Abstract
Background
To evaluate whether P2X receptors are involved in responses to noxious pulp stimulation, the P2X3 and P2X2/3 receptor agonist α,β-methyleneATP (α,β-meATP) was applied to the molar tooth pulp and nocifensive behavior and extracellular-signal regulated kinase (ERK) phosphorylation in trigeminal spinal subnucleus caudalis (Vc), trigeminal spinal subnucleus interpolaris (Vi), upper cervical spinal cord (C1/C2) and paratrigeminal nucleus (Pa5) neurons were analyzed in rats.
Results
Genioglossus (GG) muscle activity was evoked by pulpal application of 100 mM α,β-meATP and was significantly larger than GG activity following vehicle (phosphate-buffered saline PBS) application (p < 0.01). The enhanced GG muscle activity following 100 mM α,β-meATP was significantly reduced (p < 0.05) by co-application of 1 mM TNP-ATP (P2X1, P2X3 and, P2X2/3 antagonist). A large number of pERK-LI cells were expressed in the Vc, Vi/Vc, C1/C2 and Pa5 at 5 min following pulpal application of 100 mM α,β-meATP compared to PBS application to the pulp (p < 0.05). The pERK-LI cell expression and GG muscle activity induced by 100 mM α,β-meATP pulpal application were significantly reduced after intrathecal injection of the MAPK/ERK kinase (MEK) inhibitor PD 98059 and by pulpal co-application of 1 mM TNP-ATP (p < 0.05).
Conclusions
The present findings suggest that activation of P2X3 and P2X2/3 receptors in the tooth pulp is sufficient to elicit nociceptive behavioral responses and trigeminal brainstem neuronal activity.
Similar content being viewed by others
Background
Adenosine 5'-triphosphate (ATP) is considered a neuro-modulator in primary afferent neurons. Release of ATP from sympathetic nerve terminals, endothelial, Merkel or tumor cells is known to be involved in excitation of unmyelinated primary afferent neurons [1]. One of ATP receptors activated by ATP binding is the P2X family of ATP receptors; it has been classified into seven subtypes, P2X1-7 (for review, see [2–8]). All of them, except the P2X7 receptor, are expressed in various primary sensory neurons including tooth pulp neurons [9–13]. In particular, the P2X3 homomeric and P2X2/3 heteromeric receptors have been associated with peripheral nociceptive mechanisms, since these subtypes occur in a subset of putative nociceptive sensory neurons [10–12, 14–16], and their activation produces nocifensive behavior that can be attenuated by peripheral [17–20] or central [16, 21] administration of P2X3,2/3 receptor antagonists. Also activation of pulpal P2X3,2/3 receptors produces central sensitization in functionally identified nociceptive brainstem neurons in the trigeminal subnucleus caudalis (Vc) [16, 22]. Pulpal administration of capsaicin, mustard oil or other inflammatory substances are known to strongly activate Vc neurons suggesting that TRPV1 and TRPA1 receptors or other receptors related to inflammation are involved in tooth pulp pain [23, 24]. It is very important to know which receptors in the pulpal nerve terminals are involved and how these receptors mediate tooth pulpal pain, in order to understand the neuronal mechanisms of tooth pulp inflammatory pain. ATP is known as one of the important neuro-modulators involved in tooth pulp inflammatory pain [9–12]. However, how ATP is involved in pulpal pain during inflammation remains unclear. We thus introduced α,β-meATP as a P2X2/3 receptor agonist to exclude the effect of substances other than ATP.
The phosphorylated extracellular signal-regulated kinase (pERK), one of the mitogen-activated protein kinases (MAPKs), has been recognized as a marker of activation of spinal dorsal horn (DH) neurons following a variety of noxious stimuli applied to peripheral tissues [25, 26]. ERK can be phosphorylated in Vc and upper cervical spinal cord (C1/C2) neurons within 10 min following peripheral noxious stimulation, and the number of pERK-positive neurons progressively increases as stimulus intensity is increased. We have recently reported that pERK-positive neurons are expressed in Vc and the Vc/C1 transition region as well as in the transition zone between the trigeminal spinal subnucleus interpolaris (Vi) and Vc (Vi/Vc) following noxious stimulation of orofacial regions including the tooth pulp [23, 27, 28]. These findings suggest that the phosphorylation of ERK is strongly correlated with excitation of Vi/Vc, Vc and C1/C2 neurons following noxious stimulation of the orofacial region.
Given these various findings, it is of interest to study the distribution pattern of Vi/Vc, Vc and C1/C2 nociceptive neurons that can be activated by specific stimulation of tooth pulp purinergic receptors, in order to clarify the involvement of these receptors in tooth pulp-induced V central sensitization. However, the distribution pattern of the Vc, Vi/Vc and C1/C2 neurons sensitized by the activation of tooth pulp purinergic receptors is unknown. Therefore, we have investigated the effects of pulpal application of purinergic receptor agonist on the expression of pERK in Vi/Vc, Vc and C1/C2 neurons and if their activation is associated with nocifensive behavior reflected as an increase in electromyographic (EMG) activity of masticatory muscles. Some of the data have been published in abstract form [29].
Methods
Animals
Seventy-five male Sprague-Dawley adult rats (300-360 g) were used in this study. The rats were housed in individual cages (27 × 45 × 20 cm) in a temperature-controlled (21 ± 1°C) and humidity-controlled (50 ± 5%) room under a 12 hr light/dark cycle (lights on at 07:00 am) with free access to food and water. All procedures were approved by the University of Toronto Animal Care Committee in accordance with the regulations of the Ontario Animal Research Act (Canada) and animal experimentation committee in Nihon University School of Dentistry.
Drugs
The drugs used were α,β-methylene adenosine 5'-triphosphate, (α,β-meATP, Sigma-Aldrich, St. Louis, MO, USA), a P2X1, P2X3 and, P2X2/3 agonist, and 2',3'-O-(2,4,6-trinitrophenyl) adenosine 5'-triphosphate (TNP-ATP, Sigma-Ardrich), a P2X1, P2X3 and, P2X2/3 antagonist; both were dissolved in 1 mM phosphate-buffered saline (PBS; pH = 7.4). The agonist and antagonist (or vehicle PBS) were cocktailed for combined application. The solution (approximately 0.2 μl) was applied to the tooth pulp by a soaked dental paper point. The mitogen-activated extracellular signal-regulated kinase (MEK) inhibitor PD 98059 (Calbiochem, La Jolla, CA, USA) was dissolved into 10% Dimethyl sulfoxide (DMSO) and the solution placed into a mini-osmotic pump (model 2001, Durect Co. Cupertino, CA, USA) that was implanted under the skin of rat neck, so that PD 98059 could be continuously (1 μl/h) diffused intrathecally for one week before EMG and/or immunohistochemical experiment [27, 30, 31].
Electromyographic (EMG) recording
Anesthesia was maintained by 1-2% halothane (Halocarbon Products Corp., River Edge, NJ, USA) for the implantation of bipolar EMG electrode wires and for preparation of an occlusal cavity in the right maxillary first molar [23]. During the period of the subsequent experiment, the concentration of halothane was reduced to < 0.9%. Body temperature was maintained at 37-38°C by a feedback-controlled blanket (Model 73A, YSI, Yellow Springs, OH, USA). Heart rate was continuously monitored and maintained at physiological levels of 330-430 beats/min. Pairs of EMG electrodes (40-gauge, Teflon®-insulated stainless wires; Cooner wire, Chatsworth, CA, USA) were implanted into the right masseter (MA), anterior digastric (AD) and genioglossus (GG) muscles to record any tooth pulp-evoked muscle activities (Fig. 1 and Table 1), as previously described [24]. The placement of EMG electrodes was confirmed by muscle twitches induced by electrical stimulation of the EMG electrodes (12 × 0.2 ms pulses, 333 Hz, 100-200 μA).
GG muscle EMG activity induced by pulpal application of α⊠β-meATP. A: GG muscle EMG activity induced by pulpal application of α,β-meATP (100 mM). The arrow indicates the timing for drug application. Aa: Onset latency was determined from the time lag between the drug application and onset of EMG activity above 2 SDs. B: Relative area under curve of genioglossus muscle EMG activity induced by pulpal application of α,β-meATP. The data were obtained during 1 min after pulpal application of α,β-meATP (0, 10 and 100 mM). Data were analyzed using one-way ANOVA with repeated measures followed by Bonferroni multiple comparison test (**: P < 0.01).
The occlusal cavity (< 0.7 mm in diameter and 0.6-0.8 mm in depth) in the molar tooth pulp was prepared by a dental drill (at low speed) carrying a carbide bur (0.5 mm in diameter). During drilling, the molar occlusal surface was cooled by a cotton pellet soaked with cool saline. Then, the cavity was immediately filled with a small piece of cotton pellet soaked with isotonic saline. Any exposed pulp manifesting intense bleeding was considered to be severely injured and was excluded from the subsequent experiments. To allow for unrestricted orofacial muscle movements, the rat's head was held upright, with the rat in the prone position, by means of metal rods which were fixed on the skull with stainless-steel screws and dental acrylic. The mandible could thus be gently pulled down to obtain enough space to access the maxillary molar, so that prior to drug application the saline soaked cotton pellet could be removed from the cavity and a segment of dental paper point soaked with drugs or vehicle could be applied into the tooth pulp through the cavity and filled with dental temporary cement (Cavit®, 3 M, St. Paul, MN, USA) [16, 24]. Either 10 mM (n = 2) or 100 mM (n = 8) α,β-meATP, 1 mM TNP-ATP (n = 2), 100 mM α,β-meATP with 0.1 mM TNP-ATP (n = 3), 100 mM α,β-meATP with 1 mM TNP-ATP (n = 3) or PBS (n = 5) was applied to the cavity. Since two of rats in each group were received drug application to both side of maxillary first molar, respectively, the sample number of each group for data analysis was 10 (n = 4) or 100 (n = 10) mM α,β-meATP, 1 mM TNP-ATP (n = 4), 100 mM α,β-meATP with 0.1 mM TNP-ATP (n = 5), 100 mM α,β-meATP with 1 mM TNP-ATP (n = 5) and PBS (n = 7) (Table 1). An additional 6 rats pretreated with PD 98059 also received 100 mM α,β-meATP. Muscle activities were continuously recorded before, during and after PBS or α,β-meATP administration to the pulp, and the mean value of EMG activity was calculated at every 1 min before and after drug application.
In order to test whether the pulpal application of the P2X3 and P2X2/3 receptor agonist α,β-meATP causes an increase in the EMG activity in MA, AD or GG under our anesthetic condition, noxious mechanical stimulus was applied to the facial skin and the threshold intensity for evoking EMG activity in each muscle was measured in another 6 rats. Animals were placed in a stereotaxic apparatus immediately after EMG wire implantation and filaments of varying forces were applied to the surface of the left temporomandibular joint (TMJ) region in ascending order. Since it has reported that the TMJ is an appropriate region for stimulation to determine the reflex threshold (reflex sensitivity) of each orofacial muscle [32, 33]. Each filament was applied for a maximum of 10 s and for 5 stimulus trials. When the EMG activity induced in each muscle by such stimulation exceeded mean baseline amplitude over +2SD after drug application was considered to be positive response and the force which induced 3 positive responses of 5 trials was defined as the threshold for that muscle.
As previously described [34, 35], the EMG activity was amplified (gain 1000×, filtered [bandpass 100-5 kHz]; model 1700, A-M systems, Carlsborg, WA, USA) and digitized (10 × 103 samples/s) by an A/D converter and Spike2 software (CED 1401 plus, Cambridge Electronic Design, Cambridge, UK) with a PC and continuously recorded for more than 40 min during mechanical stimulation or application of drugs, then was processed off-line analysis. For each contralateral muscle of pulpal drug(s) application, EMG activities were rectified and digitally smoothed (moving average, 4-ms window). The mean baseline activity was obtained from the 10 ms period before drug application. When the EMG activity exceeded mean baseline amplitude over +2SD after drug application, it was defined as a positive response and its onset latency and duration were determined. The area under the curve (AUC) of EMG activity was measured for 10 min before and after drug application (Fig. 1 and Table 1).
pERK immunohistochemistry
For immunohistochemical experiments, 42 rats were divided to 7 groups (n = 6/group). Under general anesthesia by 1-2% halothane, the pulp of the right maxillary first molar was exposed, and drugs (PBS, 100 mM α,β-meATP, 1 mM TNP-ATP and 1 mM TNP-ATP + 100 mM α,β-meATP) were applied to the cavity as described above. To test whether jaw opening procedure caused an activation of TMJ noxious afferents affecting Vc neuronal excitability lidocaine (50 μl) was injected into the ipsilateral TMJ capsule in the halothane-anesthetized rats. Thirty min after TMJ injections, rats received 100 mM α,β-meATP pulpal application. After appropriate survival times (5 min), rats were perfused transcardially with 500 ml 0.9% saline followed by 500 ml 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The same procedure was carried out on the animals which received infusion pump implantation one week before and those were received 100 mM α,β-meATP pulpal application. The whole brain including medulla and upper cervical cord was removed and post-fixed in the same fixative for three days at 4°C. The tissues were then transferred to 20% sucrose (w/v) in PBS for several days for cryoprotection. Thirty-micron-thick sections were cut from the brain stem including trigeminal spinal subnucleus caudalis and upper cervical spinal cord with a freezing microtome, and every 4th section was collected in PBS. Free-floating tissue sections were rinsed in PBS, 10% normal goat serum in PBS for 1 h, and then incubated in rabbit anti-phospho-p44/42 MAP kinase antibody (1:1000, Cell Signaling Technology Inc., Danvers, MA, USA) for 72 h at 4°C. Next, the sections were incubated in biotinylated goat anti-rabbit IgG (1:600; Vector Labs, Burlingame, CA, USA) for 2 h at room temperature. After washing, the sections were incubated in peroxidase-conjugated avidin-biotin complex (1:100; Vector Labs) for 2 h at room temperature. After washing in 0.05 M Tris buffer (TB), the sections were incubated in 0.035% 3,3'-diaminobenzidine-tetra HCl (DAB, Sigma-Ardrich), 0.2% nickel ammonium sulfate and 0.05% peroxide in 0.05 M TB (pH 7.4). The sections were washed in PBS, serially mounted on gelatin-coated slides, dehydrated in alcohols and cover slipped. The pERK-LI cells were drawn 1 or more investigators under blind design to avoid inter experimenter error under a light microscope using camera-lucida drawing tube. The number of pERK-LI cells was counted from every eighth section. The total number of pERK-LI cells from three of every section was calculated, and the mean number of pERK-LI cells (three sections/rat) was obtained from each animal in order to avoid the variability of the number of immunoreactive neurons in each section. Double-immunofluorescence histochemistry was also used to determine whether the pERK-LI cells expressed a neuronal label NeuN in the Vc and C1-C2 of 100 mM α,β-meATP-treated rats.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance followed by Bonferroni multiple comparison tests. Student's and Paired t-test were used for comparison between two groups when appropriate. Differences were considered significant at P < 0.05. Results are presented as mean ± SEM.
Results
EMG activities following pulpal P2X receptor stimulation
A number of studies have reported that the transient increase in the MA and AD muscle activities can be recorded following noxious stimulation of the orofacial region in anesthetized rats [23, 24, 36, 37]. To define threshold intensity to evoke EMG activity in MA, AD or GG muscles under the present anesthetic condition, noxious mechanical stimulus was applied to the facial skin to measure mechanical threshold evoking EMG activity in MA, AD and GG muscles. The mechanical threshold evoking EMG activity was significantly lower in GG compared with AD and MA (GG: 54.3 ± 5.7 g, AD: 126.7 ± 39.6 g, MA: 166.7 ± 33.7 g, respectively, F(2,15) = 4.15, P < 0.05; ANOVA followed by Bonferroni multiple t-test). Incidence, onset latency and duration of GG, AD and MA activity following pulpal application of α,β-meATP and/or TNP-ATP are shown in Table 1. Consistent EMG activity could only be recorded in GG following pulpal application of 100 mM α,β-meATP (Table 1). Thus, GG activity was analyzed as the indicator of the reflex response following stimulation of the tooth pulp in the present study.
Typical GG muscle activity and mean EMG activity are illustrated in Fig. 1A. A rapid and significant increase in EMG activity could be recorded in GG (2.15 + 0.53 s) after 100 mM α,β-meATP application, but not 10 mM α,β-meATP, application compared to PBS administration (F(2,20) = 14.09, P < 0.01, ANOVA followed by Bonferroni multiple t-test) (Table 1, Fig. 1A, B).
pERK-LI neurons following P2X receptor stimulation
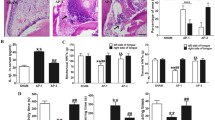
A number of pERK-like immunoreactive (LI) cells were expressed in Vc, Vi/Vc, C1/C2, paratrigeminal nucleus (Pa5), caudal ventral reticular nucleus (CVR) and nucleus tractus solitarii (NTS) 5 min after PBS or 100 mM α,β-meATP application into the right maxillary first molar pulp (Fig. 2A). The pERK-LI cells showed round soma and many fibers distributed around the soma as illustrated in Fig. 2Ba; all of them showed NeuN immunoreactivity (e.g. as shown by the arrows in Fig. 2Bb, Bc and 2Bd), suggesting that all pERK-LI cells observed in the present study could be classified as neurons. The rostro-caudal distribution of pERK-LI cells and the mean number of pERK-LI cells are illustrated in Fig. 3. In all of these areas, pERK-LI cells were observed in both ipsilateral and contralateral sides to the α,β-meATP application to the pulp. The number of pERK-LI cells was larger in Vc and C1/C2 regions compare to those in the contralateral side to injection. The pERK-LI cells were distributed in Vc and C1/C2 rostro-caudally, with a peak at about 0.0-0.7 mm caudal to the obex (Fig. 3A). At the obex level, many pERK-LI cells were observed from the dorsal to ventral portion of the Vc. On the other hand, there were no obvious peaks in the rostro-caudal distribution of pERK-LI cells in Pa5 (Fig. 3B). While pERK-LI cells were also expressed after PBS injection into the tooth pulp, pulpal application of 100 mM α,β-meATP produced a significantly larger number of pERK-LI cells in the Vc and Pa5 compared to PBS-injected rats (Vc ipsi: F(3, 23) = 20.08, P < 0.01; Vc cont: F(3, 23) = 11.70, P < 0.01; Pa5 ipsi: F(3, 23) = 7.49, P < 0.01; Pa5 cont: F(3, 23) = 3.93, P < 0.05, ANOVA; Vc ipsi, Vc cont and Pa5 ipsi: P < 0.01, respectively, Bonferroni multiple comparison test, Fig. 3C and 3D).
pERK-LI cells in the bilateral medulla and upper cervical cord following pulpal administration of PBS or α, β-meATP. A: Camera-lucida drawings of pERK-LI cells in the ipsilateral (ipsi) and contralateral (cont) medulla and upper cervical cord following pulpal administration of PBS or α,β-meATP (100 mM). Vc; trigeminal spinal subnucleus caudalis. NTS; nucleus tractus solitarii. CVR; caudal ventral reticular nucleus. Ba: Photomicrograph of pERK-LI cells in Vc. Bb: NeuN-Li cells (red) in Vc, Bc: pERK-Li cells (green) in Vc, Bd: Double labeling of pERK (green) and NeuN (red) in Vc. Arrows indicate both NeuN and pERK double-labeled cells in Vc.
Effect of TNP-ATP on mean number of pERK-LI cells in the bilateral medulla and upper cervical cord following pulpal α ,β-meATP administratio n. Mean number and rostro-caudal distribution of pERK-LI cells in ipsilateral (ipsi) and contralateral (cont) Vc and C1/C2 (A), and Pa5 (C) region induced by pulpal drug application (PBS, α,β-meATP and α,β-meATP +TNP-ATP). Mean number of pERK-LI cells in ipsilateral and contralateral Vc and C1/C2 (B), and Pa5 (D) region induced by pulpal drug application (PBS, α,β-meATP and α,β-meATP +TNP-ATP) or α,β-meATP application following lidocaine injection into TMJ. Data were analyzed using one-way ANOVA with repeated measures followed by Bonferroni multiple comparison test (*: P < 0.05, **: P < 0.01).
In order to elucidate whether the sensory input from the TMJ could have influenced the Vc and C1/C2 neuronal activity since the mouth had to be widely opened to allow access to the pulp, we also analyzed pERK-LI cell expression in Vc and Pa5 following lidocaine injection into the TMJ region bilaterally. No differences were observed in the number of pERK-LI cells in Vc and Pa5 between TMJ-anesthetized rats and intact rats following 100 mM α,β-meATP into the tooth pulp (data not shown).
Effect of TNP-ATP and PD98059 administration on pERK-LI cell expression
The number of pERK-LI cells was significantly larger in both sides of Vc and in ipsilateral Pa5 following pulpal application of α,β-meATP compared to PBS-injected rats (Fig. 3C). The numbers of pERK-LI cells in Vc and Pa5 were significantly smaller in those rats receiving co-application of 1 mM TNP-ATP (P2X1, P2X3 and, P2X2/3 antagonist) with 100 mM α,β-meATP compared with those receiving 100 mM α,β-meATP application alone (see above for detail of ANOVA; Vc ipsi, Vc cont and Pa5 ipsi: P < 0.01, respectively; Pa5 cont: P < 0.05, Bonferroni multiple t-test, Fig. 3C and 3D).
Furthermore, the numbers of pERK-LI cells on both sides of Vc and Pa5 were significantly smaller in those rats receiving pretreatment of intrathecal administration of PD 98059 and α,β-meATP pulpal application compared to those receiving 100 mM α,β-meATP pulpal application alone (Vc ipsi: P < 0.01, Vc cont: P < 0.01, Pa5 ipsi: P < 0.01, Pa5 cont: P < 0.05, Student's t-test, Fig. 4).
Mean total number of pERK-LI cells in ipsilateral (ipsi) and contralateral (cont) Vc and C1/C2 (A, C), and Pa5 (B, D) region induced by pulpal application of α,β-meATP (100 mM) following intrathecal administration of PD98059. Data were analyzed using one-way ANOVA with repeated measures followed by Bonferroni multiple comparison test (*: P < 0.05, **: P < 0.01).
Effect of TNP-ATP and PD98059 on GG EMG activity
Onset latency of GG muscle activity was not significantly different between α,β-meATP-injected and α,β-meATP +TNP-ATP-injected rats (Table 1). On the other hand, the duration of EMG activity was significantly longer in GG following pulpal administration of 100 mM α,β-meATP (F(2,16) = 7.62, P < 0.01; ANOVA followed by Bonferroni multiple t-test) compared to that following co-administration of 100 mM α,β-meATP with 0.1 mM (P < 0.05) or 1 mM (P < 0.01) of TNP-ATP. The GG activity following 10 mM α,β-meATP application was significantly smaller in those rats receiving pulpal co-application of 1 mM TNP-ATP than that in rats receiving α,β-meATP application alone (F(2,18) = 4.69, P < 0.05, ANOVA followed by Bonferroni multiple t-test). However, we could not observe any significant effect or GG activity of pulpal co-application of 0.1 mM TNP-ATP with 100 mM α,β-meATP compared to 100 mM α,β-meATP application alone (Fig. 5A). GG EMG activity following 100 mM α,β-meATP application was significantly smaller in those rats receiving intrathecal administration of PD98059 compared with that of the rats receiving α,β-meATP pulpal application alone (P < 0.01, Student's t-test, Fig. 5B).
Relative area under curve of GG muscle EMG activity induced by pulpal application of α,β-meATP (100 mM) together with TNP-ATP (A), or following intrathecal administration of PD98059 (B). The data were obtained during 1 min after pulpal application of α,β-meATP. Data were analyzed using one-way ANOVA with repeated measures followed by Bonferroni multiple comparison test (*: P < 0.05).
Discussion
Both P2X3 homomultimer and P2X2/3 heteromultimer receptors have been reported in a variety of tissues, including tooth pulp [9–13], stimulation of which causes a barrage of action potentials in tooth pulp afferents that are conveyed to the rat Vc and C1/C2, resulting in a purinergic-dependent central sensitization of Vc and C1/C2 nociceptive neurons [16, 38–40]. Recently it has been found that Vc central sensitization can be produced by specifically stimulating these P2X receptors in the pulp [22].
In the present study, we observed that GG muscle activity could be evoked by pulpal application of the P2X3,2/3 receptor agonist α,β-meATP 100 mM and was significantly larger in the rats when compared with PBS-administration to the pulp. The GG activity was significantly reduced in the rats receiving co-application of 100 mM α,β-meATP with the P2X3,2/3 receptor inhibitor TNP-ATP (1 mM). A large number of pERK-LI cells were also expressed in the Vc, Vi/Vc, C1/C2 and Pa5 5 min after pulpal application of 100 mM α,β-meATP. The pERK-LI cells and GG activity were significantly reduced after intrathecal injection of the MEK inhibitor PD98059. In addition, the number of pERK-LI cells was significantly reduced in rats receiving pulpal co-application of 100 mM α,β-meATP with 1 mM TNP-ATP compared with rats receiving 100 mM α,β-meATP alone.
Technical considerations
In the present study, dental paper points soaked with PBS, α,β-meATP or TNP-ATP were inserted into the tooth pulp. The insertion of a paper point into the tooth pulp may itself cause strong mechanical stimulation of pulpal nerve fibers. We observed some GG activity and many pERK-LI cells were expressed in the Vc, C1/C2 and Pa5 following pulpal insertion of a PBS-dipped paper point. Thus, the GG activity and pERK-LI cells expression were likely a reflection of both mechanical stimulation of the tooth pulp as well as activation of purinergic receptors when α,β-meATP was applied. Nonetheless, the use of PBS as a vehicle control allowed for separation of the effects of α,β-meATP per se. Indeed, the GG activity was significantly larger following pulpal application of 100 mM (but not 10 mM) α,β-meATP when compared with PBS application. Likewise, pERK expression was significantly larger with 100 mM α,β-meATP than PBS. The dose of α,β-meATP required was higher than that reported in other parts of the body (e.g., reduction of hind paw withdrawal threshold, hind paw lifting and licking) [41–43]. This may be due to damage of ATP receptors in tooth pulp nerve fibers during the preparation of the pulp for subsequent drug administration since it has been reported that pulpal P2X3 receptors are highly concentrated in the odontoblastic layer [9, 12] and thus susceptible to damage by cavity preparation. This may explain why a high concentration of ATP was necessary to produce a reflex effect and ERK phosphorylation. Thus, the high concentration of ATP is needed for reflex and other effects relative to other body regions.
We could not observe significant activation of MA or DA muscle following α,β-meATP administration. Primary afferent activity is not sufficiently strong to activate MA motor neurons relative to GG muscle, following noxious mechanical stimulation of facial skin, based on our EMG recordings as described in the Results section. Furthermore, P2X receptor density is lower in the tooth pulp relative to other receptors [13]. Therefore, the tooth pulp-MA and tooth pulp-DA pathways have a higher threshold for activation by tooth pulpal administration of ATP agonists compared with the tooth pulp-GG pathway.
Involvement of P2X receptor activation of tooth pulpal afferents
It is well known that peripheral inflammation or tissue injury causes peripheral ATP release from non-neuronal cells in the injured region [9, 10, 17]. The ATP released from the non-neuronal cells binds to purinergic receptors such as P2X3 and/or P2X2/3 receptors in C-fiber terminals, resulting in peripheral sensitization of the primary afferent neurons [1, 10, 11, 17, 44]. We observed strong activation of GG activity following pulpal application of α,β-meATP, indicating that activation of pulpal P2X3 and/or P2X2/3 receptors in the tooth pulp is sufficient for activation of the GG reflex. The increase in GG muscle activity with α,βme-ATP and its 1-2 sec latency are consistent with other studies of muscle (MA or DA muscle) responses evoked by tooth pulp stimulation [24] or other orofacial stimuli, eg. TMJ [36, 37]. Shigenaga et al. [45, 46] have reported that tooth pulp afferents project to the Vc and trigeminal spinal subnucleus interpolaris (Vi), and many Vi neurons send projection axons to GG muscle motor neurons [47]. Furthermore, previous electrophysiological studies have also reported that GG or hypoglossal motor neuron activity is modulated by trigeminal nerve stimulation, suggesting that the trigeminal afferent is involved in modulation of GG muscle activity [48, 49]. Previous studies have also reported that the pulpal application of mustard oil or capsaicin induces EMG activity in the MA and DA muscles simultaneously, and these excitations could last more than 1 min [23, 24]. On the other hand, pulpal application of α,β-meATP in the present study caused a significant increase in EMG activity only in the GG muscle, and the duration of GG activity was less than 1 min. These differences between the studies might be explained by different experimental conditions in the studies or that the tooth pulp afferent-GG reflex pathway especially involves P2X3,2/3 receptors.
Together with previous results our findings suggested that tooth pulp nerve fibers were sensitized by ATP released from the non-neuronal pulpal cells following tooth pulp inflammation or injury, resulting in the barrage of action potentials in the tooth pulp nerve fibers which were conveyed to Vc and C1/C2 neurons.
Sensitization of Vc, C1/C2 and Pa5 neurons
It has been documented that noxious inputs from the orofacial region are somatotopically organized in the Vc complex and C1/C2 regions, and nociceptive neurons in these areas are involved in the localization of orofacial pain [50]. Anterograde tracing studies have revealed that the rat's tooth pulp afferents are distributed in the ipsilateral Vc, C1/C2 and Pa5 [45, 46, 51–55]. In particular, the maxillary first molar pulp afferent projects to the ipsilateral Vc, Vi/Vc, Pa5 and C1/C2 regions [54]. Shimizu et al. have also reported that pulpal application of capsaicin produces pERK-LI cell expression in these regions suggesting that neurons in Pa5, Vc, Vi/Vc and Vc/C2 regions are involved in tooth pulp nociceptive processing [23]. Consistent with these previous studies, the present study documented expression of pERK-LI cells following α,β-meATP pulpal application in the dorsal portion of the ipsilateral Vc, Vi/Vc, C1/C2 and bilaterally in the Pa5 region. It is well established that Vc, Vi/Vc and C1/C2 nociceptive neurons manifest marked central sensitization following orofacial inflammation or trigeminal nerve injury [40, 56, 57]. It is also known that a barrage of action potentials is elicited in primary afferent fibers following tooth pulp inflammation [58]. Peripheral inflammation is thought to be involved in enhancement of a variety of receptor activities, including purinergic receptor in peripheral nerve terminals [42], which results in the peripheral and central sensitization of the trigeminal nociceptive system [16, 22]. Together, these data and the present results suggest that purinergic receptors in pulpal nerve terminals are involved in peripheral sensitization of the trigeminal nociceptive system. It has also been reported that Pa5 neurons are involved in autonomic regulation as well as in nociceptive processes [59].
ERK is one of the MAPK families, and has been documented in the spinal DH as well as dorsal root ganglion neurons that are phosphorylated by noxious peripheral stimulation in an intensity-related manner [60–64]. ERK has also be shown to be phosphorylated in Vc and C1/C2 neurons within 5 min after noxious stimulation of the orofacial region [28], strongly suggesting that ERK phosphorylation is involved in the activation of nociceptive neurons in the Vc and C1/C2 soon after orofacial noxious stimulation. Consistent with these findings, we observed that many pERK-LI cells were expressed in Vc, Vi/Vc, C1/C2 and Pa5 regions 5 min after pulpal application of α,β-meATP, and showed that the pERK-LI cells in Vc showed NeuN immunoreactivity, indicating that the phosphorylation of ERK occurred in neurons. Furthermore, following intrathecal administration of the MEK inhibitor PD 98059 [27, 30, 31, 65], the pERK-LI cells were significantly reduced in Vc, Vi/Vc, C1/C2 and Pa5 in rats receiving pulpal administration of α,β-meATP, suggesting that the intracellular ERK cascade is involved in the activation of Vc, Vi/Vc, C1/C2 and Pa5 nociceptive neurons. We also observed that the GG activity evoked by pulpal application α,β-meATP was significantly suppressed by intrathecal administration of PD 98059. Since it has been reported that Vc neurons are involved in masticatory muscle activity evoked by noxious stimulation of orofacial tissues [66, 67], the ERK phosphorylation in Vc neurons was likely involved in our documented enhancement of GG activity following pulpal administration of α,β-meATP.
Conclusions
The present findings suggest that P2X3 and P2X2/3 receptors may be involved in the activation of tooth pulpal nerve fibers following tooth pulp injury, resulting in central sensitization of Vc, Vi/Vc, C1/C2 and Pa5 neurons through the intracellular MAP kinase cascade. The findings underscore the importance of purinergic receptor mechanisms in tooth pulp nociceptive processes.
References
Burnstock G: Purinergic receptors and pain. Curr Pharm Des 2009, 15: 1717–1735. 10.2174/138161209788186335
Brake AJ, Julius D: Signaling by extracellular nucleotides. Annu Rev Cell Dev Biol 1996, 12: 519–541. 10.1146/annurev.cellbio.12.1.519
Ding Y, Cesare P, Drew L, Nikitaki D, Wood JN: ATP, P2X receptors and pain pathways. J Auton Nerv Syst 2000, 81: 289–294. 10.1016/S0165-1838(00)00131-4
Burnstock G: P2X receptors in sensory neurones. Br J Anaesth 2000, 84: 476–488.
Burnstock G: Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci 2001, 22: 182–188. 10.1016/S0165-6147(00)01643-6
Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP: International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev 2001, 53: 107–118.
Ralevic V, Burnstock G: Receptors for purines and pyrimidines. Pharmacol Rev 1998, 50: 413–492.
Robertson SJ, Ennion SJ, Evans RJ, Edwards FA: Synaptic P2X receptors. Curr Opin Neurobiol 2001, 11: 378–386. 10.1016/S0959-4388(00)00222-1
Alavi AM, Dubyak GR, Burnstock G: Immunohistochemical evidence for ATP receptors in human dental pulp. J Dent Res 2001, 80: 476–483. 10.1177/00220345010800021501
Burnstock G: Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther 2006, 110: 433–454. 10.1016/j.pharmthera.2005.08.013
Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW: Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature 1997, 387: 505–508. 10.1038/387505a0
Jiang J, Gu J: Expression of adenosine triphosphate P2X3 receptors in rat molar pulp and trigeminal ganglia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002, 94: 622–626. 10.1067/moe.2002.128973
Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P: Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain 2003, 17: 245–250.
Burnstock G, Wood JN: Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol 1996, 6: 526–532. 10.1016/S0959-4388(96)80060-2
Dunn PM, Zhong Y, Burnstock G: P2X receptors in peripheral neurons. Prog Neurobiol 2001, 65: 107–134. 10.1016/S0301-0082(01)00005-3
Hu B, Chiang CY, Hu JW, Dostrovsky JO, Sessle BJ: P2X receptors in trigeminal subnucleus caudalis modulate central sensitization in trigeminal subnucleus oralis. J Neurophysiol 2002, 88: 1614–1624.
Dai Y, Fukuoka T, Wang H, Yamanaka H, Obata K, Tokunaga A, Noguchi K: Contribution of sensitized P2X receptors in inflamed tissue to the mechanical hypersensitivity revealed by phosphorylated ERK in DRG neurons. Pain 2004, 108: 258–266. 10.1016/j.pain.2003.12.034
Seino D, Tokunaga A, Tachibana T, Yoshiya S, Dai Y, Obata K, Yamanaka H, Kobayashi K, Noguchi K: The role of ERK signaling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain 2006, 123: 193–203. 10.1016/j.pain.2006.02.032
Wu G, Whiteside GT, Lee G, Nolan S, Niosi M, Pearson MS, Ilyin VI: A-317491, a selective P2X 3 /P2X 2/3 receptor antagonist, reverses inflammatory mechanical hyperalgesia through action at peripheral receptors in rats. Eur J Pharmacol 2004, 504: 45–53. 10.1016/j.ejphar.2004.09.056
Shinoda M, Ozaki N, Asai H, Nagamine K, Sugiura Y: Changes in P2X3 receptor expression in the trigeminal ganglion following monoarthritis of the temporomandibular joint in rats. Pain 2005, 116: 42–51. 10.1016/j.pain.2005.03.042
Chiang CY, Zhang S, Xie YF, Hu JW, Dostrovsky JO, Salter MW, Sessle BJ: Endogenous ATP involvement in mustard-oil-induced central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn). J Neurophysiol 2005, 94: 1751–1760. 10.1152/jn.00223.2005
Cherkas PS, Dostrovsky JO, Sessle BJ: Peripheral P2X mechanisms are involved in the production of central sensitization in rat dorsal horn nociceptive neurons. Program No2667Abstract Viewer/Itinerary Planner Washington, DC: Society for Neuroscience 2009. Online
Shimizu K, Asano M, Kitagawa J, Ogiso B, Ren K, Oki H, Matsumoto M, Iwata K: Phosphorylation of Extracellular Signal-Regulated Kinase in medullary and upper cervical cord neurons following noxious tooth pulp stimulation. Brain Res 2006, 1072: 99–109. 10.1016/j.brainres.2005.12.040
Sunakawa M, Chiang CY, Sessle BJ, Hu JW: Jaw electromyographic activity induced by the application of algesic chemicals to the rat tooth pulp. Pain 1999, 80: 493–501. 10.1016/S0304-3959(98)00241-3
Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO: Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci 2001, 21: 6933–6939.
Rosen LB, Ginty DD, Weber MJ, Greenberg ME: Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 1994, 12: 1207–1221. 10.1016/0896-6273(94)90438-3
Honda K, Kitagawa J, Sessle BJ, Kondo M, Tsuboi Y, Yonehara Y, Iwata K: Mechanisms involved in an increment of multimodal excitability of medullary and upper cervical dorsal horn neurons following cutaneous capsaicin treatment. Mol Pain 2008, 4: 59. 10.1186/1744-8069-4-59
Noma N, Tsuboi Y, Kondo M, Matsumoto M, Sessle BJ, Kitagawa J, Saito K, Iwata K: Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J Comp Neurol 2008, 507: 1428–1440. 10.1002/cne.21620
Adachi K, Shimizu K, Hu JW, Iwata K, Koshikawa N, Sessle BJ: ATP tooth pulp stimulation evokes brainstem reflexes and ERK phosphorylation. J Dental Res 2008., 84: #316.3521 [http://www.dentalresearch.org]
Tsujimura T, Kitagawa J, Ueda K, Iwata K: Inhibition of swallowing reflex following phosphorylation of extracellular signal-regulated kinase in nucleus tractus solitarii neurons in rats with masseter muscle nociception. Neurosci Lett 2009, 450: 361–364. 10.1016/j.neulet.2008.12.008
Tsujimura T, Kondo M, Kitagawa J, Tsuboi Y, Saito K, Tohara H, Ueda K, Sessle BJ, Iwata K: Involvement of ERK phosphorylation in brainstem neurons in modulation of swallowing reflex in rats. J Physiol 2009, 587: 805–817. 10.1113/jphysiol.2008.165324
Broton JG, Hu JW, Sessle BJ: Effects of temporomandibular joint stimulation on nociceptive and nonnociceptive neurons of the cat's trigeminal subnucleus caudalis (medullary dorsal horn). J Neurophysiol 1988, 59: 1575–1589.
Broton JG, Sessle BJ: Reflex excitation of masticatory muscles induced by algesic chemicals applied to the temporomandibular joint of the cat. Arch Oral Biol 1988, 33: 741–747. 10.1016/0003-9969(88)90008-8
Adachi K, Lee JC, Hu JW, Yao D, Sessle BJ: Motor cortex neuroplasticity associated with lingual nerve injury in rats. Somatosens Mot Res 2007, 24: 97–109. 10.1080/08990220701470451
Adachi K, Murray GM, Lee JC, Sessle BJ: Noxious lingual stimulation influences the excitability of the face primary motor cerebral cortex (face MI) in the rat. J Neurophysiol 2008, 100: 1234–1244. 10.1152/jn.90609.2008
Yu XM, Sessle BJ, Vernon H, Hu JW: Effects of inflammatory irritant application to the rat temporomandibular joint on jaw and neck muscle activity. Pain 1995, 60: 143–149. 10.1016/0304-3959(94)00104-M
Cairns BE, Sessle BJ, Hu JW: Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J Neurosci 1998, 18: 8056–8064.
Chiang CY, Hu B, Hu JW, Dostrovsky JO, Sessle BJ: Central sensitization of nociceptive neurons in trigeminal subnucleus oralis depends on integrity of subnucleus caudalis. J Neurophysiol 2002, 88: 256–264.
Chiang CY, Park SJ, Kwan CL, Hu JW, Sessle BJ: NMDA receptor mechanisms contribute to neuroplasticity induced in caudalis nociceptive neurons by tooth pulp stimulation. J Neurophysiol 1998, 80: 2621–2631.
Xie YF, Zhang S, Chiang CY, Hu JW, Dostrovsky JO, Sessle BJ: Involvement of glia in central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn). Brain Behav Immun 2007, 21: 634–641. 10.1016/j.bbi.2006.07.008
Bland-Ward PA, Humphrey PP: Acute nociception mediated by hindpaw P2X receptor activation in the rat. Br J Pharmacol 1997, 122: 365–371. 10.1038/sj.bjp.0701371
Hamilton SG, Wade A, McMahon SB: The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br J Pharmacol 1999, 126: 326–332. 10.1038/sj.bjp.0702258
Tsuda M, Koizumi S, Kita A, Shigemoto Y, Ueno S, Inoue K: Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons. J Neurosci 2000, 20: RC90.
Burnstock G: Purinergic signalling. Br J Pharmacol 2006,147(Suppl 1):S172–181. 10.1038/sj.bjp.0706429
Shigenaga Y, Nishimura M, Suemune S, Nishimori T, Doe K, Tsuru H: Somatotopic organization of tooth pulp primary afferent neurons in the cat. Brain Res 1989, 477: 66–89. 10.1016/0006-8993(89)91395-4
Shigenaga Y, Suemune S, Nishimura M, Nishimori T, Sato H, Ishidori H, Yoshida A, Tsuru K, Tsuiki Y, Dateoka Y, et al.: Topographic representation of lower and upper teeth within the trigeminal sensory nuclei of adult cat as demonstrated by the transganglionic transport of horseradish peroxidase. J Comp Neurol 1986, 251: 299–316. 10.1002/cne.902510303
Bae YC, Kim JP, Choi BJ, Park KP, Choi MK, Moritani M, Yoshida A, Shigenaga Y: Synaptic organization of tooth pulp afferent terminals in the rat trigeminal sensory nuclei. J Comp Neurol 2003, 463: 13–24. 10.1002/cne.10741
Sauerland EK, Mizuno N: A protective mechanism for the tongue: suppression of genioglossal activity induced by stimulation of trigeminal proprioceptive afferents. Experientia 1970, 26: 1226–1227. 10.1007/BF01897980
Takata M: Two types of inhibitory postsynaptic potentials in the hypoglossal motoneurons. Prog Neurobiol 1993, 40: 385–411. 10.1016/0301-0082(93)90016-L
Liang YF, Terashima S: Physiological properties and morphological characteristics of cutaneous and mucosal mechanical nociceptive neurons with A-delta peripheral axons in the trigeminal ganglia of crotaline snakes. J Comp Neurol 1993, 328: 88–102. 10.1002/cne.903280107
Pertovaara A, Huopaniemi T, Aukee K, Carlson S: Tooth pulp-evoked activity in the spinal trigeminal nucleus caudalis of cat: comparison to primary afferent fiber, reflex, and sensory responses. Exp Neurol 1987, 95: 155–166. 10.1016/0014-4886(87)90014-8
Sessle BJ: The neurobiology of facial and dental pain: present knowledge, future directions. J Dent Res 1987, 66: 962–981.
Takemura M, Sugimoto T, Shigenaga Y: Difference in central projection of primary afferents innervating facial and intraoral structures in the rat. Exp Neurol 1991, 111: 324–331. 10.1016/0014-4886(91)90099-X
Marfurt CF, Turner DF: The central projections of tooth pulp afferent neurons in the rat as determined by the transganglionic transport of horseradish peroxidase. J Comp Neurol 1984, 223: 535–547. 10.1002/cne.902230406
Clements JR, Magnusson KR, Hautman J, Beitz AJ: Rat tooth pulp projections to spinal trigeminal subnucleus caudalis are glutamate-like immunoreactive. J Comp Neurol 1991, 309: 281–288. 10.1002/cne.903090209
Dubner R, Ren K: Brainstem mechanisms of persistent pain following injury. J Orofac Pain 2004, 18: 299–305.
Sessle BJ: Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med 2000, 11: 57–91. 10.1177/10454411000110010401
Matthews B, Sessle BJ: Peripheral mechanisms of orofacial pain. In Orofacial Pain. 2nd edition. Edited by: Seesle BJ, Lavigne GJ, Lund JP, Dubner R. Illinois: Quintessence Publishing; 2008:27–34.
Yamazaki Y, Ren K, Shimada M, Iwata K: Modulation of paratrigeminal nociceptive neurons following temporomandibular joint inflammation in rats. Exp Neurol 2008, 214: 209–218. 10.1016/j.expneurol.2008.08.005
Ji RR, Baba H, Brenner GJ, Woolf CJ: Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999, 2: 1114–1119. 10.1038/16040
Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K: Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci 2002, 22: 7737–7745.
Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR: Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci 2004, 24: 8310–8321. 10.1523/JNEUROSCI.2396-04.2004
Liu Y, Obata K, Yamanaka H, Dai Y, Fukuoka T, Tokunaga A, Noguchi K: Activation of extracellular signal-regulated protein kinase in dorsal horn neurons in the rat neuropathic intermittent claudication model. Pain 2004, 109: 64–72. 10.1016/j.pain.2004.01.010
Wang H, Dai Y, Fukuoka T, Yamanaka H, Obata K, Tokunaga A, Noguchi K: Enhancement of stimulation-induced ERK activation in the spinal dorsal horn and gracile nucleus neurons in rats with peripheral nerve injury. Eur J Neurosci 2004, 19: 884–890. 10.1111/j.0953-816X.2004.03203.x
Igwe OJ: c-Src kinase activation regulates preprotachykinin gene expression and substance P secretion in rat sensory ganglia. Eur J Neurosci 2003, 18: 1719–1730. 10.1046/j.1460-9568.2003.02878.x
Tsai CM, Chiang CY, Yu XM, Sessle BJ: Involvement of trigeminal subnucleus caudalis (medullary dorsal horn) in craniofacial nociceptive reflex activity. Pain 1999, 81: 115–128. 10.1016/S0304-3959(99)00009-3
Cairns BE, Sessle BJ, Hu JW: Temporomandibular-evoked jaw muscle reflex: role of brain stem NMDA and non-NMDA receptors. Neuroreport 2001, 12: 1875–1878. 10.1097/00001756-200107030-00022
Acknowledgements
This study was supported in part by Sato and Uemura Funds from Nihon University School of Dentistry, and a grant from the Dental Research Center, Nihon University School of Dentistry; Nihon University multidisciplinary research grant for KI; grants from the Ministry of Education, Culture, Sports, Science, and Technology to promote multidisciplinary research project "Translational Research Network on Orofacial Neurological Disorders" at Nihon University School of Dentistry and the Japan-Canada Joint Health Research Program 167458, NIH grant DE04786, and CHIR grants MOP-43095 and MOP-82831.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors read and approved the final manuscript. KA carried out the experiments and data analysis. KS, IS and MM helped the experiments, data analysis and paper writing. BJS, MS and JWH provided data interpretation and helped to finalize the manuscript. HS, KH and NK provided data interpretation. KI conceptualized the hypothesis, designed and supervised the experiments, directed the data analysis, and finalized the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Adachi, K., Shimizu, K., Hu, J.W. et al. Purinergic receptors are involved in tooth-pulp evoked nocifensive behavior and brainstem neuronal activity. Mol Pain 6, 59 (2010). https://doi.org/10.1186/1744-8069-6-59
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-6-59