Abstract
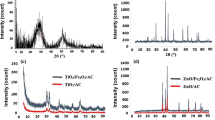
Titanium dioxide is an effective adsorbent on the removal of arsenic from drinking water. Using three different methods: (a) directly using TiOSO4 solution, (b) using the precursor of TiO2, (c) using TiO2 sol-gel, one composite adsorbent was synthesized by loading titanium dioxide on activated carbon (TiO2/AC) with titanyl sulfate (TiOSO4) and activated carbon as raw materials. The phase compositions and morphology of the adsorbent were characterized by using XRD and SEM. According to the TG/DTA results of TiOSO4, it is easy to see that there is no TiO2 crystalline phase appears below 400 °C, and only anatase phase can be found at 400–700 °C. The presence of SO4 2− restrained the transformation of anatase to rutile. The TiO2 loading on the AC surface is anatase phase by using the precursor of TiO2 and TiO2 sol-gel, when the calcination temperature is 400 °C. At the same time, the distribution of TiO2 is more uniform and immobilized on the AC surface by using TiO2 sol-gel than using other two methods.
Similar content being viewed by others

References
Pokhrel D., Viraraghavan T., and Braul L., Evaluation of treatment systems for the removal of arsenic from groundwater, Pract. Period. Hazard. Toxic Radioact., Waste Manage., 2005, 9(3): 152.
Liu G.J., Zhang X.R., Mcwilliams L., Talley J.W., and Neal C. R., Influence of ionic strength, electrolyte type, and NOM on As(V) adsorption onto TiO2, J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng., 2008, 43(4): 430.
Smith A.H., Lingas E.O., and Rahman M., Contamination of drinking water by arsenic in Bangladesh: a public health emergency, Bull. World Heal. Org., 2000, 78(9): 1093.
Galal-Gorchev H., WHO guidelines for drinking-water quality, Water Supply, 1993, 11(3–4): 1.
Jing C.Y., Meng X.G., Calvache E., and Jiang G.B., Remediation of organic and inorganic arsenic contaminated groundwater using a nanocrystalline TiO2-based adsorbent, Environ. Pollut., 2009, 157(8–9): 2514.
Hristovski K., Baumgardner A., and Westerhoff P., Selecting metal oxide nanomaterials for arsenic removal in fixed bed columns: From nanopowders to aggregated nanoparticle media, J. Hazard. Mater., 2007, 147(1–2): 265.
Yang H., Lin, W.Y., and Rajeshwar K., Homogeneous and heterogeneous photocatalytic reactions involving As(III) and As(V) species in aqueous media, J. Photochem. Photobiol. A: Chem., 1999, 123(1–3): 137.
Bissen M., Vieillard-Baron M.M., Schindelin A.J., and Frimmel F.H., TiO2-catalyzed photooxidation of arsenite to arsenate in aqueous samples, Chemosphere, 2001, 44(4): 751.
Ray A. K., Design, modelling and experimentation of a new large-scale photocatalytic reactor for water treatment, Chem. Eng. Sci.. 1999, 54(15–16): 3113.
Yao S.H., Li J.Y., Shi Z.L., Immobilization of TiO2 nanoparticles on activated carbon fiber and its photodegradation performance for organic pollutants, Particuology., 2010, 8(3): 272.
Carpio E., Zuniga P., Ponce S., Solis J., Rodriguez J., and Estrada W., Photocatalytic degradation of phenol using TiO2 nanocrystals supported on activated carbon, J. Mol. Catal. A: Chem., 2005, 228(1–2): 293.
Zhang L., Zhu Y., Li H.M., Liun N., Liu X.Y., and Guo X.J., Kinetic and thermodynamic studies of adsorption of gallium(III) on nano-TiO2, Rare Met., 2010, 29(1): 16.
Li Y.X., Zhang M., Guo M.., and Wang X.D., Preparation and properties of a nano TiO2/Fe3O4 composite superparamagnetic photocatalyst, Rare Met., 2009, 28(5): 423.
Matos J., Laine J., and Herrmann J.M., Effect of the type of activated carbons on the photocatalytic degradation of aqueous organic pollutants by UV-irradiated titania, J. Catal., 2001, 200(1): 10.
Colon G., Hidalgo M.C., Macias M., Navío J.A., and Doña J.M.., Influence of residual carbon on the photocatalytic activity of TiO2/samples for phenol oxidation, Appl. Catal. B: Enviro., 2003, 43(2): 163.
Arana J., Dona-Rodriguez J.M., Tello Rendon E., Garrigai Cabo C., González-Díaz O., Herrera-Melián J.A., Pérez-Peña J., Colón G., and Navío J.A., TiO2 activation by using activated carbon as a support — Part II. Photoreactivity and FTIR study, Appl. Catal. B: Environ. 2003, 44(2): 153.
Arana J., Dona-Rodriguez J.M., Tello Rendon E., Garriga i Cabo C., González-Díaz O., Herrera-Melián J. A., Pérez-Peña J., Colón G., and Navío J. A., TiO2 activation by using activated carbon as a support — Part I. Surface characterisation and decantability study, Appl. Catal. B: Environ. 2003, 44(2): 161.
Reddy K.M., Reddy C.V.G., and Manorama S.V., Preparation, characterization, and spectral studies on nanocrystalline anatase TiO2, J. Solid State Chem., 2001, 158(2): 180.
Li Y.Z., Lee N.H., Hwang D.S., Song J.S., Lee E.G., and Kim S.-J., Synthesis and characterization of nano titania powder with high photoactivity for gas-phase photo-oxidation of benzene from TiOCl2 aqueous solution at low temperatures, Langmuir, 2004, 20(25): 10838.
Cullity B.D., Elements of X-Ray Diraction, Adison-Wesley Publishing Company, Inc. Reading, Massachusetts, 1978.
Nagaveni K., Hegde M. S., and Madras G., Structure and photocatalytic activity of Ti1−x MxO2δ (M=W, V, Ce, Zr, Fe, and Cu) synthesized by solution combustion method, J. Phys. Chem. B, 2004, 108(52): 20204.
Fu P.F., Luan Y., and Dai X.G., Preparation of activated carbon fibers supported TiO2 photocatalys and evaluation of its photocatalytic reactivity, J. Mol. Catal. A: Chem., 2004, 221(1–2): 81.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, L., Xie, D., Qu, Y. et al. Preparation of activated carbon (AC)-loaded TiO2 adsorbent. Rare Metals 30 (Suppl 1), 217–220 (2011). https://doi.org/10.1007/s12598-011-0272-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-011-0272-y