Abstract
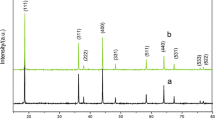
Li1.3Al0.3Ti1.7(PO4)3-coated LiMn2O4 was prepared by wet chemical route. The phase, surface morphology, and electrochemical properties of the prepared powders were characterized by X-ray diffraction, scanning electron micrograph, and galvanostatic charge-discharge experiments. Li1.3Al0.3Ti1.7(PO4)3-coated LiMn2O4 has similar X-ray diffraction patterns as LiMn2O4. The corner and border of Li1.3Al0.3Ti1.7(PO4)3-coated LiMn2O4 particles are not as clear as the uncoated one. The two powders show similar values of lithium-ion diffusion coefficient. When cycled at room temperature and 55°C for 40 times at the charge-discharge rate of 0.2C, Li1.3Al0.3Ti1.7(PO4)3-coated LiMn2O4 shows the capacity retentions of 98.2% and 93.9%, respectively, which are considerably higher than the values of 85.4% and 79.1% for the uncoated one. Both the capacity retention differences between Li1.3Al0.3Ti1.7(PO4)3-coated LiMn2O4 and LiMn2O4 cycling at room temperature and 55°C become larger with the increase of charge-discharge rate. When the charge-discharge rate reaches 2C, the capacity retention of LATP-coated LiMn2O4 becomes 8.4% higher than the uncoated LiMn2O4 for room temperature cycling, and it becomes 11.1% higher than the latter when cycled at 55°C.
Similar content being viewed by others
References
Jiang C.H., Dou S.X., Liu H.K., Ichihara M., and Zhou H.S., Synthesis of spinel LiMn2O4 nanoparticles through one-step hydrothermal reaction, J. Power Sources, 2007, 172: 410.
Cabana J., Valdes S., Palacin T., Oro-Sole M.R., Fuertes J., Marban A., and Fuertes G., Enhanced high rate performance of LiMn2O4 spinel nanoparticles synthesized by a hard-template route, J. Power Sources, 2007, 166: 492.
Zhao M.S. and Song X.P., Synthesizing kinetics and characteristics for spinel LiMn2O4 with the precursor using as lithium-ion battery cathode material, J. Power Sources, 2007, 164: 822.
Amatucci G.G., Schmutz C.N., Blyr A., Sigala C., Gozdz A.S., Larcher D., and Tarascon J.M., Materials’ effects on the elevated and room temperature performance of C/LiMn2O4 Li-ion batteries, J. Power Sources, 1997, 69: 11.
Wohlfahrt-Mehrens M., Vogler C., and Garche J., Aging mechanisms of lithium cathode materials, J. Power Sources, 2004, 127: 58.
Xia Y., Zhou Y., and Yoshio M., Capacity fading on cycling of 4 V Li/LiMn2O4 cells, J. Electrochem. Soc., 1997, 144: 2593.
Lee K.S., Myung S.T., Bang H.J., Chung S., and Sun Y.K., Co-precipitation synthesis of spherical Li1.05M0.05Mn1.9O4 (M = Ni, Mg, Al) spinel and its application for lithium secondary battery cathode, Electrochim. Acta, 2007, 52: 5201.
Eftekhari A., Moghaddam A.B., Yazdani B., and Moztarzadeh F., Effects of metal source in metal substitution of lithium manganese oxide spinel, Electrochim. Acta, 2006, 52: 1491.
Shi S., Ouyang C., Wang D.S., Chen L., and Huang X., The effect of cation doping on spinel LiMn2O4: a first-principles investigation, Solid State Commun., 2003, 126: 531.
Gnanaraj J.S., Pol V.G., Gedanken A., and Aurbach D., Improving the high-temperature performance of LiMn2O4 spinel electrodes by coating the active mass with MgO via a sonochemical method, Electrochem. Commun., 2003, 5: 940.
Ha H.W., Yun N.J., and Kim K., Improvement of electrochemical stability of LiMn2O4 by CeO2 coating for lithium-ion batteries, Electrochim. Acta, 2007, 52: 3236.
Liu H., Cheng C., Zong Q., and Zhang K., The effect of ZnO coating on LiMn2O4 cycle life in high temperature for lithium secondary batteries, Mater. Chem. Phys., 2007, 101: 276.
Wu X.M., Li X.H., Wang S.W., Wang Z., Zhang Y.H., Xu M.F., and He Z.Q., Preparation and characterization of lithium-ion-conductive Li1.3Al0.3Ti1.7(PO4)3 thin films by the solution deposition, Thin Solid Films, 2003, 425: 103.
Fu Z.W. and Qin Q.Z., Electrochemical and electrochromic properties of niobium oxide thin films fabricated by pulsed laser deposition, J. Electrochem. Soc., 1999, 146: 3914.
Jang D.H., Shin Y.J., and Oh S.M., Dissolution of spinel oxides and capacity losses in 4 V Li/LixMn2O4 cells, J. Electrochem. Soc., 1996, 143: 2204.
Aurbach D., Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries, J. Power Sources, 2000, 89: 206.
Wang E., Ofer D., Bowden W., Iltchev N., Moses R., and Brandt K., Stability of lithium ion spinel cells. III. Improved life of charged cells, J. Electrochem. Soc., 2000, 147: 4023.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Li, R., Chen, S. et al. Synthesis and characterization of Li1.3Al0.3Ti1.7(PO4)3-coated LiMn2O4 by wet chemical route. Rare Metals 28, 122–126 (2009). https://doi.org/10.1007/s12598-009-0024-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-009-0024-4