Abstract
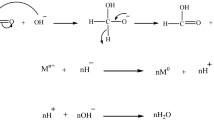
Recent studies suggested that the interactions between particles can induce aggregative nucleation and growth processes beyond those predicted by the traditional LaMer model of nanoparticle formation, but their nucleation and growth processes are still unclear. Here, we report a simple way to control the interaction between nanoparticles by manipulating the oleylamine (OAm) adsorbed on the surface of the nanoparticles. The size distributions of Ag nanoparticles produced at different reaction pressures were monitored as evidence for aggregative growth. From these kinetic data, the aggregative nucleation rate (Γ) of primary Ag nanoparticles under a 0.01 MPa was demonstrated to be faster than that under atmospheric pressure. This leads to a higher uniformity of Ag nanoparticles in a shorter time (10 min) than that achievable with previous methods. Furthermore, Ag nanoparticles supported on TiO2 exhibited a remarkable performance in the catalytic reduction of 4-nitrophenol (4-NP). After 4 min, 4-NP was completely reduced into 4-aminophenol (4-AP).









Similar content being viewed by others
References
Nicoletti O, de la Peña F, Leary RK, Holland DJ, Ducati C, Midgley PA. Three-dimensional imaging of localized surface plasmon resonances of metal nanoparticles. Nature. 2013;502(7469):80.
Cheng ZP, Chu XZ, Wu X, Xu JM, Zhong H, Yin JZ. Controlled synthesis of silver nanoplates and nanoparticles by reducing silver nitrate with hydroxylamine hydrochloride. Rare Met. 2017;36(10):799.
Guo J, Zhang Y, Shi L, Zhu YF, Mideksa MF, Hou K, Zhao WS, Wang DW, Zhao MT, Zhang XF, Lv JW, Zhang JQ, Wang XL, Tang ZY. Boosting hot electrons in hetero-superstructures for plasmon-enhanced catalysis. J Am Chem Soc. 2017;139(49):17964.
Dumrongrojthanath P, Phuruangrat A, Thongtem S, Thongtem T. Facile sonochemical synthesis and photocatalysis of Ag nanoparticle/ZnWO4-nanorod nanocomposites. Rare Met. 2019;38(7):601.
Wang FD, Richards VN, Shields SP, Buhro WE. Kinetics and mechanisms of aggregative nanocrystal growth. Chem Mater. 2014;26(1):5.
Baek IC, Seok SI, Pramanik NC, Jana S, Lim MA, Ahn BY, Lee CJ, Jeong YJ. Ligand-dependent particle size control of PbSe quantum dots. J Colloid Interfaces Sci. 2007;310(1):163.
Luo BB, Pu YC, Lindley SA, Yang Y, Lu LQ, Li Y, Li XM, Zhang JZ. Organolead halide perovskite nanocrystals: branched capping ligands control crystal size and stability. Angew Chem Int Ed. 2016;55(31):8864.
Sánchez-Iglesias A, Grzelczak M, Altantzis T, Goris B, Perez-Juste J, Bals S, Van Tendeloo G, Donaldson SH Jr, Chmelka BF, Israelachvili JN, Liz-marzan LM. Hydrophobic interactions modulate self-assembly of nanoparticles. ACS Nano. 2012;6(12):11059.
Shields SP, Richards VN, Buhro WE. Nucleation control of size and dispersity in aggregative nanoparticle growth. A study of the coarsening kinetics of thiolate-capped gold nanocrystals. Chem Mater. 2010;22(10):3212.
Richards VN, Rath NP, Buhro WE. Pathway from a molecular precursor to silver nanoparticles: the prominent role of aggregative growth. Chem Mater. 2010;22(11):3556.
Van Hyning DL, Klemperer WG, Zukoski CF. Silver nanoparticle formation: predictions and verification of the aggregative growth model. Langmuir. 2001;17(11):3128.
Narayanaswamy A, Xu HF, Pradhan N, Peng XG. Crystalline nanoflowers with different chemical compositions and physical properties grown by limited ligand protection. Angew Chem Int Ed. 2006;45(32):5361.
Liu LT, Gao ZP, Jiang BL, Bai YC, Wang WS, Yin YD. Reversible assembly and dynamic plasmonic tuning of Ag nanoparticles enabled by limited ligand protection. Nano Lett. 2018;18(8):5312.
Lei Y, Mehmood F, Lee S, Greeley J, Lee B, Seifert S, Winans RE, Elam JW, Meyer RJ, Redfern PC, Teschner D, Schlögl R, Pellin MJ, Curtiss LA, Vajda S. Increased silver activity for direct propylene epoxidation via subnanometer size effects. Science. 2010;328(5975):224.
Li YN, Wu YL, Ong BS. Facile synthesis of silver nanoparticles useful for fabrication of high-conductivity elements for printed electronics. J Am Chem Soc. 2005;127(10):3266.
Rycenga M, Cobley CM, Zeng J, Li WY, Moran CH, Zhang Q, Qin D, Xia YN. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem Rev. 2011;111(6):3669.
Quang Huy T, Van Quy N, Anh-Tuan L. Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Adv Nat Sci Nanosci Nanotechnol. 2013;4(3):033001.
Zhang P, Shao CL, Zhang ZY, Zhang MY, Mu JB, Guo ZC, Liu YC. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale. 2011;3(8):3357.
Koh AL, Bao K, Khan I, Smith WE, Kothleitner G, Nordlander P, Maier SA, McComb DW. Electron energy-loss spectroscopy (EELS) of surface plasmons in single silver nanoparticles and dimers: influence of beam damage and mapping of dark modes. ACS Nano. 2009;3(10):3015.
Camden JP, Dieringer JA, Wang YM, Masiello DJ, Marks LD, Schatz GC, Van Duyne RP. Probing the structure of single-molecule surface-enhanced Raman scattering hot spots. J Am Chem Soc. 2008;130(38):12616.
Wiley B, Sun YG, Xia YN. Synthesis of silver nanostructures with controlled shapes and properties. Accounts Chem Res. 2007;40(10):1067.
Tao A, Sinsermsuksakul P, Yang PD. Polyhedral silver nanocrystals with distinct scattering signatures. Angew Chem Int Ed. 2006;45(28):4597.
Pietrobon B, McEachran M, Kitaev V. Synthesis of size-controlled faceted pentagonal silver nanorods with tunable plasmonic properties and self-assembly of these nanorods. ACS Nano. 2009;3(1):21.
Zhang Q, Li WY, Moran C, Zeng J, Chen JY, Wen LP, Xia YN. Seed-mediated synthesis of Ag nanocubes with controllable edge lengths in the range of 30–200 nm and comparison of their optical properties. J Am Chem Soc. 2010;132(32):11372.
Chen M, Feng YG, Wang X, Li TC, Zhang JY, Qian DJ. Silver nanoparticles capped by oleylamine: formation, growth, and self-organization. Langmuir. 2007;23(10):5296.
Peng S, McMahon JM, Schatz GC, Gray SK, Sun YG, Sun Y. Reversing the size-dependence of surface plasmon resonances. Proc Natl Acad Sci. 2010;107(33):14530.
Sawczyk M, Klajn R. Out-of-equilibrium aggregates and coatings during seeded growth of metallic nanoparticles. J Am Chem Soc. 2017;139(49):17973.
Pradhan N, Reifsnyder D, Xie RG, Aldana J, Peng XG. Surface ligand dynamics in growth of nanocrystals. J Am Chem Soc. 2007;129(30):9500.
Bealing CR, Baumgardner WJ, Choi JJ, Hanrath T, Hennig RG. Predicting nanocrystal shape through consideration of surface–ligand interactions. ACS Nano. 2012;6(3):2118.
Xia YN, Xia XH, Peng HC. Shape-controlled synthesis of colloidal metal nanocrystals: thermodynamic versus kinetic products. J Am Chem Soc. 2015;137(25):7947.
Liu R, Mahurin SM, Li C, Unocic RR, Idrobo JC, Gao HJ, Pennycook SJ, Dai S. Dopamine as a carbon source: the controlled synthesis of hollow carbon spheres and yolk-structured carbon nanocomposites. Angew Chem Int Ed. 2011;50(30):6799.
Tang SC, Vongehr S, Meng XK. Carbon spheres with controllable silver nanoparticle doping. J Phys Chem C. 2010;114(2):977.
Gu Y, Jiao YQ, Zhou XG, Wu AP, Buhe B, Fu HG. Strongly coupled Ag/TiO2 heterojunctions for effective and stable photothermal catalytic reduction of 4-nitrophenol. Nano Res. 2018;11(1):12.
Acknowledgements
This work was financially supported by the Shanghai Pujiang Program (No. 17PJD012) and the Science and Technology Commission of Shanghai Municipality (No. 16ZR1407900).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, J., Shu, Y., Xia, Q. et al. Pressure control as an effective method to modulate aggregative growth of nanoparticles. Rare Met. 40, 1808–1816 (2021). https://doi.org/10.1007/s12598-020-01484-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-020-01484-4