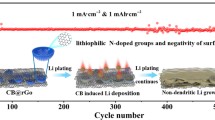
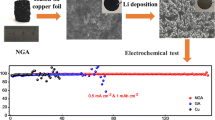
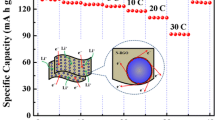
Abstract
Uncontrolled growth of lithium dendrite will lead to low Coulombic efficiency and poor cycle stability, which hinders the commercialization of lithium metal batteries. Herein, a novel modified lithium anode with reduced graphene oxide conductive network containing trace lithiophilic phosphorus (P-rGO/Cu) is prepared by electrospraying technique combined with heat treatment process. The rGO layer has a concave and undulating conductive structure, which can significantly improve the effective electrical contact between lithium metal and the current collector, speed up the kinetics of interfacial electron transport and reaction, and improve the resistance of the negative electrode to the internal stress caused by volume change of the lithium, which is advantageous for the stability of the SEI film. The extremely small and uniformly distributed red phosphorus element avoids the volume change caused by lithiation to the maximum extent. Lithiophilic two-phase compound Li3P obtained by alloying P with Li can directionally induce the homogeneous nucleation and dense deposition of lithium metal, address the issue of lithium dendrites and extend the cycle life of the batteries. The obtained P-rGO/Cu exhibits excellent electrochemical performance with an average Coulombic efficiency (CE) of 98% at a current density of 1 mA·cm−2 for 400 cycles, and the capacity retention rate of the full cell matched with lithium iron phosphate (LFP) is 83% after 400 cycles at 1C.
Graphical abstract

摘要
锂枝晶的不受控生长会导致电池的库仑效率降低, 循环稳定性变差, 从而阻碍锂金属电池的商业化进程。本文采用电喷与热处理制得了亲锂微量磷元素夹杂分布的还原氧化石墨烯导电网络改性锂负极 (P-rGO/Cu) 。研究表明rGO层凹凸起伏的导电结构显著增强金属锂与集流体间有效电接触, 加速界面电子传输与反应动力学; 以及增强负极承受锂体积变化产生内应力的能力, 有利于SEI膜的稳定; 微量P元素引入rGO层避免了锂化带来的体积变化; 利用P与Li合金化得到的亲锂两相化合物Li3P对锂金属的均匀形核与致密沉积进行定向诱导, 解决了锂枝晶问题, 提升了锂电池循环寿命。得到的P-rGO/Cu锂负极表现出优异的电化学性能, 在1 mA·cm−2电流密度下循环400圈的平均库伦效率(CE) 高达98%, 匹配磷酸铁锂 (LFP) 的全电池1C(170 mA·g−1) 循环400圈后容量保持率为83%。






Similar content being viewed by others
References
Goodenough JB. Evolution of strategies for modern rechargeable batteries. Acc Chem Res. 2013;46(5):1053. https://doi.org/10.1021/ar2002705.
Niu CJ, Pan HL, Xu W, Xiao J, Zhang JG, Luo L, Wang CM, Mei DH, Meng JS, Wang XP. Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat Nanotechnol. 2019;14(6):594. https://doi.org/10.1038/s41565-019-0427-9.
Grey CP, Tarascon JM. Sustainability and in situ monitoring in battery development. Nat Mater. 2017;16(1):45. https://doi.org/10.1038/nmat4777.
Xu W, Wang JL, Ding F, Chen XL, Nasybulin E, Zhang YH, Zhang JG. Lithium metal anodes for rechargeable batteries. Energy Environ Sci. 2014;7(2):513. https://doi.org/10.1039/C3EE40795K.
Cheng XB, Zhang R, Zhao CZ, Zhang Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem Rev. 2017;117(15):10403. https://doi.org/10.1021/acs.chemrev.7b00115.
Lin DC, Liu YY, Cui Y. Reviving the lithium metal anode for high-energy batteries. Nat Nanotechnol. 2017;12(3):194. https://doi.org/10.1038/nnano.2017.16.
Fengwei L, Yuhua X, Yulong Z, Shupeng Z, Shichun Y, Xinhua L. Lithium plating mechanism, model and fast charging strategy of lithium-ion batteries under fast charging condition. Chin J Rare Met. 2022;46(9):1235.
Liu YD, Liu Q, Xin L, Liu YZ, Yang F, Stach EA, Xie J. Making Li-metal electrodes rechargeable by controlling the dendrite growth direction. Nat Energy. 2017;2(7):1. https://doi.org/10.1038/nenergy.2017.83.
Yang ZJ, Qin XY, Lin K, Cai QC, Han CP, Kang FY, Li BH. Realizing ultra-stable SnO2 anodes via in-situ formed confined space for volume expansion. Carbon. 2022;187:321. https://doi.org/10.1016/j.carbon.2021.10.065.
Jiao SH, Zheng JM, Li QY, Li X, Engelhard MH, Cao RG, Zhang JG, Xu W. Behavior of lithium metal anodes under various capacity utilization and high current density in lithium metal batteries. Joule. 2018;2(1):110. https://doi.org/10.1016/j.joule.2017.10.007.
Huang HF, Gui YN, Sun F, Liu ZJ, Ning HL, Wu C, Chen LB. In situ formed three-dimensional (3D) lithium–boron (Li–B) alloy as a potential anode for next-generation lithium batteries. Rare Met. 2021;40(12):3494. https://doi.org/10.1007/s12598-021-01708-1.
Yang ZJ, Song HF, Chen JX, Lin K, Cai QC, Li T, Zhao DH, Liu M, Qin XY, Kang FY, Li BH. Free-standing stable silicon-based anode with exceptional flexibility realized by a multifunctional structure design in multiple dimensions. ACS Appl Mater Interfaces. 2022;14(41):46439. https://doi.org/10.1021/acsami.2c09668.
Yan K, Lu Z, Lee HW, Xiong F, Hsu PC, Li YZ, Zhao J, Chu S, Cui Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat Energy. 2016. https://doi.org/10.1038/nenergy.2016.10.
Kushima A, So KP, Su C, Bai P, Kuriyama N, Maebashi T, Fujiwara Y, Bazant MZ, Li J. Liquid cell transmission electron microscopy observation of lithium metal growth and dissolution: root growth, dead lithium and lithium flotsams. Nano Energy. 2017;32:271. https://doi.org/10.1016/j.nanoen.2016.12.001.
Goodenough JB, Park KS. The Li-ion rechargeable battery: a perspective. J Am Chem Soc. 2013;135(4):1167. https://doi.org/10.1021/ja3091438.
Zheng JM, Engelhard MH, Mei DH, Jiao SH, Polzin BJ, Zhang JG, Xu W. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat Energy. 2017;2(3):1. https://doi.org/10.1038/nenergy.2017.12.
Lu YY, Tu ZY, Archer LA. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat Mater. 2014;13(10):961. https://doi.org/10.1038/nmat4041.
Liu W, Xia YT, Wang WW, Wang YZ, Jin JL, Chen YG, Paek E, Mitlin D. Pristine or highly defective? Understanding the role of graphene structure for stable lithium metal plating. Adv Energy Mater. 2019;9(3):1802918. https://doi.org/10.1002/aenm.201802918.
Zheng GY, Lee SW, Liang Z, Lee HW, Yan K, Yao HB, Wang HT, Li WY, Chu S, Cui Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat Nanotechnol. 2014;9(8):618. https://doi.org/10.1038/nnano.2014.152.
Yan K, Lee HW, Gao T, Zheng GY, Yao HB, Wang HT, Lu ZD, Zhou Y, Liang Z, Liu ZF, Chu S, Cui Y. Ultrathin two-dimensional atomic crystals as stable interfacial layer for improvement of lithium metal anode. Nano Lett. 2014;14(10):6016. https://doi.org/10.1021/nl503125u.
Liu YY, Lin DC, Liang Z, Zhao J, Yan K, Cui Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat commun. 2016;7(1):1. https://doi.org/10.1038/ncomms10992.
Huang ZM, Ren J, Zhang W, Xie ML, Li YK, Sun D, Shen Y, Huang YH. Protecting the Li-metal anode in a Li-O2 battery by using boric acid as an SEI-forming additive. Adv Mater. 2018;30(39):1803270. https://doi.org/10.1002/adma.201803270.
Li L, Basu S, Wang YP, Chen ZZ, Hundekar P, Wang BW, Shi J, Shi YF, Narayanan S, Koratkar N. Self-heating-induced healing of lithium dendrites. Science. 2018;359(6383):1513. https://doi.org/10.1126/science.aap8787.
Li T, Liu H, Shi P, Zhang Q. Recent progress in carbon/lithium metal composite anode for safe lithium metal batteries. Rare Met. 2018;37(6):449. https://doi.org/10.1007/s12598-018-1049-3.
Yang QY, Yu Z, Li Y, Zhang W, Yuan HW, Li HJ, MaZhu WSM, Li S. Understanding and modifications on lithium deposition in lithium metal batteries. Rare Met. 2022;41(8):2800. https://doi.org/10.1007/s12598-022-01994-3.
Dudney NJ, Li JC. Using all energy in a battery. Science. 2015;347(6218):131. https://doi.org/10.1126/science.aaa2870.
Manthiram A, Yu XW, Wang SF. Lithium battery chemistries enabled by solid-state electrolytes. Nat Rev Mater. 2017;2(4):1. https://doi.org/10.1038/natrevmats.2016.103.
Li X, Deng SX, Banis MN, Doyle-Davis K, Zhang DX, Zhang TY, Yang J, Divigalpitiya R, Brandys F, Li RY, Sun XL. Suppressing corrosion of aluminum foils via highly conductive graphene-like carbon coating in high-performance lithium-based batteries. ACS Appl Mater Interfaces. 2019;11(36):32826. https://doi.org/10.1021/acsami.9b06442.
Zhu YW, Murali S, Cai WW, Li XS, Suk JW, Potts JR, Ruoff RS. Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater. 2010;22(35):3906. https://doi.org/10.1002/adma.201001068.
Lin K, Qin XY, Liu M, Xu XF, Liang GM, Wu JX, Kang FY, Chen GH, Li BH. Ultrafine titanium nitride sheath decorated carbon nanofiber network enabling stable lithium metal anodes. Adv Funct Mater. 2019;29(46):1903229. https://doi.org/10.1002/adfm.201903229.
Lin K, Xu XF, Qin XY, Zhang GQ, Liu M, Lv FZ, Xia Y, Kang FY, Chen GH, Li BH. Restructured rimous copper foam as robust lithium host. Energy Stor Mater. 2020;26:250. https://doi.org/10.1016/j.ensm.2020.01.001.
Miao G, Xiaoyu Z, Maodong L, Junlu Z, Guojia H, Yunyong L. Electrostatic self-assembly preparation of three-dimensional graphene coated red phosphorus for lithium-ion battery anode. Chin J Rare Met. 2022;46(8):1048.
Ye L, Zhang CY, Zhou Y, Ülgüt B, Zhao Y, Qian JF. Guided lithium nucleation and growth on lithiophilic tin-decorated copper substrate. J Energy Chem. 2022;74:412. https://doi.org/10.1016/j.jechem.2022.07.027.
Qiu XG, Liu W, Liu JD, Li JZ, Zhang K, Cheng FY. Nucleation mechanism and substrate modification of lithium metal anode. Acta Phys Chim Sin. 2021;37(1):2009012. https://doi.org/10.3866/PKU.WHXB202009012.
Lin DC, Liu YY, Liang Z, Lee HW, Sun J, Wang HT, Yan K, Xie J, Cui Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat Nanotechnol. 2016;11(7):626. https://doi.org/10.1038/nnano.2016.32.
Liu L, Yin YX, Li JY, Li NW, Zeng XX, Ye H, Guo YG, Wan LJ. Free-standing hollow carbon fibers as high-capacity containers for stable lithium metal anodes. Joule. 2017;1(3):563. https://doi.org/10.1016/j.joule.2017.06.004.
Ye H, Zheng ZJ, Yao HR, Liu SC, Zuo TT, Wu XW, Yin YX, Li NW, Gu JJ, Cao FF. Guiding uniform Li plating/stripping through lithium–aluminum alloying medium for long-life Li metal batteries. Angew Chem Int Ed. 2019;58(4):1094. https://doi.org/10.1002/anie.201811955.
Chen L, Fan XL, Ji X, Chen J, Hou S, Wang CS. High-energy Li metal battery with lithiated host. Joule. 2019;3(3):732. https://doi.org/10.1016/j.joule.2018.11.025.
Xia JH, Liu ZJ, Li D, Lu ZC, Zhou SX. Effect of current collector on electrochemical performance of alloy anodes of lithium ion batteries. Rare Met. 2011;30(S1):48. https://doi.org/10.1007/s12598-011-0235-3.
Jiao C, Sun HB, Zhang L, Zhao SQ, Pang GY, Zhao CR, Lu SG. A high-performance lithium anode based on N-doped composite graphene. Rare Met. 2019. https://doi.org/10.1007/s12598-019-01263-w.
Sun YM, Wang L, Li YB, Li YZ, Lee HR, Pei A, He XM, Cui Y. Design of red phosphorus nanostructured electrode for fast-charging lithium-ion batteries with high energy density. Joule. 2019;3(4):1080. https://doi.org/10.1016/j.joule.2019.01.017.
Sun J, Zheng GY, Lee HW, Liu N, Wang HT, Yao HB, Yang WS, Cui Y. Formation of stable phosphorus–carbon bond for enhanced performance in black phosphorus nanoparticle–graphite composite battery anodes. Nano Lett. 2014;14(8):4573. https://doi.org/10.1021/nl501617j.
Sun J, Lee HW, Pasta M, Yuan HT, Zheng GY, Sun YM, Li YZ, Cui Y. A phosphorene–graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat Nanotechnol. 2015;10(11):980. https://doi.org/10.1038/nnano.2015.194.
Nazri G. Preparation, structure and ionic conductivity of lithium phosphide. Solid State Ionics. 1989;34(1–2):97. https://doi.org/10.1016/0167-2738(89)90438-4.
Kim MS, Ryu JH, Deepika LYR, Nah IW, Lee KR, Archer LA, Il Cho W. Langmuir-Blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat Energy. 2018;3(10):889. https://doi.org/10.1038/s41560-018-0237-6.
Zhang C, Lyu RY, Lv W, Li H, Jiang W, Li J, Gu SC, Zhou GM, Huang ZJ, Zhang YB, Wu JQ, Yang QH, Kang FY. A lightweight 3D Cu nanowire network with phosphidation gradient as current collector for high-density nucleation and stable deposition of lithium. Adv Mater. 2019;31(48):1904991. https://doi.org/10.1002/adma.201904991.
Alam SN, Sharma N, Kumar L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene. 2017;6(1):1. https://doi.org/10.4236/graphene.2017.61001.
Voiry D, Yang J, Kupferberg J, Fullon R, Lee C, Jeong HY, Shin HS, Chhowalla M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science. 2016;353(6306):1413. https://doi.org/10.1016/j.cej.2022.140826.
Bhaviripudi S, Jia XT, Dresselhaus MS, Kong J. Role of kinetic factors in chemical vapor deposition synthesis of uniform large area graphene using copper catalyst. Nano Lett. 2010;10(10):4128. https://doi.org/10.1021/nl102355e.
Acknowledgements
This study was financially supported by the Key-Area Research and Development Program of Guangdong Province (No. 2020B090919003), the National Natural Science Foundation of China (Nos. 52261160384, 51872157 and 52072208), the Fundamental Research Project of Shenzhen (No. JCYJ20190808153609561), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (No. 2017BT01N111) and the Support Plan for Shenzhen Manufacturing Innovation Center (No. 20200627215553988). Authors thank the Materials and Devices Testing Center of Tsinghua University Shenzhen International Graduate School (Tsinghua SIGS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, JX., Zhang, GQ., Qin, XY. et al. Lithium-induced graphene layer containing Li3P alloy phase to achieve ultra-stable electrode interface for lithium metal anode. Rare Met. 43, 562–574 (2024). https://doi.org/10.1007/s12598-023-02433-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02433-7