Abstract
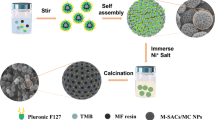
Herein, we successfully prepare highly dispersed and uniform small nano-size nickel nanoparticles embedded on core–shell carbon spheres by confined-deposition method. The mesoporous silica layer containing surfactant coated on the surface of the polymer sphere provides confined space and effectively controls the growth of nickel nanoparticles during pyrolysis. At the same time, the introduction of nickel species has an impact on structure of the obtained carbon spheres, and it can promote the deposition of carbon to realize the adjustment from hollow to core–shell and then to solid spheres. Owing to the uniform distribution of Ni nanoparticles with small size, mesoporous structure, N-doping groups, high specified surface areas, and core–shell structure, the obtained catalyst shows exciting ability for the production of CO by reduction of CO2 with a maximum CO Faradaic efficiency of 98%, indicating its promising prospect in electro-reduction of CO2.
Graphical Abstract

摘要
通过限域沉积法成功地制备了氮掺杂核壳结构介孔碳球负载的高度分散小纳米尺寸的镍颗粒。涂覆在聚合物球表 面上的介孔二氧化硅层提供了受限空间, 并在热解过程中有效地控制了镍纳米粒子的生长。同时, 镍的引入可以 促进碳的沉积, 实现从空心到核壳再到实心球的调整。由于小尺寸镍纳米粒子的均匀分布、介孔结构、氮掺杂基 团、高比表面积和核壳结构, 所获得的催化剂对CO2 还原制备CO 表现出良好的活性。CO 法拉第效率最大为98%, 表明其在电还原CO2 中具有良好的应用前景。





Similar content being viewed by others
References
Zhang S, Gao XT, Hou PF, Zhang TR, Kang P. Nitrogen-doped Zn–Ni oxide for electrochemical reduction of carbon dioxide in sea water. Rare Met. 2021;40(11):3117. https://doi.org/10.1007/s12598-021-01774-5.
Wang JJ, Li XP, Cui BF, Zhang Z, Hu XF, Ding J, Deng YD, Han XP, Hu WB. A review of non-noble metal-based electrocatalysts for CO2 electroreduction. Rare Met. 2021;40(11):3019. https://doi.org/10.1007/s12598-021-01736-x.
Zhang H, Liang Z, Huang C, Xie L, Wang H, Hu J, Jiang Z, Song F. Enhanced dissociation activation of CO2 on the Bi/Cu(111) interface by the synergistic effect. J Catal. 2022;410:1. https://doi.org/10.1016/j.jcat.2022.04.001.
Zhou AQ, Yang JM, Zhu XW, Zhu XL, Liu JY, Zhong K, Chen HX, Chu JY, Du YS, Song YH, Qian JC, Li HM, Xu H. Self-assembly construction of NiCo LDH/ultrathin g-C3N4 nanosheets photocatalyst for enhanced CO2 reduction and charge separation mechanism study. Rare Met. 2022;41(6):2118. https://doi.org/10.1007/s12598-022-01960-z.
Ma W, Bu J, Liu Z, Yan C, Yao Y, Chang N, Zhang H, Wang T, Zhang J. Monoclinic scheelite bismuth vanadate derived bismuthene nanosheets with rapid kinetics for electrochemically reducing carbon dioxide to formate. Adv Funct Mater. 2020;31(4):2006704. https://doi.org/10.1002/adfm.202006704.
Lin L, Liu T, Xiao J, Li H, Wei P, Gao D, Nan B, Si R, Wang G, Bao X. Enhancing CO2 electroreduction to methane with a cobalt phthalocyanine and zinc-nitrogen-carbon tandem catalyst. Angew Chem Int Ed. 2020;59(50):22408. https://doi.org/10.1002/anie.202009191.
Wang J, Wang G, Zhang J, Wang Y, Wu H, Zheng X, Ding J, Han X, Deng Y, Hu W. Inversely tuning the CO2 electroreduction and hydrogen evolution activity on metal oxide via heteroatom doping. Angew Chem Int Ed. 2021;60(14):7602. https://doi.org/10.1002/anie.202016022.
Wei F, Wang T, Jiang X, Ai Y, Cui A, Cui J, Fu J, Cheng J, Lei L, Hou Y, Liu S. Controllably engineering mesoporous surface and dimensionality of SnO2 toward high-performance CO2 electroreduction. Adv Funct Mater. 2020;30(39):2002092. https://doi.org/10.1002/adfm.202002092.
Zou J, Lee CY, Wallace GG. Boosting formate production from CO2 at high current densities over a wide electrochemical potential window on a SnS catalyst. Adv Sci. 2021. https://doi.org/10.1002/advs.202004521.
Yun R, Zhan F, Wang X, Zhang B, Sheng T, Xin Z, Mao J, Liu S, Zheng B. Design of binary Cu-Fe sites coordinated with nitrogen dispersed in the porous carbon for synergistic CO2 electroreduction. Small. 2021;17(4):e2069501. https://doi.org/10.1002/smll.202006951.
Li S, Alfonso D, Nagarajan AV, House SD, Yang JC, Kauffman DR, Mpourmpakis G, Jin R. Monopalladium substitution in gold nanoclusters enhances CO2 electroreduction activity and selectivity. ACS Catal. 2020;10(20):12011. https://doi.org/10.1021/acscatal.0c02266.
Niu ZZ, Gao FY, Zhang XL, Yang PP, Liu R, Chi LP, Wu ZZ, Qin S, Yu X, Gao MR. Hierarchical copper with inherent hydrophobicity mitigates electrode flooding for high-rate CO2 electroreduction to multicarbon products. J Am Chem Soc. 2021;143(21):8011. https://doi.org/10.1021/jacs.1c01190.
Yang D, Zuo S, Yang H, Wang X. Single-unit-cell catalysis of CO2 electroreduction over sub-1 nm Cu9S5 nanowires. Adv Energy Mater. 2021;11(16):2100272. https://doi.org/10.1002/aenm.202100272.
Yi JD, Xie R, Xie ZL, Chai GL, Liu TF, Chen RP, Huang YB, Cao R. Highly selective CO2 electroreduction to CH4 by in situ generated Cu2O single-type sites on a conductive MOF: stabilizing key intermediates with hydrogen bonding. Angew Chem Int Ed. 2020;59(52):23641. https://doi.org/10.1002/anie.202010601.
Song Y, Chen W, Zhao C, Li S, Wei W, Sun Y. Metal-free nitrogen-doped mesoporous carbon for electroreduction of CO2 to ethanol. Angew Chem Int Ed. 2017;56(36):10840. https://doi.org/10.1002/anie.201706777.
Li Z, Yang Y, Yin Z, Wei X, Peng H, Lyu K, Wei F, Xiao L, Wang G, Abruña HD, Lu J, Zhuang L. Interface-enhanced catalytic selectivity on the C2 products of CO2 electroreduction. ACS Catal. 2021;11(5):2473. https://doi.org/10.1021/acscatal.0c03846.
Duan X, Xu J, Wei Z, Ma J, Guo S, Wang S, Liu H, Dou S. Metal-free carbon materials for CO2 electrochemical reduction. Adv Mater. 2017;29(41):1701784. https://doi.org/10.1002/adma.201701784.
Li J, Zan WY, Kang H, Dong Z, Zhang X, Lin Y, Mu YW, Zhang F, Zhang XM, Gu J. Graphitic-N highly doped graphene-like carbon: a superior metal-free catalyst for efficient reduction of CO2. Appl Catal B-Environ. 2021. https://doi.org/10.1016/j.apcatb.2021.120510.
Wu J, Sharifi T, Gao Y, Zhang T, Ajayan PM. Emerging carbon-based heterogeneous catalysts for electrochemical reduction of carbon dioxide into value-added chemicals. Adv Mater. 2019;31(13):e1804257. https://doi.org/10.1002/adma.201804257.
Kim JY, Hong D, Lee JC, Kim HG, Lee S, Shin S, Kim B, Lee H, Kim M, Oh J, Lee GD, Nam DH, Joo YC. Quasi-graphitic carbon shell-induced Cu confinement promotes electrocatalytic CO2 reduction toward C2+ products. Nat Commun. 2021;12(1):3765. https://doi.org/10.1038/s41467-021-24105-9.
Tan X, Yu C, Song X, Zhao C, Cui S, Xu H, Chang J, Guo W, Wang Z, Xie Y, Qiu J. Toward an understanding of the enhanced CO2 electroreduction in NaCl electrolyte over CoPc molecule-implanted graphitic carbon nitride catalyst. Adv Energy Mater. 2021. https://doi.org/10.1002/aenm.202100075.
Wu D, Wang X, Fu XZ, Luo JL. Ultrasmall Bi nanoparticles confined in carbon nanosheets as highly active and durable catalysts for CO2 electroreduction. Appl Catal B-Environ. 2021;284:119723. https://doi.org/10.1016/j.apcatb.2020.119723.
Ni W, Liu Z, Guo X, Zhang Y, Ma C, Deng Y, Zhang S. Dual single-cobalt atom-based carbon electrocatalysts for efficient CO2-to-syngas conversion with industrial current densities. Appl Catal B-Environ. 2021;291:120092. https://doi.org/10.1016/j.apcatb.2021.120092.
Iwase K, Ebner K, Diercks JS, Saveleva VA, Ünsal S, Krumeich F, Harada T, Honma I, Nakanishi S, Kamiya K, Schmidt TJ, Herranz J. Effect of cobalt speciation and the graphitization of the carbon matrix on the CO2 electroreduction activity of Co/N-doped carbon materials. ACS Appl Mater Interfaces. 2021;13(13):15122. https://doi.org/10.1021/acsami.0c21920.
Lu P, Tan X, Zhao H, Xiang Q, Liu K, Zhao X, Yin X, Li X, Hai X, Xi S, Wee ATS, Pennycook SJ, Yu X, Yuan M, Wu J, Zhang G, Smith SC, Yin Z. Atomically dispersed indium sites for selective CO2 electroreduction to formic acid. ACS Nano. 2021;15(3):5671. https://doi.org/10.1021/acsnano.1c00858.
Sui R, Pei J, Fang J, Zhang X, Zhang Y, Wei F, Chen W, Hu Z, Hu S, Zhu W, Zhuang Z. Engineering Ag–Nx single-atom sites on porous concave N-doped carbon for boosting CO2 electroreduction. ACS Appl Mater Interfaces. 2021;13(15):17736. https://doi.org/10.1021/acsami.1c03638.
Zhao Y, Hao L, Ning J, Zhu H, Vijayakumar A, Wang C, Wallace GG. A versatile transition metal ion-binding motif derived from covalent organic framework for efficient CO2 electroreduction. Appl Catal B-Environ. 2021;291:119915. https://doi.org/10.1016/j.apcatb.2021.119915.
Zhou Y, Zheng L, Yang D, Yang H, Wang X. Boosting CO2 electroreduction via the synergistic effect of tuning cationic clusters and visible-light irradiation. Adv Mater. 2021;33(27):e2101886. https://doi.org/10.1002/adma.202101886.
Zhang X, Wang Y, Gu M, Wang M, Zhang Z, Pan W, Jiang Z, Zheng H, Lucero M, Wang H, Sterbinsky GE, Ma Q, Wang YG, Feng Z, Li J, Dai H, Liang Y. Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2 reduction. Nat Energy. 2020;5(9):684. https://doi.org/10.1038/s41560-020-0667-9.
Li H, Liu T, Wei P, Lin L, Gao D, Wang G, Bao X. High-rate CO2 electroreduction to C2+ products over a copper-copper iodide catalyst. Angew Chem Int Ed. 2021;60(26):14329. https://doi.org/10.1002/anie.202102657.
Wang J, Dou S, Wang X. Structural tuning of heterogeneous molecular catalysts for electrochemical energy conversion. Sci Adv. 2021;7(13):3989. https://doi.org/10.1126/sciadv.abf3989.
Wang Z, Hou P, Wang Y, Xiang X, Kang P. Acidic Electrochemical reduction of CO2 using nickel nitride on multiwalled carbon nanotube as selective catalyst. ACS Sustain Chem Eng. 2019;7(6):6106. https://doi.org/10.1021/acssuschemeng.8b06278.
Jia M, Choi C, Wu TS, Ma C, Kang P, Tao H, Fan Q, Hong S, Liu S, Soo YL, Jung Y, Qiu J, Sun Z. Carbon-supported Ni nanoparticles for efficient CO2 electroreduction. Chem Sci. 2018;9(47):8775. https://doi.org/10.1039/C8SC03732A.
Zhang M, Wu TS, Hong S, Fan Q, Soo YL, Masa J, Qiu J, Sun Z. Efficient electrochemical reduction of CO2 by Ni–N catalysts with tunable performance. ACS Sus Chem Eng. 2019;7(17):15030. https://doi.org/10.1021/acssuschemeng.9b03502.
Alqahtani MF, Katuri KP, Bajracharya S, Yu Y, Lai Z, Saikaly PE. Porous hollow fiber nickel electrodes for effective supply and reduction of carbon dioxide to methane through microbial electrosynthesis. Adv Funct Mater. 2018;28(43):1804860. https://doi.org/10.1002/adfm.201804860.
Li D, Liu T, Yan Z, Zhen L, Liu J, Wu J, Feng Y. MOF-derived Cu2O/Cu Nanospheres anchored in nitrogen-doped hollow porous carbon framework for increasing the selectivity and activity of electrochemical CO2-to-formate conversion. ACS Appl Mater Interfaces. 2020;12(6):7030. https://doi.org/10.1021/acsami.9b15685.
Hao L, Sun Z. Metal oxide-based materials for electrochemical CO2 reduction. Acta Phys -Chim Sin. 2021;37:2009033. https://doi.org/10.3866/PKU.WHXB202009033.
Gong YN, Jiao L, Qian Y, Pan CY, Zheng L, Cai X, Liu B, Yu SH, Jiang HL. Regulating the coordination environment of MOF-templated single-atom nickel electrocatalysts for boosting CO2 reduction. Angew Chem Int Ed. 2020;59(7):2705. https://doi.org/10.1002/anie.201914977.
Liu S, Yang HB, Hung SF, Ding J, Cai W, Liu L, Gao J, Li X, Ren X, Kuang Z, Huang Y, Zhang T, Liu B. Elucidating the electrocatalytic CO2 reduction reaction over a model single-atom nickel catalyst. Angew Chem Int Ed. 2020;59(2):798. https://doi.org/10.1002/anie.201911995.
Zhang H, Li J, Xi S, Du Y, Hai X, Wang J, Xu H, Wu G, Zhang J, Lu J, Wang J. A graphene-supported single-atom FeN5 catalytic site for efficient electrochemical CO2 reduction. Angew Chem Int Ed. 2019;58(42):14871. https://doi.org/10.1002/anie.201906079.
Zhang T, Han X, Yang H, Han A, Hu E, Li Y, Yang XQ, Wang L, Liu J, Liu B. Atomically dispersed nickel(i) on an alloy-encapsulated nitrogen-doped carbon nanotube array for high-performance electrochemical CO2 reduction reaction. Angew Chem Int Ed. 2020;59(29):12055. https://doi.org/10.1002/anie.202002984.
Niu K, Xu Y, Wang H, Ye R, Xin HL, Lin F, Tian C, Lum Y, Bustillo KC, Doeff MM, Koper MTM, Ager J, Xu R, Zheng H. A spongy nickel-organic CO2 reduction photocatalyst for nearly 100% selective CO production. Sci Adv. 2017;3(7):1700921. https://doi.org/10.1126/sciadv.1700921.
Guo JH, Zhang XY, Dao XY, Sun WY. Nanoporous metal–organic framework-based ellipsoidal nanoparticles for the catalytic electroreduction of CO2. ACS Appl Nano Mater. 2020;3(3):2625. https://doi.org/10.1021/acsanm.0c00007.
Büchele S, Martín AJ, Mitchell S, Krumeich F, Collins SM, Xi S, Borgna A, Pérez-Ramírez J. Structure sensitivity and evolution of nickel-bearing nitrogen-doped carbons in the electrochemical reduction of CO2. ACS Catal. 2020;10(5):3444. https://doi.org/10.1021/acscatal.9b05333.
Gang Y, Pan F, Fei Y, Du Z, Hu YH, Li Y. Highly efficient nickel, iron, and nitrogen codoped carbon catalysts derived from industrial waste petroleum coke for electrochemical CO2 reduction. ACS Sustain Chem Eng. 2020;8(23):8840. https://doi.org/10.1021/acssuschemeng.0c03054.
Ye S, Fan G, Xu J, Yang L, Li F. Nickel-nitrogen-modified porous carbon/carbon nanotube hybrid with necklace-like geometry: an efficient and durable electrocatalyst for selective reduction of CO2 to CO in a wide negative potential region. Electrochim Acta. 2020;334:135583. https://doi.org/10.1016/j.electacta.2019.135583.
Zhang T, Lin L, Li Z, He X, Xiao S, Shanov VN, Wu J. Nickel–nitrogen–carbon molecular catalysts for high rate CO2 electro-reduction to CO: on the role of carbon substrate and reaction chemistry. ACS Appl Energy Mater. 2020;3(2):1617. https://doi.org/10.1021/acsaem.9b02112.
Roy S, Sharma B, Pecaut J, Simon P, Fontecave M, Tran PD, Derat E, Artero V. Molecular cobalt complexes with pendant amines for selective electrocatalytic reduction of carbon dioxide to formic acid. J Am Chem Soc. 2017;139(10):3685. https://doi.org/10.1021/jacs.6b11474.
Chen C, Sun X, Yan X, Wu Y, Liu H, Zhu Q, Bediako BBA, Han B. Boosting CO2 electroreduction on N, P-co-doped carbon aerogels. Angew Chem Int Ed. 2020;59(27):11123. https://doi.org/10.1002/anie.202004226.
Gao D, Sinev I, Scholten F, Arán-Ais RM, Divins NJ, Kvashnina K, Timoshenko J, Roldan CB. Selective CO2 electroreduction to ethylene and multicarbon alcohols via electrolyte-driven nanostructuring. Angew Chem Int Ed. 2019;58(47):17047. https://doi.org/10.1002/anie.201910155.
Hursán D, Samu AA, Janovák L, Artyushkova K, Asset T, Atanassov P, Janáky C. Morphological attributes govern carbon dioxide reduction on N-doped carbon electrodes. Joule. 2019;3(7):1719. https://doi.org/10.1016/j.joule.2019.05.007.
Acknowledgements
This work was financially supported by the Natural Science Foundation of Hebei (Nos. B02020208088, H2020206514 and B2021208074), S&T Program of Hebei (Nos. 20544401D, 20314401D, 206Z4406G, 21314402D, 22344402D, 22373709D, 22284601Z and 21344601D), Tianjin Science and Technology Project (No. 19YFSLQY00070).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, J., Lin, QY., Zhang, JQ. et al. N-doped core–shell mesoporous carbon spheres embedded by Ni nanoparticles for CO2 electroreduction. Rare Met. 42, 2284–2293 (2023). https://doi.org/10.1007/s12598-023-02317-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02317-w