mAh·g−1, finally, after 1200 cycles with only 0.019% capacity fading per cycle. The morphological and structural alterations of the N–Co3O4–C@Cu and N–CoSe/CoSe2–C@Cu electrodes were shown in order to make the structural stability clearer. The ex situ SEM image reveals the changes of structure integrity after long-term cycling, proving the excellent stability of N–CoSe/CoSe2–C@Cu electrodes (Fig. 5b, c). The interior of Co3O4 nanospheres is solid and composed of one-dimensional needle-like nanowires, which causes huge deformation of the external structure during cycling, serious solvation, and significant agglomeration of the structure after 100 cycles (Fig. 5b). In contrast to that, the N–CoSe/CoSe2–C@Cu consisting of multistage necklace-like microsphere has a hollow structure (Fig. 5c). The N–CoSe/CoSe2–C@Cu composite deforms structurally, but no surface agglomeration is observed after 10 cycles, implying that the SEI layer forms frequently, which promotes the change of morphology and structure and leads to the capacity fading of electrode during the initial cycles [56]. It is remarkable that the N–CoSe/CoSe2–C@Cu electrode retains the whole structural framework and loose pore structure after 1200 cycles (Fig. 5c).
a Cycling performance of N–CoSe/CoSe2–C@Cu and N–Co3O4–C@Cu at 2000 mA·g−1 for 1200 cycles; b ex situ SEM images of N–Co3O4–C@Cu before and after 10 and 100 cycles at 2000 mA·g−1 and (inset) corresponding large-size images; and c ex situ SEM images of N–CoSe/CoSe2–C@Cu before and after 10 and 1200 cycles at 2000 mA·g−1 and (inset) corresponding large-size images
The thickness of cycled N–CoSe/CoSe2–C@Cu electrode is only raised from 9.68 to 10.75 μm with a small frame expansion rate of 11% (Fig. S9). Consequently, the data of N–CoSe/CoSe2–C@Cu obtained in the potassiation/depotassiation process identify the remarkable reversibility of the current collector-integrated electrode. An advanced anode for potassium-ion battery (PIBs) was designed in the form of a current collector-integrated electrode, specifically the N–CoSe/CoSe2–C@Cu composite. The conductive CoSe/CoSe2 heterojunction with rich Se vacancy defects, conductive sp2 N-doped carbon layer, and the elastic copper foil matrix can greatly accelerate the electron transfer and enhance the structural stability.
4 Conclusion
To summarize, a hierarchical CoSe/CoSe2 architectures coated with N-doped carbon framework have been synthesized as a current collector-integrated electrode for PIBs. Among them, the copper foil matrix performs multiple actions in this work, including elastic skeleton, restraining agglomeration, and current collector. The well-designed current collector-integrated electrode N–CoSe/CoSe2–C@Cu with rich Se vacancy defects, conductive sp2 N-doped carbon layer, and the elastic copper foil matrix can greatly accelerate the electron transfer and enhance the structural stability. Among these, the copper foil matrix serves as the elastic skeleton and current collector. In the meantime, each CoSe/CoSe2 particle in the hierarchical designs aligns itself as a series of nanowires, creating zigzag empty spaces between the particles that can support volume expansion and enhance structural stability during cycling. As expected, the well-designed current collector-integrated anode delivers a notable K+ storage capacity with extraordinary rate capability (223.5 mAh·g−1 at 2000 mA·g−1) and remarkable long-term cycling stability (a capacity fading of only 0.019% per cycle over 1200 cycles at 2000 mA·g−1). Ex situ characterizations demonstrate that the well-designed N–CoSe/CoSe2–C@Cu undergoes the evolutions of structure before and after long-term cycling with the K+ insertion, proving that the multistep transformation process benefited from the metal conversion reactions of variable valence Co. The designed approaches of the current collector-integrated electrode for PIBs offer a practical strategy for constructing progressive electrode materials with long cycle life for the next-generation rechargeable batteries.
References
Dong YF, Shi HD, Wu ZS. Recent advances and promise of MXene-based nanostructures for high-performance metal ion batteries. Adv Funct Mater. 2020;30(47):2000706. https://doi.org/10.1002/adfm.202000706.
Pomerantseva E, Bonaccorso F, Feng XL, Cui Y, Gogotsi Y. Energy storage: the future enabled by nanomaterials. Science. 2019;366:eaan8285. https://doi.org/10.1126/science.aan8285.
Chen Jiaxing Su, Zilong Zhao Ting, Ganggang Pu, Ang Li, Lve Wang. Performance of cathode material of high-power lithium-ion battery. Chin J Rare Met. 2023;47(12):1756. https://doi.org/10.13373/j.cnki.cjrm.XY21010027.
Wang XT, Yang Y, Guo JZ, Gu ZY, Ang EH, Sun ZH, Li WH, Liang HJ, Wu XL. An advanced cathode composite for co-utilization of cations and anions in lithium batteries. J Mater Sci Technol. 2022;102:72. https://doi.org/10.1016/j.jmst.2021.05.074.
He J, Wang HP, Zhou Q, Qi SH, Wu MG, Li F, Hu W, Ma JM. Unveiling the role of Li+ solvation structures with commercial carbonates in the formation of solid electrolyte interphase for lithium metal batteries. Small Methods. 2021;5(8):2100441. https://doi.org/10.1002/smtd.202100441.
Wang D, Zhang W, Zheng W, Cui X, Rojo T, Zhang Q. Towards high-safe lithium metal anodes: suppressing lithium dendrites via tuning surface energy. Adv Sci. 2017;4(1):1600168. https://doi.org/10.1002/advs.201600168.
Rajagopalan R, Tang Y, Ji X, Jia C, Wang H. Advancements and challenges in potassium ion batteries: a comprehensive review. Adv Funct Mater. 2020;30(12):1909486. https://doi.org/10.1002/adfm.201909486.
Xu X, Zhang Y, Sun H, Zhou J, Liu Z, Qiu Z, Wang D, Yang C, Zeng Peng Z. Orthorhombic cobalt ditelluride with Te vacancy defects anchoring on elastic MXene enables efficient potassium-ion storage. Adv Funct Mater. 2021;33(31):2100272. https://doi.org/10.1002/adma.202100272.
Zhao R, Di H, Hui X, Zhao D, Wang R, Wang C, Yin L. Self-assembled Ti3C2 MXene and N-rich porous carbon hybrids as superior anodes for high-performance potassium-ion batteries. Energy Environ Sci. 2020;13(1):246. https://doi.org/10.1039/C9EE03250A.
Zhong L, Zhang W, Sun S, Zhao L, Jian W, He X, Xing Z, Shi Z, Chen Y, Alshareef H. Engineering of the crystalline lattice of hard carbon anodes toward practical potassium-ion batteries. Adv Funct Mater. 2023;33(8):2211872. https://doi.org/10.1002/adfm.202211872.
Geng S, Zhou T, Jia M, Shen X, Gao P, Tian S, Zhou P, Liu B, Zhou J, Zhuo S, Li F. Carbon-coated WS2 nanosheets supported on carbon nanofibers for high-rate potassium-ion capacitors. Energy Environ Sci. 2021;14(5):3184. https://doi.org/10.1039/D1EE00193K.
Dai J, Jing J, Yang JP, Zhang W, Liu SX, Wang QF, Wen JX, Hu H, Li HF, Liu L. Flexible and free-standing La0.33Ti2(PO4)3/C nanofibers film as a novel high-performance anode for sodium-and potassium-ion batteries. Rare Metals. 2023;42(10):3387. https://doi.org/10.1007/s12598-023-02332-x.
Wu CY, Zhang Y, Xiao WP. Design and synthesis of PtNi/MXene nanocomposites and its electrocatalytic performance for oxygen reduction reaction. Chin J Rare Metals. 2023;47(12):1747–55. https://doi.org/10.13373/j.cnki.cjrm.XY22060042.
Wu YL, Hong JB, Zhong WX, Wang CX, Li ZF, Dmytro S. Auxiliary ball milling to prepare WS2/graphene nanosheets composite for lithium-ion battery anode materials. Tungsten. 2023. https://doi.org/10.1007/s42864-023-00216-2.
Yang M, Kong Q, Feng W, Yao W. N/O double-doped biomass hard carbon material realizes fast and stable potassium ion storage. Carbon. 2021;176:71. https://doi.org/10.1016/j.carbon.2021.01.114.
Yi X, Rao AM, Zhou J, Lu B. Trimming the degrees of freedom via a K+ flux rectifier for safe and long-life potassium-ion batteries. Nano-Micro Lett. 2023;15(1):200. https://doi.org/10.1007/s40820-023-01178-3.
Yu LH, Tao X, Feng SR, Liu JT, Zhang LL, Zhao GZ, Zhu G. Recent development of three-dimension printed graphene oxide and MXene-based energy storage devices. Tungsten. 2022;10:1. https://doi.org/10.1007/s42864-022-00181-2.
Yang H, Xu R, Yao Y, Ye S, Zhou X, Yu Y. Multicore-shell Bi@ N-doped carbon nanospheres for high power density and long cycle life sodium-and potassium-ion anodes. Adv Funct Mater. 2019;29(13):1809195. https://doi.org/10.1002/adfm.201809195.
Ge J, Wang B, Wang J, Zhang Q, Lu B. Nature of FeSe2/N-C anode for high performance potassium ion hybrid capacitor. Adv Energy Mater. 2020;10(4):1903277. https://doi.org/10.1002/aenm.201903277.
Zhang X, Xiong T, He B, Feng S, Wang X, Mai L. Recent advances and perspectives in aqueous potassium-ion batteries. Energy Environ Sci. 2022;15:3750. https://doi.org/10.1039/D2EE01573K.
Huang ZX, Gu ZY, Heng YL, Ang EH, Geng HB, Wu XL. Advanced layered oxide cathodes for sodium/potassium-ion batteries: development, challenges and prospects. Chem Eng J. 2023;452:139438. https://doi.org/10.1016/j.cej.2022.139438.
Dong S, Yu D, Yang J, Jiang L, Wang J, Cheng L, Zhou Y, Yue H, Wang H, Guo L. Tellurium: a high-volumetric-capacity potassium-ion battery electrode material. Adv Mater. 2020;32(23):1908027. https://doi.org/10.1002/adma.201908027.
Yu Q, Jiang B, Hu J, Lao CY, Gao Y, Li P, Liu Z, Suo G, He D, Wang W. Metallic octahedral CoSe2 threaded by N-doped carbon nanotubes: a flexible framework for high-performance potassium-ion batteries. Adv Sci. 2018;5(10):1800782. https://doi.org/10.1002/advs.201800782.
Ma X, Fu H, Shen J, Zhang D, Zhou J, Tong C, M RA, Zhou J, Fan L, Lu B. Green ether electrolytes for sustainable high-voltage potassium ion batteries. Angew Chem Int Ed Engl. 2023;17:e202312973. https://doi.org/10.1002/anie.202312973.
Yang H, Wu Y, Hu LY, Wang JJ, Wang F, Xu XH. Controlled synthesis of GeSe2 and GeSe nanostructures induced by TBAB. Rare Met. 2023;42(1):189. https://doi.org/10.1007/s12598-022-02148-1.
Pan Y, Cheng X, Gao M, Fu Y, Feng J, Gong L, Ahmed H, Zhang H, Battaglia V. Cagelike CoSe2@ N-doped carbon aerogels with pseudocapacitive properties as advanced materials for sodium-ion batteries with excellent rate performance and cyclic stability. ACS Appl Mater Interfaces. 2020;12(30):33621. https://doi.org/10.1021/acsami.0c06296.
Wu YL, Hong JB, Zhong WX, Wang CX, Li ZF, Dmytro S. Auxiliary ball milling to prepare WS2/graphene nanosheets composite for lithium-ion battery anode materials. Tungsten. 2023;27:1. https://doi.org/10.1007/s42864-023-00216-2.
Liu M, Pasanen H, Ali-Löytty H, Hiltunen A, Lahtonen K, Qudsia S, Smått JH, Valden M, Tkachenko NV, Vivo P. B-site Co-alloying with germanium improves the efficiency and stability of all-inorganic tin-based perovskite nanocrystal solar cells. Angew Chem Int Ed. 2020;59(49):22117. https://doi.org/10.1002/anie.202008724.
Liu Y, Deng Q, Li Y, Li Y, Zhong W, Hu J, Ji X, Yang C, Lin Z, Huang K. CoSe@ N-doped carbon nanotubes as a potassium-ion battery anode with high initial coulombic efficiency and superior capacity retention. ACS Nano. 2021;15(1):1121. https://doi.org/10.1021/acsnano.0c08094.
Chen YF, Zheng YT, Zhang FY, Liu ZG, Zhang LY, Yang L, Sun XD, Deng Y, Wang Y. High-temperature polymer-based nanocomposites for high energy storage performance with robust cycling stability. Rare Met. 2023;42(11):3682–91. https://doi.org/10.1007/s12598-023-02312-1.
Shan H, Qin J, Ding Y, Sari HMK, Song X, Liu W, Hao Y, Wang J, Xie C, Zhang J, Li X. Controllable heterojunctions with a semicoherent phase boundary boosting the potassium storage of CoSe2/FeSe2. Adv Mater. 2021;33(37):2102471. https://doi.org/10.1002/adma.202102471.
Gu X, Zhang L, Zhang W, Liu S, Wen S, Mao X, Dai P, Li L, Liu D, Zhao X. A CoSe–C@ C core-shell structure with stable potassium storage performance realized by an effective solid electrolyte interphase layer. J Mater Chem A. 2021;9(18):11397. https://doi.org/10.1039/D1TA01107C.
Ramachandran K, Subburam G, Liu XH, Huang MG, Xu C, Ng DHL, Cui YX, Li GC, Qiu JX, Wang C, Lian JB. Nitrogen-doped porous carbon nanofoams with enhanced electrochemical kinetics for superior sodium-ion capacitor. Rare Met. 2022;41(7):2481–90. https://doi.org/10.1007/s12598-022-01992-5.
Zhu G, Xie X, Li X, Liu Y, Shen X, Xu K, Chen S. Nanocomposites based on CoSe2-decorated FeSe2 nanoparticles supported on reduced graphene oxide as high-performance electrocatalysts toward oxygen evolution reaction. ACS Appl Mater Interfaces. 2018;10(22):19258. https://doi.org/10.1021/acsami.8b04024.
Liang H, Zhang Y, Hao S, Cao L, Li Y, Li Q, Chen D, Wang X, Guo X, Li H. Fast potassium storage in porous CoV2O6 nanosphere@graphene oxide towards high-performance potassium-ion capacitors. Energy Storage Mater. 2021;40:250. https://doi.org/10.1016/j.ensm.2021.05.013.
Chong S, Ma M, Yuan L, Qiao S, Dong S, Liu H, Dou S. Hierarchical encapsulation and rich sp2 N assist Sb2Se3-based conversion-alloying anode for long-life sodium-and potassium-ion storage. Energy Environ Sci. 2023. https://doi.org/10.1002/eem2.12458.
Zhang W, Ming J, Zhao W, Dong X, Hedhili MN, Costa PM, Alshareef H. Graphitic nanocarbon with engineered defects for high-performance potassium-ion battery anodes. Adv Funct Mater. 2019;29(35):1903641. https://doi.org/10.1002/adfm.201903641.
Liang HJ, Gu ZY, Zhao XX, Guo JZ, Yang JL, Li WH, Li B, Liu ZM, Sun ZH, Zhang JP. Advanced flame-retardant electrolyte for highly stabilized K-ion storage in graphite anode. Sci Bull. 2022;67(15):1581. https://doi.org/10.1016/j.scib.2022.07.002.
Zhang W, Sun M, Yin J, Wang W, Huang G, Qiu X, Schwingenschlögl U, Alshareef H. Rational design of carbon anodes by catalytic pyrolysis of graphitic carbon nitride for efficient storage of Na and K mobile ions. Nano Energy. 2021;87:106184. https://doi.org/10.1016/j.nanoen.2021.106184.
Zhang W, Yin J, Sun M, Wang W, Chen C, Altunkaya M, Emwas AH, Han Y, Schwingenschlögl U, Alshareef H. Direct pyrolysis of supermolecules: an ultrahigh edge-nitrogen doping strategy of carbon anodes for potassium-ion batteries. Adv Mater. 2020;32(25):2000732. https://doi.org/10.1002/adma.202000732.
Chu K, Zhang X, Yang Y, Li Z, Wei L, Yao G, Zheng F, Chen Q. Edge-nitrogen enriched carbon nanosheets for potassium-ion battery anodes with an ultrastable cycling stability. Carbon. 2021;184:277. https://doi.org/10.1016/j.carbon.2021.08.015.
Lee JS, Saroha R, Cho JS. Porous microspheres comprising CoSe2 nanorods coated with N-doped graphitic C and polydopamine-derived C as anodes for long-lived Na-ion batteries. Nano-Micro Lett. 2022;14(1):113. https://doi.org/10.1007/s40820-022-00855.
Yang C, Wang M, Chang M, Yuan M, Zhang W, Tan J, Ding B, Ma P, Lin J. heterostructural nanoadjuvant CuSe/CoSe2 for potentiating ferroptosis and photoimmunotherapy through intratumoral blocked lactate efflux. J Am Chem Soc. 2023;145(13):7205. https://doi.org/10.1021/jacs.2c12772.
Li Y, Wang X, Sun M, Xiao J, Zhang B, Ai L, Zhao Z, Qiu J. CoSe Nanoparticle embedded B, N-codoped carbon nanotube array as a dual-functional host for a high-performance Li-S full battery. ACS Nano. 2022;16(10):17008. https://doi.org/10.1021/acsnano.2c07137.
Zhang KY, Gu ZY, Ang EH, Guo JZ, Wang XT, Wang Y, Wu XL. Advanced polyanionic electrode materials for potassium-ion batteries: progresses, challenges and application prospects. J Mater Today. 2022;54:189. https://doi.org/10.1016/j.mattod.2022.02.013.
Zhang R, Pan L, Guo B, Huang ZF, Chen Z, Wang L, Zhang X, Guo Z, Xu W, Loh KP. Tracking the role of defect types in Co3O4 structural evolution and active motifs during oxygen evolution reaction. J Am Chem Soc. 2023;145(4):2271. https://doi.org/10.1021/jacs.2c10515.
Feng J, Luo SH, Lin YC, Zhan Y, Yan SX, Hou PQ, Wang Q, Zhang YH. Metal-organic framework derived CoSe2/N-doped carbon core-shell nanoparticles encapsulated in porous N-doped carbon nanotubes as high-performance anodes for sodium-ion batteries. J Power Sources. 2022;535:231444. https://doi.org/10.1016/j.jpowsour.2022.231444.
Feng J, Luo SH, Yan SX, Zhan Y, Wang Q, Zhang YH, Liu X, Chang LJ. Rational design of yolk-shell ZnCoSe@N-doped dual carbon architectures as long-life and high-rate anodes for half/full Na-ion batteries. Small. 2021;17(46):2101887. https://doi.org/10.1002/smll.202101887.
Hu H, Zhang J, Guan B, Lou XW. Unusual formation of CoSe@ carbon nanoboxes, which have an inhomogeneous shell, for efficient lithium storage. Angew Chem Int Ed. 2016;55(33):9514. https://doi.org/10.1002/anie.201603852.
Ko YN, Choi SH, Kang YC. Hollow cobalt selenide microspheres: synthesis and application as anode materials for Na-ion batteries. ACS Appl Mater Interfaces. 2016;8(10):6449. https://doi.org/10.1021/acsami.5b11963.
Chu J, Zhang C, Wu X, Xing L, Zhang J, Zhang L, Wang H, Wang W, Yu Q. Short-range graphitic nanodomains in hypocrystalline carbon nanotubes realize fast potassium ion migration and multidirection stress release. Small. 2023. https://doi.org/10.1002/smll.202304406.
Sun X, Zeng S, Man R, Wang L, Zhang B, Tian F, Qian Y, Xu L. Yolk–shell structured CoSe2/C nanospheres as multifunctional anode materials for both full/half sodium-ion and full/half potassium-ion batteries. Nanoscale. 2021;13(23):10385. https://doi.org/10.1039/D1NR01227D.
Huang Q, Fan X, Ou X, Wang H, Wu L, Yang C. Fabrication of CoSe@ NC nanocubes for high performance potassium ion batteries. J Colloid Interf Sci. 2021;604:157. https://doi.org/10.1016/j.jcis.2021.06.162.
Yu N, Zou L, Li C, Guo K. In-situ growth of binder-free hierarchical carbon coated CoSe2 as a high performance lithium ion battery anode. Appl Surf Sci. 2019;483:85. https://doi.org/10.1016/j.apsusc.2019.03.258.
Ding Y, Wang W, Bi M, Guo J, Fang Z. CoTe nanorods/rGO composites as a potential anode material for sodium-ion storage. Electrochim Acta. 2019;313:331. https://doi.org/10.1016/j.electacta.2019.05.047.
Wang H, Zhai D, Kang F. Solid electrolyte interphase (SEI) in potassium ion batteries. Energy Environ Sci. 2020;13(12):4583. https://doi.org/10.1039/D0EE01638A.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 52371131), Beijing Nova Program (No. Z211100002121082), the Interdisciplinary Research Project for Young Teachers of University of Science and Technology Beijing (No. FRF-IDRY-21-013), the Project of State Key Laboratory of Explosion Science and Technology (No. QNKT23-05), and Xiaomi Young Scholar Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mu, ZJ., Gao, YJ., Dong, WS. et al. A N–CoSe/CoSe2–C@Cu hierarchical architecture as a current collector-integrated anode for potassium-ion batteries. Rare Met. (2024). https://doi.org/10.1007/s12598-024-02788-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12598-024-02788-5