Abstract
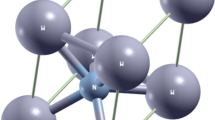
The valence electron structure and theoretical cohesive energy of Mg2Ni were calculated by means of bond length difference method (BLD) in the Empirical Electron Theory of Solids and Molecules (EET). Ionicity of chemical bonds in Mg2Ni was given by EET together with PVL valence bond theory. Results show that, the theoretical cohesive energy of Mg2Ni is 746.5 kJ/mol, in good agreement with the experimental value. In Mg2Ni, the interactions between atoms along c-axis are stronger than those along a-axis and b-axis, taking on obvious anisotropy, so that the pulverization easily occurs after repeated absorption and desorption of hydrogen. In the alloy, the interaction between Ni-Mg atoms dominates and tetrahedral clusters made of Ni-Mg atoms provide hydrogen proper interstices. Chemical bonds of Mg2Ni are so complicated that there are not only covalent components but also metallic one as well as ionic ones. The ionicity of Mg2Ni is very small with the value of only 0.04.
Similar content being viewed by others
References
Schlapbach L., Stucki F., Seiler A., and Siegmann H.C., Magnetism and hydrogen storage in LaNi5, FeTi and Mg2Ni, Journal of Magnetism and Magnetic Materials, 1980, 15–18(3): 1271.
Takahashi Y., Yukawa H., and Morinaga M., Alloying effects on the electronic structure of Mg2Ni intermetallic hydride, Journal of Alloys and Compounds, 1996, 242(1–2): 98.
Prigent J., and Gupta M., Ab initio study of the hydrogenation properties of Mg-based binary and ternary compounds Mg2X (X = Ni, Si) and YMgNi4, Journal of Alloys and Compounds, 2007, 446–447: 90.
Michiel J.V.S., and Gilles A.D.W., Ab initio study of the effects of transition metal doping of Mg2NiH4, Physical Review B, 2007, 76: 1.
Myers W.R., Wang L.W., Richardson T.J., and Rubin M.D., Calculation of thermodynamic, electronic, and optical properties of monoclinic Mg2NiH4, Journal of applied physics, 2002, 91(8): 4879.
Zhang J., Zhou D.W., He L.P., Peng P., and Liu J.S., First-principles investigation of Mg2Ni phase and high/low temperature Mg2NiH4 complex hydrides, Journal of Physics and Chemsitry of solids, 2009, 70(1): 32.
Ma S.Y., Huang D., Zheng D.S., Xiao R.J., and Guo J., First principles calculation on Mg2Ni alloy and its hydride electronic structure and bond characteristics, The Chinese Journal of Nonferrous Metals, 2007, 17(1): 1118.
Morinaga M., Yukawa H., Nakatsuka K., and Takagi M., Roles of constituent elements and design of hydrogen storage alloys, Journal of Alloys and Compounds, 2002, 330–332: 20.
Soubeyroux J.L., Fruchart D., Mikou A., Pezat M., and Darriet B., Etude structurale du systeme Mg2Ni - H2. I - La solution solide Mg2NiHx (x = 0.30), Material Research Bulletin, 1984, 19: 895.
Yü R.H., The empirical electron theory of solids and molecules, Chinese Science Bulletin, 1978, 23(4): 217.
Pauling L., The Nature of the Chemical Bond, Cornell University Press, London, 1960.
Zhang R.L., The Empirical Electron Theory of Solids and Molecules, Ji Lin Press of Science of Technology, Changchun, 1993. 231.
Xu W.D., Zhang R.L., and Yü R.H., Calculation of cohesive energy for transitional metal compound, Science China Series A, 1988, 18(3): 323
King R.C., and Kleppa O.J., A theoretical study of some related laves phases, Acta Metallurgy, 1964, 12(1): 87.
Kittel C., Solid State Physics, John Wiley and Sons Inc, New York, 1979. 84.
Yukawa H., Takagi M., and Morinaga M., Design of hydrogen storage alloys with the aid of molecular orbital method, Bulletin of Materials Science, 1999, 22(5): 885.
Levine B.F., Bond susceptibilities and ionicities in complex crystal structures, Journal of Chemistry and Physics, 1973, 59(3): 1463.
Fan C.Z., Sun L.L., and Wei Z.J., Valence electron structure of TaC and TaN, Science China Series G, 2008, 38(2): 120.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Li, S. Valence electron structure and hydrogen storage properties analysis of Mg2Ni. Rare Metals 30 (Suppl 1), 71–76 (2011). https://doi.org/10.1007/s12598-011-0241-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-011-0241-5