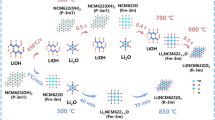
Abstract
Spinel-type cathodes are considered an optimal substitute for conventional layered oxide cathodes owing to their use of inexpensive and earth-abundant manganese as the redox-active element. Moreover, the introduction of cation disorder can effectively suppress the detrimental two-phase reaction to realize high capacities in a wide voltage range. However, the continuous capacity decay during cycles has hindered the widespread application of these cathode materials. Inorganic fluorides exhibit excellent electrochemical stability at high voltage; therefore, in this study, the direct F2 gas reaction with a partially disordered spinel cathode (Li1.6Mn1.6O3.7F0.3, LMOF1.6) was initially applied to investigate the impacts of fluorination on the surface structure and electrochemical performances. The inorganic fluorinated layer, mainly containing LiF, was distributed uniformly on the surface of LMOF1.6 nanoparticles after fluorination for an appropriate time without the turbulence caused by the valency of manganese cation, which improved the capacity retention and rate capability by the suppression of structural damage, parasitic reaction, and cation dissolution. The LMOF1.6 cathode fluorinated for 0.5 h exhibited a capacity of 283.6 mAh·g−1 at 50 mA·g−1 and an enhanced capacity retention of 29.6% after 50 cycles in the voltage range of 1.5–4.8 V, as compared to the pristine LMOF1.6 with only 27.9% capacity retention.
Graphical abstract

摘要
尖晶石型正极被认为是传统层状氧化物正极的最佳替代品, 它们使用廉价且储量丰富的锰作为氧化还原活性元素。此外, 阳离子无序体系的引入可以有效地抑制有害的两相反应, 从而在宽电压范围内实现高容量。然而, 循环过程中的连续容量衰减阻碍了这些正极材料的广泛应用。无机氟化物在高电压下表现出优异的电化学稳定性; 因此, 在本研究中, 应用F2气体与部分无序尖晶石正极 (Li1.6Mn1.6O3.7F0.3, LMOF1.6) 的直接反应来研究氟化对表面结构和电化学性能的影响。氟化适当时间后, 主要含有LiF的无机氟化层均匀分布在LMOF1.6纳米颗粒表面, 没有锰阳离子化合价引起的湍流, 通过抑制结构损伤、寄生反应和阳离子溶解, 提高了容量保持和倍率能力。氟化0.5小时的LMOF1.6正极在50 mA g−1下表现出283.6 mAh g−1的容量, 并且在1.5–4.8 V的电压范围内进行50次循环后, 与仅具有27.9%容量保持的原始LMOF1.6相比, 容量保持率提升了29.6%





Similar content being viewed by others
References
Xu B, Qian DN, Wang ZY, Meng YS. Recent progress in cathode materials research for advanced lithium ion batteries. Mater Sci Eng A R Rep. 2012;73(5–6):51. https://doi.org/10.1016/j.mser.2012.05.003.
Zhu XF, Li X, Liang TQ, Liu XH, & Ma JM. 2023. Electrolyte perspective on stabilizing LiNi0.8Co0.1Mn0.1O2 cathode for lithium-ion batteries. Rare Met. 2023;42(2):387. https://doi.org/10.1007/s12598-022-02101-2.
Ma LX, Chen TD, Hai CX, Dong SD, He X, Xu Q, Feng H, Xin A, Chen JT, Zhou Y. Surface engineering of Li-and Mn-rich layered oxides for superior Li-ion battery. Tungsten. 2022; 1. https://doi.org/10.1007/s42864-022-00187-w.
Ji H, Wu J, Cai Z, Liu J, Kwon DH, Kim H, Urban A, Papp JK, Foley E, Tian Y, Balasubramanian M, Kim H, Clément RJ, McCloskey BD, Yang W, Ceder G. Ultrahigh power and energy density in partially ordered lithium-ion cathode materials. Nat Energy. 2020;53:213. https://doi.org/10.1038/s41560-020-0573-1.
Jaffe S. Vulnerable links in the lithium-ion battery supply chain. Joule. 2017;12:225. https://doi.org/10.1016/j.joule.2017.09.021.
Hsieh IYL, Pan MS, Chiang Y-M, Green WH. Learning only buys you so much: practical limits on battery price reduction. Appl Energy. 2019;239:218. https://doi.org/10.1016/j.apenergy.2019.01.138.
Lun Z, Ouyang B, Kwon DH, Ha Y, Foley EE, Huang TY, Cai Z, Kim H, Balasubramanian M, Sun Y, Huang J, Tian Y, Kim H, McCloskey BD, Yang W, Clement RJ, Ji H, Ceder G. Cation-disordered rocksalt-type high-entropy cathodes for Li-ion batteries. Nat Mater. 2021;202:214. https://doi.org/10.1038/s41563-020-00816-0.
Lee J, Kitchaev DA, Kwon DH, Lee CW, Papp JK, Liu YS, Lun Z, Clement RJ, Shi T, McCloskey BD, Guo J, Balasubramanian M, Ceder G. Reversible Mn(2+)/Mn(4+) double redox in lithium-excess cathode materials. Nature. 2018;556(7700):185. https://doi.org/10.1038/s41586-018-0015-4.
Lee J, Urban A, Li X, Su D, Hautier G, Ceder G. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science. 2014;3436170:519. https://doi.org/10.1126/science.1246432.
Urban A, Lee J, Ceder G. The configurational space of rocksalt-type oxides for high-capacity lithium battery electrodes. Adv Energy Mater. 2014;413:1400478. https://doi.org/10.1002/aenm.201400478.
Geng KQ, Yang MQ, Meng JX, Zhou LF, Wang YQ, Dmytro S, Zhang Q, Zhong SW, Ma QX. (2022). Engineering layered/spinel heterostructure via molybdenum doping towards highly stable Li-rich cathodes. Tungsten. 2022;4(4): 323. https://doi.org/10.1007/s42864-022-00173-2.
Urban A, Matts I, Abdellahi A, Ceder G. Computational design and preparation of cation-disordered oxides for high-energy-density Li-ion batteries. Adv Energy Mater. 2016;615:1600488. https://doi.org/10.1002/aenm.201600488.
Thackeray MM. Exploiting the spinel structure for Li-ion battery applications: a tribute to John B. Goodenough Adv Energy Mater. 2021;112:2001117. https://doi.org/10.1002/aenm.202001117.
Bianchini M, Suard E, Croguennec L, Masquelier C. Li-rich Li1+xMn2–xO4 spinel electrode materials: an operando neutron diffraction study during Li+ extraction/insertion. J Phys Chem C. 2014;11845:25947. https://doi.org/10.1021/jp509027g.
Cai Z, Ji H, Ha Y, Liu J, Kwon DH, Zhang Y, Urban A, Foley EE, Giovine R, Kim H, Lun Z, Huang TY, Zeng G, Chen Y, Wang J, McCloskey BD, Balasubramanian M, Clément RJ, Yang W, Ceder G. Realizing continuous cation order-to-disorder tuning in a class of high-energy spinel-type Li-ion cathodes. Matter. 2021;412:3897. https://doi.org/10.1016/j.matt.2021.10.013.
Lee J, Seo D-H, Balasubramanian M, Twu N, Li X, Ceder G. A new class of high capacity cation-disordered oxides for rechargeable lithium batteries: Li–Ni–Ti–Mo oxides. Energy Environ. 2015;811:3255. https://doi.org/10.1039/C5EE02329G.
Seo D-H, Lee J, Urban A, Malik R, Kang S, Ceder G. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat Chem. 2016;87:692. https://doi.org/10.1038/nchem.2524.
Lun Z, Ouyang B, Kwon D-H, Ha Y, Foley EE, Huang T-Y, Cai Z, Kim H, Balasubramanian M, Sun Y, Huang J, Tian Y, Kim H, McCloskey BD, Yang W, Clément RJ, Ji H, Ceder G. Cation-disordered rocksalt-type high-entropy cathodes for Li-ion batteries. Nat Mater. 2021;202:214. https://doi.org/10.1038/s41563-020-00816-0.
Richards WD, Dacek ST, Kitchaev DA, Ceder G. Fluorination of lithium-excess transition metal oxide cathode materials. Adv Energy Mater. 2018;85:1701533. https://doi.org/10.1002/aenm.201701533.
Xue W, Huang M, Li Y, Zhu YG, Gao R, Xiao X, Zhang W, Li S, Xu G, Yu Y, Li P, Lopez J, Yu D, Dong Y, Fan W, Shi Z, Xiong R, Sun CJ, Hwang I, Lee WK, Shao-Horn Y, Johnson JA, Li J. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat Energy. 2021;65:495. https://doi.org/10.1038/s41560-021-00792-y.
Seaby T, Lin TE, Hu YX, Yuan QH, Wang LZ. An analysis of F-doping in Li-rich cathodes. Rare Met. 2022;41(6):1771. 1771–1796 (2022). https://doi.org/10.1007/s12598-021-01883-1.
Fan X, Ji X, Chen L, Chen J, Deng T, Han F, Yue J, Piao N, Wang R, Zhou X, Xiao X, Chen L, Wang C. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat Energy. 2019;410:882. https://doi.org/10.1038/s41560-019-0474-3.
Qiao ZA, Brown SS, Adcock J, Veith GM, Bauer JC, Payzant EA, Unocic RR, Dai S. A topotactic synthetic methodology for highly fluorine-doped mesoporous metal oxides. Angew Chemie. 2012;5112:2888. https://doi.org/10.1002/anie.201107812.
Li YY, Liu C, Chen L, Wu XZ, Zhou PF, & Shen XY, Zhou J. Multi-layered fluorinated graphene cathode materials for lithium and sodium primary batteries. Rare Met. 2023;42(3):940. https://doi.org/10.1007/s12598-022-02155-2.
Thapaliya BP, Self EC, Jafta CJ, Borisevich AY, Meyer HM III, Bridges CA, Nanda J, Dai S. Synthesizing high-capacity oxyfluoride conversion anodes by direct fluorination of molybdenum dioxide (MoO2). Chemsuschem. 2020;1315:3825. https://doi.org/10.1002/cssc.202001006.
Zhang Y, Self EC, Thapaliya BP, Giovine R, Meyer HM, Li L, Yue Y, Chen D, Tong W, Chen G, Wang C, Clément R, Dai S, Nanda J. Formation of LiF surface layer during direct fluorination of high-capacity Co-Free disordered rocksalt cathodes. ACS Appl Mater Inter. 2021;1332:38221. https://doi.org/10.1021/acsami.1c07882.
Myung T, Kikuchi M, Yoon CS, Yashiro H, Sun Y-K. A new synthetic method of titanium oxyfluoride and its application as an anode material for rechargeable lithium batteries. J Power Sources. 2015;288:376. https://doi.org/10.1016/j.jpowsour.2015.04.146.
Palaniyandy N, Nkosi FP, Raju K, Ozoemena KI. Fluorinated Mn3O4 nanospheres for lithium-ion batteries: low-cost synthesis with enhanced capacity, cyclability and charge-transport. Mater Chem Phys. 2018;209:65. https://doi.org/10.1016/j.matchemphys.2018.01.003.
Zhou S, Wang G, Tang W, Xiao Y, Yan K. Enhanced rate performance and high potential as well as decreased strain of LiNi0.6Co0.2Mn0.2O2 by facile fluorine modification. Electrochim Acta. 2018;261:565. https://doi.org/10.1016/j.electacta.2017.12.159.
Liu K, Zhang Q, Dai S, Li W, Liu X, Ding F, Zhang J. Synergistic effect of F– doping and LiF coating on improving the high-voltage cycling stability and rate capacity of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium-ion batteries. ACS Appl Mater Interfaces. 2018;1040:34153. https://doi.org/10.1021/acsami.8b10016.
Thapaliya BP, Borisevich AY, Meyer HM III, Sun XG, Bridges CA, Dai S. Conformal LiF stabilized interfaces via electrochemical fluorination on high voltage spinel cathodes (≈4.9 V) for lithium-ion batteries. Adv Mater. 2022;932:2201600. https://doi.org/10.1002/admi.202201600.
Kraus F, Ivlev SI, Bandemehr J, Sachs M, Pietzonka C, Conrad M, Serafin M, Müller BG. Synthesis and characterization of manganese tetrafluoride β-MnF4. Z Anorg Allg Chem. 2020;64618:1481. https://doi.org/10.1002/zaac.202000048.
Kim D, Park S, Chae OB, Ryu JH, Kim YU, Yin RZ, Oh SM. Re-deposition of manganese species on spinel LiMn2O4 electrode after Mn dissolution. J Electrochem Soc. 2012;1593:A193. https://doi.org/10.1149/2.003203jes.
Lee J, Papp JK, Clement RJ, Sallis S, Kwon DH, Shi T, Yang W, McCloskey BD, Ceder G. Mitigating oxygen loss to improve the cycling performance of high capacity cation-disordered cathode materials. Nat Commun. 2017;81:981. https://doi.org/10.1038/s41467-017-01115-0.
Lun Z, Ouyang B, Cai Z, Clément RJ, Kwon D-H, Huang J, Papp JK, Balasubramanian M, Tian Y, McCloskey BD, Ji H, Kim H, Kitchaev DA, Ceder G. Design principles for high-capacity Mn-based cation-disordered rocksalt cathodes. Chem. 2020;61:153. https://doi.org/10.1016/j.chempr.2019.10.001.
Richards WD, Dacek ST, Kitchaev DA, Ceder G. Fluorination of lithium-excess transition metal oxide cathode materials. Adv Energy Mater. 2017;3(2):518. https://doi.org/10.1002/aenm.201701533.
Zheng S, Dou A, Su M, Liu Y. Influence of Nb doping on electrochemical performance of nanostructured cation disordered Li1+x/100Ni1/2-x/100Ti1/2-x/100Nbx/100O2 composites cathode for Li-ion batteries. J Nanosci Nanotechnol. 2020;201:452. https://doi.org/10.1166/jnn.2020.16884.
Assat G, Glazier SL, Delacourt C, Tarascon J-M. Probing the thermal effects of voltage hysteresis in anionic redox-based lithium-rich cathodes using isothermal calorimetry. Nat Energy. 2019;48:647. https://doi.org/10.1038/s41560-019-0410-6.
Dai K, Wu J, Zhuo Z, Li Q, Sallis S, Mao J, Ai G, Sun C, Li Z, Gent WE, Chueh WC, Chuang YD, Zeng R, Shen ZX, Pan F, Yan S, Piper LFJ, Hussain Z, Liu G, Yang W. High reversibility of lattice oxygen redox quantified by direct bulk probes of both anionic and cationic redox reactions. Joule. 2019;32:518. https://doi.org/10.1016/j.joule.2018.11.014.
Ji H, Kitchaev DA, Lun Z, Kim H, Foley E, Kwon DH, Tian Y, Balasubramanian M, Bianchini M, Cai Z, Clément RJ, Kim JC, Ceder G. Computational investigation and experimental realization of disordered high-capacity Li-ion cathodes based on Ni redox. Chem Mater. 2019;317:2431. https://doi.org/10.1021/acs.chemmater.8b05096.
Du G, NuLi Y, Yang J, Wang J. Fluorine-doped LiNi0.5Mn1.5O4 for 5V cathode materials of lithium-ion battery. MateR Res Bull. 2008;4312:3607. https://doi.org/10.1016/j.materresbull.2008.02.025.
Yue Y, Li N, Li L, Foley EE, Fu Y, Battaglia VS, Clément RJ, Wang C, Tong W. Redox behaviors in a Li-excess cation-disordered Mn–Nb–O–F rocksalt cathode. Chem Mater. 2020;3211:4490. https://doi.org/10.1021/acs.chemmater.9b05221.
Zhao B, Si J, Cao C, Zhang J, Xia B, Xie J, Li B, Jiang Y. Enhanced electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode by reducing lithium residue with low-temperature fluorination treatment. Solid State Ion. 2019;339:114998. https://doi.org/10.1016/j.electacta.2017.02.067.
Du Z, Peng W, Wang Z, Guo H, Hu Q, Li X. Improving the electrochemical performance of Li-rich Li1.2Ni0.13Co0.13Mn0.54O2 cathode material by LiF coating. Ionics. 2018;2412:3717. https://doi.org/10.1016/j.electacta.2022.140169.
Thackeray MM, Kang S-H, Johnson CS, Vaughey JT, Benedek R, Hackney SA. Li2MnO3-stabilized LiMO2 (M=Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater. 2007;1730:3112. https://doi.org/10.1039/B702425H.
Yabuuchi N, Takeuchi M, Nakayama M, Shiiba H, Ogawa M, Nakayama K, Ohta T, Endo D, Ozaki T, Inamasu T, Sato K, Komaba S. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. PNAS. 2015;11225:7650. https://doi.org/10.1073/pnas.1504901112.
Dominko R, Bele M, Gaberscek M, Remskar M, Hanzel D, Pejovnik S, Jamnik J. Impact of the carbon coating thickness on the electrochemical performance of LiFePO4/C composites. J Electrochem Soc. 2005;1523:A607. https://doi.org/10.1149/1.1860492.
Thapaliya BP, Jafta CJ, Lyu H, Xia J, Meyer HM, III, Paranthaman M P, Sun X-G, Bridges C A, Dai S. Fluorination of MXene by elemental F2 as electrode material for lithium-ion batteries. Chemsuschem. 2019;127:1316. https://doi.org/10.1002/cssc.201900003.
Shi SJ, Tu JP, Tang YY, Zhang YQ, Liu XY, Wang XL, Gu CD. Enhanced electrochemical performance of LiF-modified LiNi1/3Co1/3Mn1/3O2 cathode materials for Li-ion batteries. J Power Sources. 2013;225:338. https://doi.org/10.1016/j.jpowsour.2012.10.065.
Breddemann U, Erickson EM, Davis V, Schipper F, Ellwanger M, Daub M, Hoffmann A, Erk C, Markovsky B, Aurbach D, Krossing I. Fluorination of Li-rich lithium-ion-battery cathode materials by fluorine gas: chemistry, characterization, and electrochemical performance in half cells. ChemElectroChem. 2019;613:3337. https://doi.org/10.1002/celc.201900733.
Liu J. Improvement of high-voltage electrochemical performance of surface modified LiNi0.6Co0.2Mn0.2O2 cathode La2O3 by coating. Int J Electrochem Sci. 2018;13(10):9816. https://doi.org/10.20964/2018.10.33.
Xiong X, Wang Z, Yin X, Guo H, Li X. A modified LiF coating process to enhance the electrochemical performance characteristics LiNi0.8Co0.1Mn0.1O2 of cathode materials. Mater Lett. 2013;110:4. https://doi.org/10.1016/j.matlet.2013.07.098.
Menetrier M, Bains J, Croguennec L, Flambard A, Bekaert E, Jordy C, Biensan P, Delmas C. NMR evidence of LiF coating rather than fluorine substitution in Li(Ni0.425Mn0.425Co0.15)O2. J Solid State Chem. 2008;18112:3303. https://doi.org/10.1016/j.jssc.2008.09.002.
He R, Bai X, Wei AJ, Zhang LH, Liu P, Liu ZF. Y2O3 modification on nickel-rich LiNi0. 8Co0. 1Mn0. 1O2 with improved electroche-mical performance in lithium-ion batteries. J Rare Earths. 2022;40(2):309. https://doi.org/10.1016/j.jre.2020.12.010.
Acknowledgements
This work was financially supported by the National Key R and D Program of China (No. 2022YFB3805702), State Key Program of the National Natural Science Foundation of China (No. 52130303), and National Natural Science Foundation of China (Nos. 51973152, 51973119, 52103093, and 52173078).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, YN., Kong, LC., Chen, SS. et al. Enhanced cyclic stability of partially disordered spinel cathodes through direct fluorination with gaseous fluorine. Rare Met. 43, 1635–1646 (2024). https://doi.org/10.1007/s12598-023-02528-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02528-1