Abstract
Peripheral tissue inflammation can alter the properties of somatic sensory pathways, causing behavioral hypersensitivity and resulting in increased responses to pain caused by noxious stimulation (hyperalgesia) and normally innocuous stimulation (allodynia). These hypersensitivities for nociception are caused by changes in the excitability of trigeminal ganglion (TG) neurons. These changes alter sensory information processing in the neurons in the medullary trigeminal nucleus of caudalis. Increasing information is becoming available regarding trigeminal neuron–neuron/neuron–satellite glial cells (SGCs) communication. The activation of intraganglionic communication plays an important role in the creation and maintenance of trigeminal pathological pain. Therefore, in this review, we focus on the recent findings for sensory functions and pharmacological modulation of TG neurons and SGCs under normal and pathological conditions, and we discuss potential therapeutic targets in glia–neuronal interactions for the prevention of trigeminal neuropathic and inflammatory pain.
Similar content being viewed by others
Introduction
It is well known that trigeminal ganglion (TG) neurons are involved in various sensory functions in the orofacial region, such as non-noxious or noxious mechanical, thermal and chemical sensations [1–3]. Following the application of noxious stimuli to the orofacial region, a barrage of action potentials is generated in small-diameter primary afferent neurons, and those are conveyed to the small TG neurons [3, 4]. On the other hand, non-noxious stimuli cause action potentials in large-diameter nerve fibers [3, 5]. Various ion channel proteins and neuropeptides are up- and down-regulated following non-noxious and/or noxious stimuli under normal conditions [1–3]. In addition to these physiological functions under normal states, TG neurons are known to change in their excitability and molecular expression under pathological conditions [3–5, 11–19]. Expression of a variety of molecules in TG neurons and changes in morphological and physiological properties of these neurons are thought to be involved in orofacial sensory dysfunctions associated with trigeminal nerve injury or orofacial inflammation [4–13]. Furthermore, satellite glial cells have been known to be involved in orofacial sensory abnormalities associated with trigeminal nerve injury or orofacial inflammation [14–19]. It has recently been reported that neuron–glia interaction is an important system involved in modulation of the excitability of TG neurons [4–19]. Based on many previous data, TG neurons and satellite glial cells (SGC) are thought to have a pivotal role in exerting these orofacial sensory functions.
Many new findings regarding sensory functions of TG neurons and satellite glial cells under normal and pathological conditions have been reported in many recent papers [4–26]. In this review, we introduce basic research findings regarding morphology and physiological features of TG neurons and satellite glial cells, and potential pharmacological approach to treat trigeminal pain, focusing on following themes: (1) The α2/δ-1 subunit L-type calcium channel of dihydropyridine receptor in the TG, (2) ATP transporter, vesicular nucleotide transporter (VNUT) regulates ATP signaling in TG, (3) modulatory mechanisms of inflammatory nociceptive signals in the trigeminal ganglia, (4) involvement of intra-TG communication in ectopic orofacial pain, (5) pharmacological action of eugenol on TG neurons.
The α2/δ-1 subunit L-type calcium channel of dihydropyridine receptor in the trigeminal ganglion
L-type voltage-dependent Ca2+ channels are heteromultimeric polypeptide complexes of α1-, α2/δ-, and β-subunits [27]. The α2/δ-1-subunit possesses a stereoselective, high-affinity binding site for gabapentin, widely used to treat epilepsy and postherpetic neuralgic pain [27]. We studied immunohistochemical analysis for α2/δ-1 subunit of L-type calcium channel on the rat TG neurons. The immunoreactivity (IR) was detected in one-third of TG neurons. A double immunofluorescence method revealed that half of α2/δ-1-immunoreactive (IR) neurons were also immunoreactive for calcitonin gene-related peptide. In addition, α2/δ-1-IR sensory neurons which contained transient receptor potential vanilloid 1 (TRPV1) were abundant. However, co-expression of α2/δ-1 with TRPV2 was infrequent in the TG. A retrograde tracing method also demonstrated that α2/δ-1-IR was common among cutaneous TG neurons and relatively rare among tooth pulp TG neurons (Fig. 1). Transection of the infraorbital nerve dramatically increased the number of α2/δ-1 subunit-IR neurons in the TG. Taken together, our findings suggest that primary nociceptors with unmyelinated axons contain a α2/δ-1 subunit of L-type calcium channel in the TG. These results suggest that the subunit in TG neurons may be associated with orofacial nociceptive transmission via augmented neurotransmitter release from trigeminal nerve terminal and/or cell soma under neuropathic condition.
Vesicular nucleotide transporter (VNUT) regulates ATP signaling in trigeminal ganglion
Although no synaptic transmission has been found in the primary sensory ganglia, it has been discovered that the activity of neighboring neurons elicits a functional cross-excitation in the somata of affected sensory neurons under normal conditions, indicating that non-synaptically released diffusible chemical messengers (ATP, substance P, cytokine) modify the neuronal excitability of the sensory ganglia [3, 28]. Anatomically, the SGCs form a distinct sheath around nearly all individual sensory neurons in the TG neurons and are associated with the transport and metabolism of various molecules, such as glutamate and glucose, and maintain ionic homeostasis, including extracellular potassium [19, 29]. Recent study focused on ATP as a neurotransmitter, and investigated the association of the VNUT ATP transporter with neurons and SGCs in TG using immunocytochemistry, reverse transcription polymerase chain reaction (RT-PCR), and in situ hybridization (ISH) in rats after rat upper molar extraction. We found the results as follows: after extraction, the activating transcription factor (ATF) 3-IR neurons appeared in the maxillary nerve region; ATF3-IR was damaged, neurons were surrounded by GFAP-IR, and active SGCs. Interestingly, ATF3 immunonegative neurons were also surrounded by GFAP-IR SGCs. The number of GFAP-IR SGCs and P2X3 receptor (P2X3R)-IR neurons was increased after extraction in a time-dependent manner. RT-PCR and ISH confirmed the expression of VNUT mRNA expression in neurons and SGCs in TG. Therefore, the results suggest that peripheral nerve injury induced the mutual activation of TG neuron and SGCs, possibly by VNUT-mediated ATP release (Fig. 2). VNUT may play an important role in the communication between neurons and satellite glial cells located at a distant area in the TG.
Modulatory mechanisms of inflammatory nociceptive signals in the trigeminal ganglia
Peripheral tissue inflammation can alter the properties of somatic sensory pathways, causing behavioral hypersensitivity and resulting in increased responses to pain caused by noxious stimulation (hyperalgesia) and normally innocuous stimulation (allodynia). We recently investigated possible mechanisms underlying mechanical allodynia/neuronal changes in sensitivity at sites remote from the temporomandibular joint (TMJ) inflammation by using behavioral, electrophysiological, immunohistochemical and molecular approaches [5, 11–13]. Both in vitro and in vivo studies revealed that, under TMJ inflammation, substance P (SP) released from the Aδ-/C-TG neuronal soma (nociceptive) innervating inflamed TMJ via the paracrine mechanism play an important role in the modification of the excitability of Aβ-TG neurons (non-nociceptive) innervating intact facial skin with up-regulated neurokinin 1 (NK1) receptors [5–12]. The enhanced excitability of TMJ nociceptive TG neurons was mainly due to the suppressing A-type voltage-gated potassium currents via a hyperpolarizing shift in the inactivation curve [13]. These results suggest that the paracrine mechanism via the SP-NK1 receptor in the trigeminal ganglia may explain the development of ectopic inflammatory mechanical allodynia without sprouting non-nociceptive fibers in the medullary dorsal horn. Peripheral inflammation also up-regulates NK1 receptor expression in satellite glial cells and SP stimulates the production of interleukin (IL)-1β in glial cells [19]. This may potentiate the synthesis and/or release of IL-1β via paracrine mechanisms in satellite glial cells [17, 18]. It is therefore possible that IL-1β suppresses voltage-gated potassium currents in TG neurons via protein kinase C/G-protein-coupled signaling pathways, and that this contributes to the potentiation of the neuronal excitability of TG neurons innervating the inflamed tissue [19]. It can be assumed that the amplified discharge frequency of Aδ TG neurons contributes to the sensitization of secondary neurons and the development of hyperalgesia [19]. Since we also obtained the evidence that early stages of blockade of NK1 receptor within the trigeminal ganglia can attenuate sensitization of trigeminal subnucleus caudalis neurons [3], these results clearly indicated that early stages of suppression of peripheral sensitization contribute to prevent central sensitization. Therefore, the modulation of the neuronal signal by cross-talk in the trigeminal ganglia contributes to inflammatory pain, and is a potential therapeutic target for the prevention of allodynia/hyperalgesia.
Involvement of intra-trigeminal ganglionic communication in ectopic orofacial pain
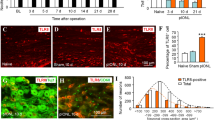
Pathological orofacial pain which spreads to a wide area in the trigeminal territory occurs with orofacial inflammation or trigeminal nerve injury. However, the peripheral mechanisms underlying such ectopic orofacial pain remain unclear. We investigated the involvement of intra-TG communication in ectopic orofacial pain via nitric oxide (NO), nerve growth factor (NGF) or calcitonin gene-related peptide (CGRP) following orofacial inflammation or trigeminal nerve injury [7–9]. Heat or mechanical hypersensitivity was induced in the ipsilateral whisker pad skin following inferior alveolar nerve transection or lower lip inflammation, and suppressed by TRPV1 or P2X3R antagonism, respectively [8]. Neuronal nitric oxide synthase (nNOS), NGF and CGRP expression in the TG was increased following lower lip inflammation [8]. Moreover, intra-trigeminal administration of tyrosine kinase receptor or nNOS inhibitor diminished the heat or mechanical hypersensitivity [9]. The lower lip inflammation increased the number of P2X3R- and TRPV1-positive TG neurons that innervate the whisker pad skin, which was attenuated by anti-NGF intra-TG administration [7–9]. The present findings suggest that intra-TG communication via NO, NGF or CGRP signaling resulted in up-regulation and/or sensitization of TRPV1 or P2X3R in TG neurons following orofacial inflammation or trigeminal nerve injury, which may develop ectopic orofacial pain.
Pharmacological action of eugenol on trigeminal ganglion neurons
It is well known that eugenol, an active ingredient of essential oil extracted from cloves and other herbs, is used extensively in dentistry as an analgesic. However, the molecular mechanism underlying the analgesic activity of eugenol is mostly unknown. A series of investigations have revealed that eugenol modulates various ion channels that are responsible for nociception, generation of neuronal spikes, and synaptic transmission in the trigeminal system [20–26]. Pharmacological action of eugenol includes inhibition of action potential firing by reducing voltage-gated sodium, calcium and potassium channels in TG neurons [23–25]. In addition, eugenol inhibits hyperpolarization-activated cyclic nucleotide-gated (HCN) channels that play a crucial role in mechanical allodynia in neuropathic pain state [21]. Concurrently, eugenol successfully reversed mechanical allodynia in an experimental trigeminal neuropathic pain animal, at much smaller dose than it blocks sodium channels [21]. Modulation of P2X3R, an ionotropic ATP receptor, might be another mechanism by which eugenol exerts its analgesic activity [22]. Activation of TRPV1 and TRP ankyrin 1 (TRPA1) by eugenol might also contribute to the analgesic effect, since pharmacological activation of TRPV1 and TRPA1 has been shown to produce biphasic action of the initial pungent and sustained inhibition of nociception [20, 26]. In conclusion, eugenol, a natural compound that displays a number of pharmacological actions for affecting the nociceptive transduction and transmission mechanisms, could have a therapeutic potential for versatile analgesic applications under pathological conditions in the trigeminal system.
Conclusions
In this review, we summarize the recent findings for sensory functions of TG neurons and SGC cells under normal and pathological conditions (see Fig. 3).
Schematic representation for a possible molecular mechanism involved in the trigeminal nociceptive and pathological pain. a Trigeminal nociceptive pain. Eugenol inhibits the generator potential by acting transient receptor potential ankyrin 1 (TRPA1) and P2X 3 receptor. Also eugenol suppress the action potential firing by reducing voltage-gated sodium (Nav), calcium (Cav) and potassium (Kv) channels and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels of the small-diameter TG neurons. Up arrows excitation, down arrows inhibition. b Trigeminal pathological pain. Possible molecular mechanism for a activation of trigeminal neuron–neuron/neuron–satellite glial cells contributes to pathological pain (ectopic allodynia and hyperalgesia) following orofacial inflammation/trigeminal nerve injury. Interaction between neuron and satellite glial cells via substance P (SP), adenosine triphosphate (ATP), and interleukin-1β (IL-1β) signaling resulted in augementation of neuronal firing in small-diameter TG neurons following orofacial inflammation or trigeminal nerve injury, which may develop hyperalgesia. Neuron–neuron communication via SP, ATP, nerve growth factor (NGF), and nitric oxicide (NO) signaling resulted in the activation of neighboring neuronal firing in medium-/large-diameter TG neurons following orofacial inflammation or trigeminal nerve injury, which may develop ectopic allodynia pain. TRPV1 transient receptor potential vanilloid 1, IL-1RI IL-receptor type I, TrkA tyrosine kinase A, NK1R neruokinine 1 receptor, VNUT vesicular nucleotide transporter. Up arrows up-regulation, down arrows down-regulation
-
Eugenol modulates various ion channels that are responsible for nociception, generation of neuronal spikes, and synaptic transmission in the trigeminal system and displays a number of pharmacological properties with therapeutic potential for versatile analgesic applications (Fig. 3a).
-
The paracrine mechanism via SP-NK1 receptor and IL-1β in satellite glial cells may contribute to the development of ectopic inflammatory mechanical allodynia/hyperalgesia. The modulation of the ganglionic neuron–glial signaling may contribute to potential therapeutic target for the prevention of pathological pain (Fig. 3b).
-
Intra-TG communication via NO, NGF or CGRP signaling resulted in up-regulation and/or sensitization of TRPV1 or P2X3R in TG neurons following orofacial inflammation or trigeminal nerve injury, which may develop ectopic orofacial pain (Fig. 3b).
-
The α2/δ-1 subunit of the L-type calcium channel in the TG neuron may be associated with orofacial nociceptive transmission via augmented neurotransmitter release from the trigeminal nerve terminal and/or cell soma under neuropathic condition.
-
Peripheral nerve injury induced the mutual activation of TG neurons and SGCs modulate ATP transportor, VNUT-related ATP release (Fig. 3b).
References
Andrea M, Harriot BS, Gold MS (2009) Contribution of primary afferent channels to neuropathic pain. Curr Pain Headache Rep 13:197–207
Sessle BJ (2005) Peripheral and central mechanism of orofacial pain and their clinical correlates. Minerva Anesthsiol 71:117–136
Takeda M, Matsumoto S, Sessle BJ, Shinoda M, Iwata K (2011) Peripheral and central mechanisms of trigeminal neuropathic and inflammatory pain. J Oral Biosci 53:318–329
Nakagawa K, Takeda M, Tsuboi Y, Kondo M, Kitagawa J, Matsumoto S, Kobayashi A, Sessle BJ, Shinoda M, Iwata K (2010) Alteration of primary afferent activity following inferior alveolar nerve transection in rats. Mol Pain 6:9
Takeda M, Tanimoto T, Nasu M, Ikeda M, Kadoi J, Matsumoto S (2010) Activation of NK1 receptor of trigeminal root ganglion via substance P paracrine mechanism contributes to the mechanical allodynia in the temporomandibular joint inflammation in rats. Pain 116:375–385
Gunjigake KK, Goto T, Nakao K, Kono T, Kobayashi S, Yamaguchi K (2006) Correlation between the appearance of neuropeptides in the rat trigeminal ganglion and reinnervation of the healing root socket after tooth extraction. Acta Histochem Cytochem 39:69–77
Shinoda M, Asano M, Omagari D, Honda K, Hitomi S, Katagiri A, Iwata K (2011) Nerve growth factor contribution via transient receptor potential vanilloid 1 to ectopic orofacial pain. J Neurosci 31:7145–7155
Yasuda M, Shinoda M, Kiyomoto M, Honda K, Suzuki A, Tamagawa T, Kaji K, Kimoto S, Iwata K (2012) P2X3 receptor mediates ectopic mechanical allodynia with inflamed lower lip in mice. Neurosci Lett 528:67–72
Sugiyama T, Shinoda M, Watase T, Honda K, Ito R, Kaji K, Urata K, Lee J, Ohara K, Takahashi O, Echizenya S, Iwata K (2013) Nitric oxide signaling contributes to ectopic orofacial neuropathic pain. J Dent Res 92:1113–1117
Adachi K, Shimizu K, Hu JW, Suzuki I, Sakagami H, Koshikawa N, Sessle BJ, Shinoda M, Miyamoto M, Honda K, Iwata K (2010) Purinergic receptors are involved in tooth-pulp evoked nocifensive behavior and brainstem neuronal activity. Mol Pain 6:59
Takeda M, Tanimoto T, Ikeda M, Nasu M, Kadoi J, Shima Y, Ohta H, Matsumoto S (2005) Temporomandibular joint inflammation potentiates the excitability of trigeminal root ganglion neurons innervating the facial skin in rats. J Neurophysiol 93:2723–2738
Takeda M, Tanimoto T, Nasu M, Matsumoto S (2008) Temporomandibular joint inflammation decreases the voltage-gated K+ channel subtype 1.4-immunoreactivity of trigeminal ganglion neurons in rats. Eur J Pain 12:189–195
Takeda M, Tanimoto T, Ikeda M, Nasu M, Kadoi J, Yoshida S, Matsumoto S (2006) Enhanced excitability of rat trigeminal root ganglion neurons via decrease in A-type potassium currents following temporomandibular joint inflammation. Neuroscience 138:621–630
Takeda M, Takahashi M, Nasu M, Matsumoto S (2011) Peripheral inflammation suppresses inward rectifying potassium currents of satellite glial cells in the trigeminal ganglia. Pain 152:2147–2156
Gunjigake KK, Goto T, Nakao K, Kobayashi S, Yamaguchi K (2009) Activation of satellite glial cells in rat trigeminal ganglion after upper molar extraction. Acta Histochem Cytochem 42:143–149
Katagiri A, Shinoda M, Honda K, Toyofuku A, Sessle BJ, Iwata K (2013) Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol Pain 8:23
Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, Matsumoto S (2007) Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain 129:155–166
Takeda M, Takahashi M, Matsumoto S (2008) Contribution of activated interleukin receptors in trigeminal ganglion neurons to hyperalgesia via satellite glial interleukin-1beta paracrine mechanism. Brain Behav Immun 22:1016–1023
Takeda M, Takahashi M, Matsumoto S (2009) Contribution of the satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev 33:784–792
Chung G, Im ST, Kim YH, Jung SJ, Rhyu MR, Oh SB (2014) Activation of transient receptor potential ankyrin 1 by eugenol. Neuroscience 261:153–160
Yeon KY, Chung G, Kim YH, Hwang JH, Davies AJ, Park MK, Ahn DK, Kim JS, Jung SJ, Oh SB (2011) Eugenol reverses mechanical allodynia after peripheral nerve injury by inhibiting hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Pain 152:2108–2116
Li HY, Lee BK, Kim JS, Jung SJ, Oh SB (2008) Eugenol inhibits ATP-induced P2X currents in trigeminal ganglion neurons. Korean J Physiol Pharmacol 12:315–321
Li HY, Park CK, Jung SJ, Choi SY, Lee SJ, Park K, Kim JS, Oh SB (2007) Eugenol inhibits K+ currents in trigeminal ganglion neurons. J Dent Res 86:898–902
Park CK, Li HY, Yeon KY, Jung SJ, Choi SY, Lee SJ, Lee S, Park K, Kim JS, Oh SB (2006) Eugenol inhibits sodium currents in dental afferent neurons. J Dent Res 85:900–904
Lee MH, Yeon KY, Park CK, Li HY, Fang Z, Kim MS, Choi SY, Lee SJ, Lee S, Park K, Lee JH, Kim JS, Oh SB (2005) Eugenol inhibits calcium currents in dental afferent neurons. J Dent Res 84:848–851
Yang BH, Piao ZG, Kim YB, Lee CH, Lee JK, Park K, Kim JS, Oh SB (2003) Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res 82:781–785
Fuller-Bicer GA, Varadi G, Koch SE, Ishii M, Bodi I, Kadeer N, Muth JN, Mikala G, Petrashevskaya NN, Jordan MA, Zhang SP, Qin N, Flores CM, Isaacsohn I, Varadi M, Mori Y, Jones WK, Schwartz A (2009) Targeted disruption of the voltage-dependent calcium channel alpha2/delta-1-subunit. Am J Physiol Heart Circ Physiol 297:H117–H124
Amir R, Devor M (2000) Functional cross-excitation between afferent A- and C-neurons in dorsal root ganglia. Neuroscience 95:189–195
Hanani M (2005) Satellite glial cells in sensory ganglia: from form to function. Brain Res Rev 48:457–476
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Goto, T., Oh, S.B., Takeda, M. et al. Recent advances in basic research on the trigeminal ganglion. J Physiol Sci 66, 381–386 (2016). https://doi.org/10.1007/s12576-016-0448-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-016-0448-1