Abstract
We compared the prognostic power of end-tidal CO2 pressure (PETCO2) during exercise, an index of arterial CO2 pressure, with those of established respiratory gas indexes during exercise testing in patients with left ventricular dysfunction. Seventy-eight consecutive patients with a left ventricular ejection fraction (LVEF) ≤40% were enrolled in the study. All the patients performed a symptom-limited incremental exercise test with respiratory gas measurements. PETCO2 at peak exercise, peak O2 uptake (\( \dot{\rm{V}} \)O2), the ratio of the increase in ventilation to the increase in CO2 output (\( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope), and the ratio of the increase in \( \dot{\rm{V}} \)O2 to the increase in work rate (∆\( \dot{\rm{V}} \)O2/∆WR) were measured. PETCO2 at peak exercise was significantly correlated with peak \( \dot{\rm{V}} \)O2, \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope and ∆\( \dot{\rm{V}} \)O2/∆WR. During a prospective follow-up period of 992 ± 570 days, 14 cardiac deaths occurred. As compared to survivors, non-survivors had a significantly lower LVEF, lower PETCO2 at peak exercise, lower peak \( \dot{\rm{V}} \)O2, lower ∆\( \dot{\rm{V}} \)O2/∆WR and a higher \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope. Among these indexes, only PETCO2 at peak exercise was found to be an independent predictor for cardiac death. PETCO2 at peak exercise is useful in predicting poor prognosis in patients with left ventricular systolic dysfunction.
Similar content being viewed by others
Introduction
Parameters obtained from cardiopulmonary exercise testing reflect the severity of heart disease and the activities of daily living in cardiac patients [1]. Among these, the peak O2 uptake (\( \dot{\rm{V}} \)O2) has traditionally been considered a gold standard for identifying patients with a poor prognosis and selecting candidates for cardiac transplantation [2]. The ratio of the increase in ventilation (\( \dot{\rm{V}} \)E) to the increase in CO2 output (\( \dot{\rm{V}} \)CO2) during exercise (\( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope) also strongly reflects the severity of heart failure and has been found to be an independent prognostic marker in cardiac patients [3].
End-tidal CO2 pressure (PETCO2) is one of the parameters obtained from respiratory gas analysis [4]. PETCO2 generally reflects arterial PCO2 (PaCO2) both at rest and during exercise in healthy subjects [5]. It has been demonstrated that PETCO2 becomes low in pulmonary vascular occlusive disease because of a lack of perfusion of ventilated lungs [5, 6]. It has also been shown that cardiac patients have an abnormally low PETCO2 during exercise, in accordance with a decreased peak \( \dot{\rm{V}} \)O2 [4, 7, 8]. In patients with previous myocardial infraction, it has been noted that physical training increases PETCO2 not at rest, but during exercise, in accordance with an improved response of cardiac output during exercise [9]. Thus, it has been speculated that PETCO2 during exercise has strong associations with cardiopulmonary function during exercise and cardiac reserve. Although very few studies have suggested the prognostic value of PETCO2 during exercise [10], it has not been established whether the measurement of PETCO2 during exercise is useful in predicting future cardiac events.
During incremental exercise, PETCO2 usually remains constant or slightly increases from rest to moderate exercise. Thereafter, it generally declines. The point at which PETCO2 starts to decline is called the respiratory compensation point [11]. In our experience, this point may not always be identified in cardiac patients. Therefore, we focused simply on PETCO2 at peak exercise. In the present study, we hypothesized that a low PETCO2 at peak exercise is related to a worse prognosis, especially cardiac death due to worsening of heart failure, and that its prognostic power might be superior to the established respiratory gas indexes during exercise in cardiac patients. In order to test this hypothesis, we compared the prognostic power of PETCO2 at peak exercise with those of the left ventricular ejection fraction (LVEF) measured at rest and respiratory gas indexes obtained during exercise testing in patients with left ventricular dysfunction, focusing on their cardiac mortality.
Methods
Study patients
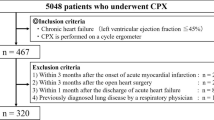
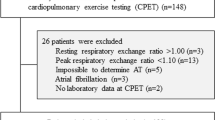
We prospectively evaluated 78 cardiac patients who performed exercise testing with a respiratory gas analysis at the Cardiovascular Institute between February 2001 and August 2003 and whose LVEF was equal to or less than 40% by echocardiography (mean LVEF = 28.6 ± 7.7%). The etiology of heart diseases was coronary artery disease in 43, valvular heart disease in 18 and idiopathic dilated cardiomyopathy in 17 patients. Among the 43 patients with coronary artery disease, 38 had previous myocardial infarction. Coronary artery disease was diagnosed by the presence of significant coronary stenosis defined as a ≥75% reduction in the luminal diameter of coronary vessels or the presence of myocardial infarction diagnosed according to the World Health Organization criteria [12]. The protocol was approved by the human subjects committee of the Cardiovascular Institute. Its purposes and risks were explained to the patients, and informed consent was obtained from each.
Follow-up
Patients were prospectively followed up at the outpatient clinic of the Cardiovascular Institute. The endpoint was defined as cardiac death. Causes of cardiac deaths were categorized into progressive heart failure, acute myocardial infarction and sudden cardiac death. Sudden cardiac death was diagnosed according to published criteria [13].
Cardiopulmonary exercise testing
An incremental symptom-limited exercise test was performed using an upright, electromagnetically-braked cycle ergometer (Corival 400, Lode, Holland). Exercise began with a 4-min warm-up at 0 W or 20 W at 60 rpm, and the load was then increased incrementally by 1 W every 6 s (10 W/min). The work rate of the warm-up exercise was selected as 0 W in 34 patients whose daily activity was assumed to be very low. In the remaining 44 patients, 20 W was used. Through a rubber mask attached to the subject’s face, breath-by-breath \( \dot{\rm{V}} \)O2, \( \dot{\rm{V}} \)CO2, \( \dot{\rm{V}} \)E, and PETCO2 were measured during the test using an Aeromonitor AE-300S (Minato Medical Science, Osaka, Japan) [8, 14]. The Aeromonitor AE-300S consists of a microcomputer, a hot wire flowmeter, and a gas analyzer, which contains a sampling tube, filter, suction pump, 02 analyzer made by a paramagnetic oxygen transducer, and an infrared CO2 analyzer. Gas was sampled through a filter by a suction pump through the gas analyzers at a rate of 170 ml/min. The system was carefully calibrated before each study.
Data analysis
Prior to any calculation of the parameters from the respiratory gas analysis, a five-point moving average of the breath-by-breath data was performed. The peak \( \dot{\rm{V}} \)O2 and PETCO2 at peak exercise were defined as the average values obtained during the last 15 s of incremental exercise. The ratio of the increase in \( \dot{\rm{V}} \)O2 to the increase in work rate (∆\( \dot{\rm{V}} \)O2/∆WR) was calculated by a least-squares linear regression from the data recorded between 30 s after the start of incremental exercise to 30 s before the end of the exercise [15]. The \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope was calculated from the start of incremental exercise to the respiratory compensation point by the least-squares linear regression [15]. The respiratory compensation point was determined using the following criteria: (1) the ratio of \( \dot{\rm{V}} \)E to \( \dot{\rm{V}} \)CO2 starts to increase after a period of decrease or stasis; and (2) the PETCO2 starts to decrease after a period of stasis. When the respiratory compensation point could not be clearly identified, the \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope was calculated from the data recorded between the start of incremental exercise to the end of the exercise [15]. PETCO2 at rest was also determined as the average of the value obtained as the subject sat on the ergometer over a 4-min period before the start of the exercise test.
Statistics
Data are presented as the mean ± SD. Linear regression analysis was used to correlate the PETCO2 at peak exercise and the CPX parameters. Intergroup differences for variables were compared using the unpaired t test or Fisher’s exact test, where appropriate. A Cox proportional hazards model was used to measure the impact of cardiopulmonary variables on event-free survival time. The optimal threshold value of PETCO2 at peak exercise for cardiac death was determined by receiver operating characteristics (ROC) curve analysis. The difference in survival between two groups divided on the basis of the optimal threshold value was detected by the Kaplan–Meier method, and compared using the logrank test. For all comparisons, P < 0.05 was considered statistically significant.
Results
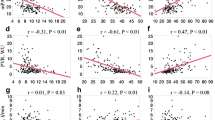
As shown in Fig. 1, PETCO2 at peak exercise showed significant positive correlations with peak \( \dot{\rm{V}} \)O2 (r = 0.60, P < 0.0001) and ∆\( \dot{\rm{V}} \)O2/∆WR (r = 0.56, P < 0.0001), and a negative correlation with the \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope (r = −0.79, P < 0.0001).
During the follow-up period of 992 ± 570 days, no patients underwent cardiac transplantation, but three patients received implantation of a cardioverter defibrillator. Among the 43 patients with coronary artery disease, four patients underwent coronary artery bypass graft surgery and two patients received percutaneous coronary intervention during the follow-up period. Among the 18 patients with valvular disease, four patients underwent replacements and/or repairs of cardiac valves during this period.
By the end of the study, 14 cardiac deaths occurred. Of these, seven patients died of progressive heart failure (five patients with coronary artery disease, one patient with valvular disease and one patient with idiopathic dilated cardiomyopathy), six patients had a sudden cardiac death (four patients with coronary artery disease and two patients with valvular disease) and one patient with coronary artery disease died of acute myocardial infarction. LVEF at rest was 24.7 ± 8.0% in non-survivors, which was lower than that of survivors (29.4 ± 7.5%, P = 0.04). As compared to survivors, non-survivors had lower heart rate and systolic blood pressure at peak exercise. Non-survivors had a lower PETCO2 both at rest (28.4 ± 5.2 vs. 33.4 ± 4.1 mmHg, P = 0.0002) and at peak exercise (28.7 ± 6.7 vs. 35.9 ± 5.6 mmHg, P < 0.0001) than survivors. Non-survivors achieved a lower peak \( \dot{\rm{V}} \)O2 than survivors (12.1 ± 3.5 vs. 15.9 ± 4.4 ml/min/kg, P = 0.004). ∆\( \dot{\rm{V}} \)O2/∆WR in non-survivors was 6.5 ± 1.9 ml/min/W, which was significantly lower than that in survivors (8.3 ± 2.6 ml/min/W, P = 0.01). The \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope was significantly higher in non-survivors than in survivors (44.5 ± 16.1 vs. 35.7 ± 11.2, P = 0.02).
In Fig. 2, ROC curve for PETCO2 at peak exercise is illustrated for cardiac death. The optimal threshold value for PETCO2 at peak exercise was 30.9 mmHg (ROC area = 0.81, 95% confidence interval = 0.68 − 0.94, P < 0.001, Sensitivity = 83%, Specificity = 79%). Table 1 shows a comparison of clinical characteristics between patients with PETCO2 ≥30.9 mmHg (n = 56) and those with PETCO2 <30.9 mmHg (n = 22). As compared to patients with PETCO2 ≥30.9 mmHg, those with PETCO2 <30.9 mmHg had lower systolic blood pressure at peak exercise. Patients with PETCO2 <30.9 mmHg had a lower PETCO2 both at rest and at peak exercise than those with PETCO2 ≥30.9 mmHg. Patients with PETCO2 <30.9 mmHg achieved a lower peak \( \dot{\rm{V}} \)O2 and ∆\( \dot{\rm{V}} \)O2/∆WR and a higher \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope.
Table 2 shows the univariate and multivariate Cox proportional hazards analyses of the association between cardiopulmonary indexes and cardiac deaths. By the univariate analysis, LVEF, heart rate and systolic blood pressure at peak exercise, PETCO2 both at rest and at peak exercise, peak \( \dot{\rm{V}} \)O2, ∆\( \dot{\rm{V}} \)O2/∆WR, and the \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope were all found to be significant indexes for survival. When these indexes were entered all together, only PETCO2 at peak exercise (P = 0.030) was found to be an independent predictor for cardiac death. Figure 3 shows the Kaplan–Meier survival in each group of subjects divided on the basis of the optimal threshold value for PETCO2 at peak exercise. Kaplan–Meier survival curves demonstrate a significantly lower survival rate in patients with PETCO2 <30.9 mmHg than that seen in those with PETCO2 ≥30.9 mmHg (P < 0.0001).
Kaplan–Meier survival curves for patients stratified into two groups on the basis of the threshold value for PETCO2 at peak exercise determined from ROC analysis: Patients with a PETCO2 at peak exercise ≥30.9 mmHg (n = 56) and those with a PETCO2 at peak exercise <30.9 mmHg (n = 22). The survival rate in the patients with a lower PETCO2 at peak exercise was significantly lower than that seen in those with a higher PETCO2 at peak exercise (P < 0.0001)
Discussion
Until 1980s, risk stratification of chronic heart disease was primarily based on the functional assessment by the New York Heart Association functional classifications and resting hemodynamic measurements. However, the former is subject to the physician’s bias, and the latter does not necessarily correlate with the clinical manifestations of heart failure [2, 16]. Hence, cardiopulmonary exercise testing has become indispensable in stratifying patients with heart failure and singling out those with poor prognosis. In the present study, we found that among patients with left ventricular systolic dysfunction, those with a relatively low PETCO2 at peak exercise have a significantly lower survival rate than those with a relatively high PETCO2 at peak exercise. By the multivariate Cox proportional hazards analysis including peak \( \dot{\rm{V}} \)O2, ∆\( \dot{\rm{V}} \)O2/∆WR, and \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope, only PETCO2 at peak exercise was found to be an independent predictor for cardiac deaths.
Parameters obtained from cardiopulmonary exercise testing
Given that \( \dot{\rm{V}} \)O2 is the product of cardiac output and the difference in the arterial and mixed venous blood O2 content, peak \( \dot{\rm{V}} \)O2 in cardiac patients reflects maximal cardiac output, i.e., the heart’s pumping reserve, along with the O2 uptake capacity in the skeletal muscle. ∆\( \dot{\rm{V}} \)O2/∆WR, which reflects the rate of the increase in cardiac output during incremental exercise, is known to be approximately 10 ml/min/W in healthy subjects [17]. The lower ∆\( \dot{\rm{V}} \)O2/∆WR in non-survivors suggests an insufficient cardiac reserve. The \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope, which ranges from approximately 24 to 34 in healthy subjects, becomes steeper in cardiac patients, according to the severity of heart failure [3, 18]. Theoretically, a steep \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope is assumed to relate to an increased ratio of pulmonary dead space to tidal volume (VD/VT) or to a decrease in the regulatory set point for PaCO2. During incremental exercise, lactic acidosis develops. This development occurs at lower intensity exercise as the heart disease worsens, and increases ventilation through stimulation of carotid bodies. Therefore, a steeper \(\dot{\rm{V}}\)E/\( \dot{\rm{V}} \)CO2 slope is also related to the increase in the blood lactate level itself. It is assumed that a higher \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope leads to a worse prognosis by these mechanisms determining the \( \dot{\rm{V}} \)E/\( \dot{\rm{V}} \)CO2 slope.
It has been reported that the values of PETCO2 at rest and at peak exercise are, on average, 34 and 40 mmHg, respectively, in healthy subjects of approximately 60 years of age [19]. Thus, PETCO2 at rest and at peak exercise in non-survivors of the present study are considerably lower than those reported in healthy subjects. The arterial−end-tidal PCO2 difference is known to reflect a ventilation-perfusion (V/Q) mismatch. In patients with left ventricular dysfunction, the failure of pulmonary blood flow (cardiac output) to increase appropriately during exercise aggravates the V/Q mismatch (high ventilation/perfusion) and the pulmonary dead space, leading to a greater arterial−end-tidal PCO2 difference. This greater difference attributes to the lower PETCO2 and becomes much greater with the increasing severity of heart failure [4, 7, 20]. Also during exercise, patients with heart failure have a lower PaCO2 as compared to healthy subjects because of their lactic acidosis-induced hyperventilation [21]. Thus, a lower PETCO2 during exercise which is determined by V/Q mismatch and degree of lactic acidosis would be tightly related to insufficient cardiac reserve. Probably by this reason, PETCO2 at peak exercise was found as a powerful prognostic index for cardiac mortality in patients with left ventricular dysfunction in whom the main cause of death was the worsening of heart failure.
Study limitations
During incremental exercise, PETCO2 usually remains constant or slightly increases from rest to moderate exercise, and then declines during heavy exercise. Since the respiratory compensation point, i.e., the point at which PETCO2 starts to decline may not always be clear, we obtained the value at peak exercise. The value of PETCO2 at peak exercise is influenced by the subjects’ motivation and the philosophy of the physician who has the responsibility to terminate the exercise. Thus, PETCO2 at the respiratory compensation point or at the anaerobic threshold rather than the point at peak exercise might have been better to discriminate cardiac patients with poor prognosis.
Although the prognostic power of the parameters may depend partly on the etiology of heart disease, the number of subjects in the present study was not sufficient to bring out disease-specific characteristics of each prognostic index. Also, the prognostic power of indexes obtained during cardiopulmonary exercise testing must depend on the severity of heart disease. Thus, the results of our present study do not refute the significance of peak \( \dot{\rm{V}} \)O2 in predicting mortality in heart failure patients and in selecting candidates for cardiac transplantation. For the future, it will be ideal to estimate the cardiopulmonary reserve and to identify high risk patients by integrating the indexes of cardiopulmonary exercise testing.
Conclusion
PETCO2 at peak exercise is useful in predicting poor prognosis in patients with left ventricular systolic dysfunction.
References
Wasserman K (1988) New concepts in assessing cardiovascular function. Circulation 78:1060–1071
Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR (1991) Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 83:778–786
Chua TP, Ponikowski P, Harrington D, Anker SD, Webb-Peploe K, Clark AL, Poole-Wilson PA, Coats AJS (1997) Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 29:1585–1590
Matsumoto A, Itoh H, Eto Y, Kobayashi T, Kato M, Omata M, Watanabe H, Kato K, Momomura S (2000) End-tidal CO2 pressure decreases during exercise in cardiac patients: association with severity of heart failure and cardiac output reserve. J Am Coll Cardiol 36:242–249
Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ (2005) Principles of exercise testing and interpretation. Lippincott, Philadelphia, pp 33–206
Taniguchi S, Irita K, Sakaguchi Y, Takahashi S (1996) Arterial to end-tidal CO2 gradient as an indicator of silent pulmonary embolism. Lancet 348:1451
Wasserman K, Zhang YY, Gitt A, Belardinelli R, Koike A, Lubarsky L, Agostoni PG (1997) Lung function and exercise gas exchange in chronic heart failure. Circulation 96:2221–2227
Koike A, Nagayama O, Goda A, Yamaguchi K, Tajima A, Uejima T, Itoh H, Aizawa T (2006) Does the severity of central sleep apnea correlate with respiratory gas indexes during cardiopulmonary exercise testing? Int Heart J 47:889–900
Eto Y, Koike A, Matsumoto A, Momomura S, Tajima A, Aizawa T, Fu LT, Itoh H (2004) Early aerobic training increases end-tidal CO2 pressure during exercise in patients after acute myocardial infarction. Circ J 68:778–783
Arena R, Guazzi M, Myers J (2007) Prognostic value of end-tidal carbon dioxide during exercise testing in heart failure. Int J Cardiol 117:103–108
Koike A, Wasserman K, Armon Y, Weiler-Ravell D (1991) The work-rate-dependent effect of carbon monoxide on ventilatory control during exercise. Respir Physiol 85:169–183
Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A (1994) Myocardial infarction and coronary deaths in the world health organization MONICA project. Circulation 90:583–612
Torp-Pedersen C, Køber L, Elming H, Burchart H (1997) Classification of sudden and arrhythmic death. Pacing Clin Electrophysiol 20:2545–2552
Koike A, Hiroe M, Adachi H, Yajima T, Yamauchi Y, Nogami A, Ito H, Miyahara Y, Korenaga M, Marumo F (1994) Oxygen uptake kinetics are determined by cardiac function at onset of exercise rather than peak exercise in patients with prior myocardial infarction. Circulation 90:2324–2332
Koike A, Itoh H, Kato M, Sawada H, Aizawa T, Fu LT, Watanabe H (2002) Prognostic power of ventilatory responses during submaximal exercise in patients with chronic heart disease. Chest 121:1581–1588
Franciosa JA, Park M, Levine TB (1981) Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardiol 47:33–39
Hansen JE, Sue DY, Oren A, Wasserman K (1987) Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. Am J Cardiol 59:669–674
Metra M, Dei Cas L, Panina G, Visioli O (1992) Exercise hyperventilation in chronic congestive heart failure, and its relation to functional capacity and hemodynamics. Am J Cardiol 70:622–628
Koike A, Itoh H, Oohara R, Hoshimoto M, Tajima A, Aizawa T, Fu LT (2004) Cerebral oxygenation during exercise in cardiac patients. Chest 125:182–190
Tanabe Y, Hosaka Y, Ito M, Ito E, Suzuki K (2001) Significance of end-tidal PCO2 response to exercise and its relation to functional capacity in patients with chronic heart failure. Chest 119:811–817
Wasserman K, Zhang YY, Riley MS (1996) Ventilation during exercise in chronic heart failure. Basic Res Cardiol 91(Suppl 1):1–11
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hoshimoto-Iwamoto, M., Koike, A., Nagayama, O. et al. Prognostic value of end-tidal CO2 pressure during exercise in patients with left ventricular dysfunction. J Physiol Sci 59, 49–55 (2009). https://doi.org/10.1007/s12576-008-0004-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-008-0004-8