Abstract
Nutritious foods are unaffordable to the majority of households in lower-income countries. Hence, starchy staples tend to be the main source of diets for men, women, and children, implying poor diet quality and deficiency in key nutrients that are crucial for child’s physical growth and cognitive development. Much of existing studies on child linear growth or stunting examined the impact of current dietary consumption on physical growth even though the effect of diets and nutrition would only be observed with time lag. This study examined the associations between sustained consumption of animal sourced foods (ASFs) and adequate nutrient intakes (measured by the mean nutrient adequacy (MPA)) and linear growth differences among children in rural and small towns in Ethiopia. Using nationally representative longitudinal data, results of fixed effects estimations suggested that a standard deviation increase in the household MPA was associated with a 10–11% increase in the height-for-age z-score; and a 10% increase in the household MPA was associated with a 3.2 – 5.6% reduction in stunting rate in children 6–59 months of age. Results also indicated some associations between past ASFs consumption and current linear growth outcomes in younger children. The gap in the household MPA was over 41%, indicating the need for nutrient-dense ASFs in the household diets despite the sustainability concerns around ASFs. Key implication is that policies and interventions aimed at improving linear growth outcomes of children in rural and small towns in Ethiopia should improve household’s access to ASFs and nutrient acquisition capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In 2021, the share of the global population who could not afford a healthy diet—that is energy sufficient, nutritionally adequate and sourced from diverse foods – was estimated at 42.2% (FAO et al., 2023). The corresponding share in low-income and lower-middle-income countries was estimated at 86.1% and 70.2%, respectively. In Ethiopia, the corresponding share was 83.8% (FAO et al., 2023). This has huge implications on undernutrition in the population in general and young children in particular. This is because undernutrition in early years is associated with shorter adult height, lower attained schooling, reduced economic productivity (Victoria et al., 2008), and can result in intergenerational transfer of poor birth outcomes in the next generation (Addo et al., 2015; Victoria et al., 2008). Child stunting (being short for their age) together with deficiencies of iodine and iron can contribute to children not reaching their developmental potential; and deficiencies of vitamin A and zinc can result in deaths (Black et al., 2013).
In Ethiopia, despite a reduction of stunting prevalence by about 14 percentage points between 2005 and 2019 among children under 5 years of age, child undernutrition remains high (EPHI & ICF, 2021). The same report indicates that, in 2019, the prevalence of stunting among children under two years of age ranged between 17 and 36%. Unless the current trend in the reduction of stunting in Ethiopia will be accelerated, the country will be offtrack to meeting one of the SDG targets and its ambitious plan to end stunting in children under two years of age by 2030, also called the Seqota Declaration (FDRE, 2016).
There is a vast literature on the determinants of child linear growth outcomes or stunting in Ethiopia. Based on a systematic literature review, Tasic et al. (2020) found that child characteristics such as age, sex, and the incidence of diarrhea; parental characteristics including maternal education and height; and household characteristics including wealth status, food security, access to improved water sources and sanitation facilities, are among major determinants of child stunting in Ethiopia. The aforementioned study further grouped stunting determinants in Ethiopia into immediate, underlying, and the enabling determinants, according to the conceptual framework on maternal and child nutrition (UNICEF, 2021) which also guides this study.
Chief among immediate causes of child stunting in Ethiopia includes inadequate dietary intakes of energy and different nutrients needed for child growth (e.g. Haile et al., 2016; Wirth et al., 2017). Various studies show that energy and nutrient intakes among young children in Ethiopia are far below the WHO recommendations (e.g. Haileselassie et al., 2022; Feyisa et al., 2020; Mengistu et al., 2017; EPHI, 2016, 2013; Baye et al., 2012). Yet, much of existing studies on the determinants of child stunting in Ethiopia examined the importance of diet quality and adequacy using proxy indicators such as dietary diversity score (e.g. Gebreayohanes & Dessie, 2022; Mohammed et al., 2019; Motbainor et al., 2015), notwithstanding the strong associations between dietary diversity and nutrient adequacy (e.g. Mekonnen et al., 2020; Arimond et al., 2010; Arimond & Ruel, 2004; Ruel, 2003). This study contributes to the literature by examining the quality of diets consumed in the household, taking into account the type of foods consumed and adequacy of nutrient intakes.
Studies elsewhere documented the importance of animal source foods (ASFs) for improving diet quality and children’s linear growth or stunting (e.g. Grillenberger et al., 2006; Headey et al., 2018; Iannotti et al., 2017, 2019; Murphy & Allen, 2003; Neumann et al., 2002; Parikh et al., 2021), and some studies also in Ethiopia (e.g. Hoddinott et al., 2015; Mekonnen et al., 2022; Omer et al., 2019, 2022). However, evidence on the longer-term effect of ASFs consumption on linear growth is inconclusive. For example, a randomized controlled trial involving egg intervention during early complementary feeding in Ecuador found improved linear growth and reduced stunting (Iannotti et al., 2017). A follow up study, however, did not find the effect of the intervention in linear growth after two years (Iannotti et al., 2019). Relatedly, using observational data from the Demographic and Health Surveys for several countries, a recent study identified the independent association of ASFs consumption and children’s linear growth or stunting (e.g. Headey et al., 2018). The aforementioned study, however, lacks information on whether sustained consumption of ASFs is associated with child linear growth. This study adds to the growing literature by considering sustained (past as well as current) consumption of ASFs.
This study is embedded in two related strands of literature on child linear growth determinants. The first is on nutrient intakes which has been found strongly linked with child linear growth outcomes (e.g. Gibson et al., 2009; Grillenberger et al., 2006; Puentes et al., 2016), even though the role of micro-nutrients is considered rather supportive for optimal functioning of immune system and optimal growth outcomes (Ramakrishnan et al., 2009; Sanin et al., 2018). The second is on the importance of ASFs as described above. This study contributes to both strands of the literature because many of previous studies drew from cross-sectional or pooled panel observations even though the effect of dietary consumption on child growth would only be observed with time lag. Studies with repeated observations over a child’s consumption and linear growth are either scarce (Puentes et al., 2016; Zaharia et al., 2021), or those available are mainly from nonrepresentative sample with limited geographic coverage (e.g. Belayneh et al., 2021; Puentes et al., 2016). Further, the aforementioned longitudinal studies, with the exception of Puentes et al. (2016), relied on proxy indicators such as dietary diversity score or indicators of consumed food groups without measuring the quantity and adequacy of nutrient intakes, even though dietary diversity is strongly associated with nutrient adequacy as described above.
In sum, the main objective of this study is to examine the associations between household’s food and nutrient acquisition capacity and linear growth in children in rural and small towns in Ethiopia. Specifically, we examine whether household’s sustained intake of multiple nutrients (measured by the mean nutrient adequacy (MPA)) is linked with child’s linear growth. In addition, we examine whether household’s sustained acquisition of animal sourced foods (ASFs) is strongly linked with child’s linear growth. This study differs from previous research in Ethiopia in at least two ways: (1) it evaluates the actual quantities consumed and their adequacy (the MPA) instead of using other proxy indicators for nutrient consumption; and (2) compares the association of past (lagged) consumption of food (including ASFs) and nutrients against current (contemporaneous) consumption on child linear growth outcomes using nationally representative longitudinal data.
2 Material and methods
2.1 Data
We used all the three waves of the Ethiopia Socioeconomic Panel Survey (ESS) collected by the Ethiopia Central Statistics Agency in collaboration with the World Bank as part of the Living Standards Measurement Study–Integrated Surveys on Agriculture (LSMS-ISA) project. The ESS was implemented every two years: the households in the survey were visited in 2011/2012, re-visited in 2013/2014, and then re-visited again in 2015/2016. The first wave was a nationally representative survey of 3,969 households living in rural and small town areas. The subsequent waves added samples from large town areas, increasing the total sample to over 5000 households in waves 2 and 3 (CSA and World Bank 2020). For the purpose of establishing associations between child linear growth and past consumption patterns, we limited our analysis to the same set of households that were interviewed at the baseline (wave 1) and subsequent waves.
This study considered 1708 households with children aged less than 59 months at the baseline (i.e. wave 1) and had complete information on other household level indicators. Some of the households had multiple children under five years of age. In total 2386 children were considered in the study, including those younger than 6 months at the baseline. The panel survey therefore interviewed each study panel household up to three times. For children 6—59 months of age we calculated two indicators of child growth status using the 2006 World Health Organization (WHO) growth standards: the height for age z scores (HAZ) and a binary indicator for stunting based on whether the HAZ score was below two standard deviations from the WHO growth standards (WHO, 2006).Footnote 1 Children aged 6—59 months with height measurement outside of the normal growth standards (i.e. less than or above five standard deviations) according to the WHO growth reference were excluded from the baseline analysis but were kept in the sample. That is, some of these children were included back in the analysis of the subsequent survey waves because their height measurement in those surveys was within the normal growth standards described above. Similarly, children younger than six months at the baseline were excluded from the baseline analysis but were included in the analysis of the last two survey waves, yielding unbalanced panel. Hence, the total number of children with HAZ scores within the WHO growth standards at the baseline, the second- and third- survey waves was 1605, 2297 and 2376, respectively. Only 1022 children were observed in all three survey waves whereas the remaining children were observed only twice (i.e. either in waves 1 and 2, waves 2 and 3, or waves 1 and 3). Finally, the overall attrition rate was quite small since it was only ten children and nine households that completely dropped out from the analysis.
Besides height measurements for children under five years of age,Footnote 2 the ESS contains information on a wide range of household socioeconomic and demographic characteristics in each round of the survey including age and education status of each household member, and household level information such as access to water and sanitation, nonfood spending, food consumption and spending, among others. We calculated the nutrient content of foods consumed in the household using the Ethiopian food composition table (Ågren & Gibson, 1968), and other sources including the USDA (2016), Langenhoven et al. (1991), West et al. (1989), where nutrient information was missing. Waste and retention factors were applied to raw foods to account for nutrient losses during preparation (USDA, 2016). As this study is concerned mainly with household nutrient and dietary consumption and child linear growth, we checked whether the daily total food consumption per adult equivalent was feasible based on criteria from a human nutrition perspective; excluding households whose estimated energy consumption per adult female equivalent (AFE) was below 500 kilocalories per day and those above 5,000 kilocalories per day (Voortman et al., 2017). More detailed procedures followed including the use of AFE are described elsewhere (Mekonnen et al., 2020).
Having calculated the nutrient contents from foods consumed at home, we estimated the mean probability of nutrient adequacy (MPA) per AFE based on 11 nutrients including protein, iron, zinc, thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, vitamin C, and vitamin A. The probability approach to calculating nutrient adequacy is considered robust to misspecification of variance so long as the distribution of requirements is symmetric (Wiesmann et al., 2009). The probability approach makes use of the estimated average requirements (EAR), which is the daily nutrient intake value estimated to meet the requirements of half of the healthy individuals in a given population (IOM, 2006). Assuming normal distribution of nutrient requirements of non-pregnant, non-lactating adult women, we calculated the probability of adequacy for each of the above nutrients (excluding iron) by solving for the standardized score (z-score):
where \(SD\) and \(CV\) respectively represent the standard deviation and coefficient of variation for nutrient \(i\). We replaced usual intake by observed daily nutrient consumption per AFE. Note that even though we used per AFE values to proxy for intra-household distribution of food (Coates et al., 2017; Sununtnasuk & Fiedler, 2017), actual food distribution may differ from that implied by the AFE scale, which is one of the limitations of this study. We used the EAR and CV values from WHO/FAO (2004) for an adult female (19–65 years) (see Supplementary Table A1). Using the standardized z-scores and the property of standard normal distribution, we computed the probability of adequacy for each nutrient. As the requirements for iron are known to be skewed for non-pregnant, non-lactating women (see IOM, 2006, 43–44), the probability of adequacy for iron was calculated using the probability of adequacy table (see Wiesmann et al., 2009, p 206, adapted from IOM, 2006) assuming a bioavailability of 5%. Last, we calculated the MPA by averaging the probability of adequacy of 11 nutrients described above.
Further, we examined which food groups were consumed in the household over the previous 7 days, based on information whether anyone in the household consumed any food during the recall period. Specifically, we considered 7 food groups which are important for child growth according to WHO guidance, including (1) cereals, roots and tubers; (2) legumes and nuts; (3) milk and its derivatives; (4) meat products (meat, poultry, offal, and fish); (5) eggs; (6) vitamin A-rich fruits and vegetables (leafy green vegetables, yellow fruits and vegetables); and (7) other fruits and vegetables (WHO, 2010). Even though the standard recall period for this type of research is twenty-four hours, our data is available for seven days. However, considering household consumption data over seven days period is likely to be closer to the household’s normal consumption pattern. In addition, the seven days reference may take into account religious restrictions among orthodox Christians in Ethiopia on the consumption of animal sourced foods on specific days in a week which otherwise a twenty-four hours recall may not capture (Haileselassie et al., 2022; Desalegn et al., 2019; Melesse & van den Berg, 2020).
3 Empirical strategy
As noted above, the effect of dietary consumption on child’s linear growth would be observed with time lag. Hence, to examine the relationship between current child outcomes and household’s dietary consumption in the previous period, we run panel regressions of the following form, following Zaharia et al. (2021) and informed by the concept of nutrition production function described in Behrman and Deolalikar (1988):
where \({Child\,outcome}_{it}\) is either the HAZ score of child \(i\) in time \(t\), or a binary variable indicating whether or not the child is stunted at time\(t\). MPA is the key variable of interest and denotes the consumption of multiple nutrients (the mean probability of nutrient adequacy) by the \(i{\prime}th\) child in the household at time \(t-1\). \(X\) measured at time \(t\) denotes a vector of controls including the child’s age (including third-degree polynomial to capture the nonlinear relationship between child growth and dietary consumption (Alderman & Headey, 2018; Haile et al., 2016; Puentes et al., 2016), sex, and whether the child recently had diarrhea, maternal education, and whether the household had access to improved water sources and improved sanitation facilities. Controls were selected based on the concept of nutrition production function (Behrman & Deolalikar, 1988), the conceptual framework on maternal and child nutrition (UNICEF, 2021), and previous studies especially in Ethiopia (Tasic et al., 2020). Also in \(X\) is the number of days between height measurements for each child across successive survey waves. This is to account for linear growth differences among children due to differences in the dates of measurement since children experience high growth and growth spurts during the first two years (Puentes et al., 2016; Victoria et al., 2010). \({e}_{i,t}\) is a random error. Since there is potential for error \({e}_{i,t}\) variances to be correlated over time for a given child or over children within a given cluster, we cluster standard errors at enumeration area level (Abadie et al., 2017).
\({\delta }_{j,t}\) denotes location by survey wave fixed effects that control for unobserved temporal characteristics such as food prices, market availability, the health environment, etc. that affect children’s outcomes that are common to all children in a given location. In some specifications we use child fixed effects to control for unobserved child characteristics that do not change over time, such as maternal height, health and nutrition during pregnancy, birth outcome and exclusive breastfeeding before the age of 6 months, together with survey wave fixed effects to account for temporal shocks that affect all children in a survey.
Further, we examine the relationships between ASFs consumption and child growth outcomes by modifying Eq. 2, and replacing MPA by ASFs and other food groups measured at time t-1:
where \({\varvec{O}}\) denotes a vector of food groups other than ASFs (including binary indicators for starchy staples, legumes, vegetables, and fruits), the controls \(X\) and \(\delta\) are the same as in Eq. 2 and \({\mu }_{i,t}\) is error terms of cluster and enumeration area fixed effects. Variants of this model consider two definitions of ASFs consumption: by type of individual ASF and the total number of ASFs consumed. The latter is considered because different ASFs contain different growth-stimulating nutrients and previous studies also found stronger associations between multiple ASFs consumption and child growth outcomes (Headey et al., 2018).
To check the robustness of results, we rerun the above models after including contemporaneous consumption in \(X\); based purely on contemporaneous consumption; by changing controls and fixed effects; and by running separate regressions for different age groups. The analysis of the longitudinal panel on contemporaneous consumption and child growth outcomes re-examines findings of previous cross-sectional and pooled panel studies that found strong links between child growth outcomes and contemporaneous consumption, since the effect of current consumption on child growth outcomes would be observed with time lag. We run regressions with user written Stata command, reghdfe, that efficiently absorbs multiple levels of fixed effects (Correia, 2014). The main advantage of this method is that it iteratively removes groups with only one observation which otherwise can overstate statistical significance and lead to incorrect inference (Correia, 2015).
Last, despite the use of lagged explanatory variables and fixed effects estimations, we interpret the results as associations rather than causal relations since lagging explanatory variables may not sufficiently address endogeneity concerns (Bellemare et al., 2017) i.e. factors that might determine the household’s food and nutrition acquisition capacity might still affect nutritional status through other channels.
4 Results
4.1 Descriptive statistics
Characteristics of the sample and variables used in this study are described in Table 1 and Figs. 1, 2 and 3. The average height-for-age z-score (HAZ) of children was -1.41, and the prevalence of stunting in children was 38%. The household’s mean nutrient adequacy (MPA) was 0.41. Figures 1 and 2 show the distribution of both the HAZ score and the MPA by survey wavesFootnote 3. The share of households who consumed only one type of animal sourced food (ASF), and two or more types of ASFs was 31% and 13%, respectively (Fig. 3). When individual food groups were evaluated, the share of households consuming starchy staples was above 60%, and those consuming legumes and vegetables was between 30%-36%. Whereas the share of households consuming eggs, dairy, and meat was between 13%—24% (Fig. 3). The average age of children was 33 months. Nearly half of the children were girls, and only about 8% of the children had experienced diarrhea in the one month preceding the survey. Only 4% of mother’s had above 8 years of education. The share of households who had access to improved water source and improved latrines were 60% and 19%, respectively. A little over 90% of households reported that all household members eat the same diet during the study period. Descriptive statistics of key variables for the sub-sample corresponding to children age 6–59 month are presented as Annex Table 9.
4.2 Econometric results
4.2.1 The average nutrient adequacy (MPA) and child linear growth outcomes
Table 2 summarizes the results of fixed effects (FE) panel estimations of variants of Eq. 2.Footnote 4 The Column headers in Table 2 indicate the dependent variables including the HAZ score (Columns 1, 2, 5, & 6) and stunting (Columns 3, 4, 7 & 8). For the binary outcome stunting, fixed effects estimations imply linear probability models. Besides the list of controls reported in the result Tables, Columns 1—4 and Columns 5—8 control for the enumeration area by survey fixed effects and child and survey fixed effects, respectively. Additional control in all specifications include the number of days between successive height measurements for each child as described in Table 1.
The results presented in columns 1–8 (with the exception of column 6) show that lagged MPA, but not contemporaneous MPA, was significantly associated with child linear growth outcomes, regardless of whether child linear growth was measured in terms of the HAZ score or stunting. The magnitude of the coefficient estimates to lagged MPA implies that, on average, a standard deviation increase in lagged consumption of an MPA was associated with a 0.14 unit increase in the HAZ score (Columns 1&2). This is roughly a 10 percent increase over the mean HAZ score. Correspondingly, results suggest that a 10% increase in lagged MPA was associated with a 1.4 percentage points reduction in the likelihood of being stunted (Columns 3&4). Since the overall stunting rate in the sample is 38 percent, these percentage points translate into a 3.7 percent reduction in stunting rate. The reduction in the stunting rate associated with a 10% increase in lagged MPA increased to 4.2–5.2 percent when child and survey fixed effects were used instead of enumeration area by survey fixed effects (Columns 7 and 8). These are all important findings as coefficient estimates remained statistically significant even after controlling for child specific unobserved heterogeneities beside other controls. Further, the null hypotheses for joint tests for equality of the estimated coefficients across specifications were rejected, suggesting that the results were not spurious significant correlations. Footnote 5 Among controls, the incidence of diarrhea and child’s age (including its polynomial roots capturing the non-linear relationships between child growth and age) were significantly associated with the HAZ score with the expected signs (see Columns 1–4). We re-run Columns 1- 8 after replacing the household’s access to improved water sources and improved sanitation facilities by the household’s socioeconomic status score. Corresponding results presented in the Annex Table 10 show that the results are robust to specification changes.
We further checked the robustness of the results by limiting our analysis to children only aged 6–59 months. Even though the number of observations reduced by over one-third, results suggest that past consumption of an MPA, but not contemporaneous MPA, was indeed significantly associated with child linear growth outcomes (Table 3). Considering the mean HAZ score of -1.47 for this age group (see Annex Table 9), Columns 1 and 2 suggest that a standard deviation increase in lagged, but not contemporaneous, MPA was associated with a 10–11 percent increase over the mean HAZ score.Footnote 6 These estimates are roughly the same as those reported above for the full sample. Similarly, Columns 3 and 4 and Columns 7 and 8 of Table 3, suggest that a 10% increase in lagged MPA was associated with a 1.3—2.3 percentage point reduction in the likelihood of being stunted. Considering the overall stunting rate of 41 percent for this age group, this translates roughly into a range of 3.2 – 5.6 percent reduction in stunting rate. This range is slightly wider than the 3.7 – 5.2 percent reduction in the stunting rate that we found for the full sample (Table 2). Overall these estimates for lagged MPA on both the HAZ score and stunting show the robustness of the results. Also, the joint hypotheses tests for the equality of the estimated coefficients across specifications were rejected, suggesting that the results were not spurious significant correlations.Footnote 7
4.2.2 Animal sourced foods consumption and child linear growth outcomes
In this section, we explore the relationships between lagged consumption of animal sourced foods to current linear growth outcomes while controlling for other food groups consumption and relevant covariates. Previous studies found that consumption of animal sourced foods is strongly associated with child’s linear growth outcomes. As noted above, however, previous studies, with a few exceptions, relied on cross-sectional or pooled panel observations that do not allow establishing associations between past consumption history to child’s current linear growth outcomes. This section attempts to add some evidence to the literature using longitudinal data. Table 4 reports the results of estimating various versions of Eq. 3, using the full sample. As in Sect. 4.1., the dependent variable takes two forms: Columns 1, 2, 5, and 6 consider the HAZ score, and Columns 3,4,7, and 8 employ a dummy variable if the child was stunted. Columns 1—4 and Columns 5—8 control for the enumeration area by survey fixed effects, and child and survey fixed effects, respectively.
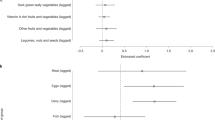
The results presented in Table 4 appear to show a lack of strong relationships between the number of ASFs consumed in the past and child’s current linear growth outcomes in any of the specifications. In contrast, there appears to be some negative association between the likelihood of the child being stunted and contemporaneous consumption of one type of ASF (Columns 4),Footnote 8 and with two or more types of ASFs, even though the association was only significant at p < 0.10 (Columns 4 and 8). Since accessibility of each ASF may not be similar and aggregation may hide some of the variations among ASF types in the data, we re-run Columns 1- 8 after replacing the number of ASFs consumed by types of ASFs (Table 5).
Results presented in Table 5 suggest that there was a strong negative relationship between lagged eggs consumption and the likelihood of being stunted (Columns 3 and 4). The results suggest that sustained consumption of eggs in the past was associated with a 7–8 percentage point reduction in the likelihood of being stunted. On average, this translates into a 18–21 percent reduction in the likelihood being stunted, since the overall stunting rate in the sample is 38 percent. In addition, contemporaneous consumption of meat and fish was strongly associated with increased HAZ score (Column 6) and with a reduced likelihood of being stunted (Column 8). Similarly, results suggest that contemporaneous consumption of dairy was associated with increased HAZ score (Column 2), and with a reduced likelihood of being stunted (Column 4). The joint hypotheses tests for the equality of the estimated coefficients across specifications (including for eggs, dairy, and meat) were rejected, suggesting that the results were not spurious significant correlations.Footnote 9
As in the preceding section, we conducted additional analysis by limiting the sample to children only aged 6–59 months. Tables 6 and 7 present the results with similar specifications corresponding to Table 4 and 5, respectively. According to Table 6, consumption of two or more ASFs in the past, but not current consumption, was strongly associated with the likelihood of being stunted (Columns 3 and 4). Considering the average stunting rate of 41 percent for this age group, the results suggest that sustained consumption of two or more types of ASFs in the past was associated with a 24–27 percent reduction in the likelihood being stunted, on average.Footnote 10
Among ASF types, lagged consumption of dairy appear to be strongly associated with the HAZ score (Table 7, Columns 1 and 2). Results suggest that sustained dairy consumption in the past was associated with about 34 percentage point increase in the HAZ score. This, on average, translates into about 23 percent increase in the HAZ score since the mean HAZ score for this age group was -1.47. Columns 3 and 4 also show negative associations between meats consumption in the past and the likelihood of being stunted, even though it was significant only at 10% level (Columns 3 and 4). The null hypotheses for joint tests for equality of the estimated coefficients across specifications for past consumption of dairy were rejected, suggesting that the results were not spurious significant correlations.Footnote 11
4.2.3 Contemporaneous consumption and child linear growth outcomes
As noted above, much of previous studies reported evidence of strong associations between child linear growth outcomes and contemporaneous consumption mainly using one-time observations. However, as shown in Sects. 4.1 and 4.2 above, such association disappears (in the case of MPA) or becomes less strong (in the case of ASFs) when past consumption status is taken into account. This section re-examines such association using longitudinal data.
Table 8 presents a summary of results from fixed effects estimations of the HAZ score and stunting for children aged 6–113 months, and 6–59 months only. Results in each panel and column in Table 8 come from separate regressions. From Panel a, there was only one out of 8 specifications that show a weakly significant association between contemporaneous MPA and child linear growth outcome (Column 2). From Panel b, there was a strong association between contemporaneous consumption of 1 ASF and child stunting (Column 2). Columns 2 and 8 also showed some weak association (that is significant only at p < 0.10) between contemporaneous consumption of two or more ASFs and linear child growth outcomes. From Panel c, consumption of meat and fish (Column 3 and 4) and consumption of dairy (Columns 2 and 7) appear to be associated with child linear growth outcomes, even though two of these four coefficients were significant only at p < 0.10. Regression results that take into account location and time fixed effects appear to show some associations between contemporaneous consumption of ASFs and child linear growth outcomes, especially when older children were included in the analysis. Nonetheless, results were not as strong as those reported in previous studies with cross-sectional data.
5 Discussion and conclusions
5.1 Discussion
Less than seven years remain till the globally agreed date to end all forms of malnutrition, including stunting in children under 5 years of age, one of the targets of the Sustainable Development Goals (SDG 2 zero hunger). However, the prevalence of child stunting in Ethiopia is still high and the progress to date in the reduction of stunting appears to be slow according to survey data used in this study and a more recent survey by MOH (MOH, 2023). Identifying optimal conditions to end hunger and improve diets within the limits of planetary boundaries is one of the evidence-based priorities that the Scientific Group to the United Nations Food Systems Summit suggested (von Braun et al., 2021). This study examined whether household’s average nutrient acquisition capacity (measured as MPA) predicts linear growth in children, and whether household’s sustained acquisition of animal sourced foods is associated with child linear growth in rural and small towns in Ethiopia. Much of previous studies on child linear growth determinants used proxy indicators such as dietary diversity scores or simple indicators of consumed food groups without measuring the quantity and adequacy of nutrient intakes, and relied mostly on cross-sectional observations even though the effect of dietary consumption on child linear growth would be observed with time lag. This study contributes to filling the gap in the literature using longitudinal data.
Results suggest that household’s lagged MPA, but not contemporaneous MPA, was strongly associated with current child growth outcomes, regardless of whether child growth was measured in terms of the HAZ score or stunting. For example, for children 6–59 months of age, a standard deviation increase in lagged MPA was associated with a 10–11 percent increase over the mean HAZ score. Similarly, a 10% increase in lagged MPA was associated with a 3.2 – 5.6 percent reduction in stunting rate. When older children (aged up to 114 months) were included in the analysis, the corresponding increase in the average HAZ score was 10 percent and the reduction in stunting rate was 4.2–5.2 percent, respectively. The results were robust to specifications even after controlling for child specific unobserved heterogeneities and survey fixed effects beside other controls. Since the MPA comprises both macro- and micronutrients, results suggest that both types of nutrients were important predictors of child growth. However, the specific contribution of micronutrients in linear growth outcomes could either be small (Ramakrishnan et al., 2009; Sanin et al., 2018), or that their role is rather indirect—via supporting the optimal functioning of immune system and development potential (Black et al., 2013). Identifying the independent roles of macro- and micronutrients for linear growth outcomes is beyond the scope of this study.
Animal source foods are known to be rich in high quality protein and other growth-stimulating nutrients (Dror & Allen, 2011). Recent observational studies such as Headey et al. (2018) found strong associations between contemporaneous ASFs consumption and child linear growth or stunting. Nonetheless, findings of some randomized control trials in low-income countries suggest that the longer-term effect of ASFs consumption on linear growth is inconclusive (Allen & Dror, 2011; Iannotti et al., 2019). In this context, this study sheds some light using longitudinal data from rural and small towns in Ethiopia. For children 6–59 months of age, results suggest that lagged (sustained) consumption of two or more ASFs was associated with an average of 24–27 percent reduction in the likelihood of being stunted. Among ASF types, lagged consumption of meats was associated with stunting reduction, and lagged consumption of dairy was associated with improvement in the HAZ score. When older children (aged up to 114 months) were included in the analysis, results did not show strong relationships between the number of ASFs consumed in the past and the child’s current linear growth outcomes. However, among ASFs results suggest that sustained consumption of eggs was associated with a 18–21 percent reduction in the likelihood of being stunted. Further, there appears to be some association between child linear growth outcomes and contemporaneous ASFs consumption.
While findings of this study provide some support to the importance of ASFs to child’s linear growth in small towns and rural Ethiopia, results were not as strong as those reported in previous studies such as Headey et al. (2018) that used cross-sectional or pooled panel observations from several countries. However, to put our results in context, first, the surveys used this study were not designed primarily for nutrition research and the consumption data was for the household. Hence, our analysis may not have fully captured intrahousehold food allocations, specifically children’s access to ASFs and the adequacy of quantity consumed. This is because previous studies suggest that intra-household food allocation is determined by relative differences in household members’ income, bargaining power, food behaviors, social status, tastes and preferences, and interpersonal relationships (Harris-Fry et al., 2017), among others. Second, our reference period of consumption was seven days unlike the twenty-four hours reference used in Headey et al., (2018). This may have minimized potential variations in the consumption data that is needed for identification. Third, due to data limitation (the fact that the surveys used in this study were conducted in about two-year intervals), we were unable to conduct separate panel data estimations for children aged 6–23 months (age category that children experience higher growth faltering (Victoria et al., 2010)) and those older than 23 months. Fourth, due to attrition and missing observations, the sample used for analysis was unbalanced panel; hence, results may suffer from the corresponding bias. However, overall results did not show major differences (at least qualitatively) across specifications and when data were disaggregated across age groups, possibly suggesting that sample attrition may not have been systematic.
The first reason, however, may not carry too much weight in explaining the less strong associations between ASFs consumption and child linear growth outcomes observed in this study. This is because, over 90% households in this study reported that all household members eat roughly the same diet regardless of age, a pattern that has changed very little between 2011/12 and 2015/16 as described above. In fact, a study in rural Ethiopia found that children are favored in intrahousehold food allocations (Coates et al., 2018). Relatedly, based on the 2015/16 survey wave used in this study, Mekonnen et al. (2020) found estimates of household nutrient adequacy which were reasonably comparable to that of EPHI (2013)—a national food consumption survey that measured individual level consumption. Nonetheless, to better understand the contribution of ASFs to child’s linear growth would require better data—designed for nutrition research—with repeated measurements of length/height across age groups and the quantities consumed (including that of other food groups) over time, besides other socioeconomic determinants which are important especially for nutrition outcomes of older children (Alderman & Headey, 2018).
On the one hand, results of this study concerning the association of MPA and ASFs with child linear growth outcomes appear to suggest that the full diet perspective needs greater attention to improve linear growth outcomes of children in small towns and rural Ethiopia. On the other hand, the mean probability of nutrient adequacy (MPA) among study households was 41% or less, indicating the burden of multiple nutrient deficiencies. Since household diets in Ethiopia are monotonous whereby grains/starchy staples constitute a major part of children’s, women’s and men’s diet (EPHI, 2013), the enormity of the gap in the household MPA indicates the need for nutrient-dense foods especially that of ASFs in the household diets. Addressing nutrient gaps using ASFs may involve tradeoffs with sustainability, since ASFs have higher environmental impacts (e.g. Willet et al., 2019). Potential solutions may include stimulating farm-livestock systems that is sustainable, e.g. circular systems where there is no food-feed competition, that avoid losses, that recycle waste and use animals to unlock biomass that humans cannot eat (Oosting et al., 2022). Note however that due to the low level of ASFs consumption in Ethiopia, sustainability issues around ASFs may not be the prime concern as much as that of high-income countries where plant-based alternatives are more available or affordable year-round (Parlasca & Qaim, 2022). Further, despite the low level of ASFs consumption in Ethiopia, recent studies show that ASFs and other nutrient-rich foods in the country have become increasingly unaffordable especially to lower-income households (Bachewe et al., 2017; Daba et al., 2021; Haileselassie et al., 2020).
5.2 Conclusions
Given the study limitations described above, findings of this study provide suggestive evidence that policies and interventions aimed at improving linear growth outcomes of children in rural and small towns in Ethiopia should improve household’s access to ASFs and nutrient acquisition capacity. Since the association of ASFs consumption and child linear growth outcomes appears to be heterogenous across age groups, targeting households with younger children may be more beneficial.
Data availability
Publicly available datasets were analyzed in this study. The data, the Living Standards Measurement Study - Integrated Surveys on Agriculture (LSMS-ISA), can be found here: https://www.worldbank.org/en/programs/lsms/initiatives/lsms-ISA#2.
Notes
For children who became older than five years during the second and third survey waves, the HAZ score and the stunting prevalence were calculated using the 2007 WHO reference for children 5–19 years (WHO, 2007).
Height and weight measurements were also taken on children who became older than 5 years during waves 2 and 3; and were included in this study.
A figure describing the distribution of the HAZ scores against the standard normal distribution is presented as supplementary material (Fig. S1).
The FE method is preferred because it allows time-invariant unobserved heterogeneities to be correlated with regressors.
F(1, 4902) = 13.58 with Prob > F = 0.0002 (columns 1 & 3); F( 1, 4900) = 13.31 with Prob > F = 0.0003 (columns 2 &4); F(1, 1794) = 3.53 with Prob > F = 0.0604 (columns 5 &7); and F(1, 1798) = 3.47 with Prob > F = 0.0625 (columns 6 &8).
As noted in Table 9, the mean (SD) of the MPA were 0.41 (0.23). Hence, a unit change in the HAZ score for a SD change in MPA were a 0.1518 (= 0.66 × 0.23) and 0.1564 (= 0.68 × 0.23) unit increase. These suggest that a SD increase in lagged MPA were associated with roughly a 10% (= 0.1518/-1.47) to 11% (= 0.1564 /-1.47) increase in the HAZ score.
F(1, 1622) = 5.69 with Prob > F = 0.0172 (columns 1 & 3); F( 1, 1620) = 0.0172 with Prob > F = 0.0003 (columns 2 &4).
The null hypothesis for joint test for equality of the estimated coefficients for 1 ASF was rejected: F( 1, 1413) = 4.16 with Prob > F = 0.0415 (columns 2 & 4).
The test statistics include: F( 1, 4890) = 5.14 with Prob > F = 0.0235 (columns 1&3, eggs_lagged); F( 1, 4876) = 4.15 with Prob > F = 0.0417 (columns 2&4, eggs_lagged); F( 1, 4876) = 11.84 with Prob > F = 0.0006 (columns 2&4, dairy); and, F( 1, 1774) = 8.79 with Prob > F = 0.0031 (columns 6&8, meat).
The null hypotheses for joint tests for equality of the estimated coefficients for two or more ASFs was rejected: F( 1, 1612) = 2.83 with Prob > F = 0.0924 (columns 1 & 3), and F(1, 2628) = 4.35 with Prob > F = 0.0371 (columns 6&8).
The null hypotheses for joint tests for equality of the estimated coefficients for two or more ASFs was rejected: F( 1, 1612) = 2.83 with Prob > F = 0.0924 (columns 1 & 3), and F(1, 2628) = 4.35 with Prob > F = 0.0371 (columns 6&8).
References
Abadie, A., Athey, S., Imbens, G. W., & Wooldridge, J. (2017). When should you adjust standard errors for clustering? NBER Working Papers 24003, National Bureau of Economic Research, Inc.
Addo, O. Y., Stein, A. D., Fall, C. H. D., Gigante, D. P., Guntupalli, A. M., Horta, B. L., Kuzawa, C. W., Lee, N., Norris, S. A., Osmond, C., Prabhakaran, P., Richter, L. M., Sachdev, H. P. S., Martorell, R., Cohorts Group. (2015). Parental childhood growth and offspring birthweight: Pooled analyses from four birth cohorts in low and middle income countries. American Journal of Human Biology. https://doi.org/10.1002/ajhb.22614
Ågren, G., & Gibson, R. (1968). Food composition table for use in Ethiopia. Swedish international development authority, Stockholm, and Ethiopian nutrition institute, Addis Ababa.
Alderman, H., & Headey, D. (2018). The timing of growth faltering has important implications for observational analyses of the underlying determinants of nutrition outcomes. PLoS ONE, 13(4), e0195904. https://doi.org/10.1371/journal.pone.0195904
Allen, L. H., & Dror, D. K. (2011). Effects of animal source foods, with emphasis on milk, in the diet of children in low-income countries. Nestlé Nutrition Workshop Series. Paediatric Programme, 67, 113–130. https://doi.org/10.1159/000325579
Arimond, M. & Ruel, M. T. (2004). Dietary diversity is associated with child nutritional status: evidence from 11 demographic and health surveys. Journal of Nutrition, 134(10), 2579–2585. https://doi.org/10.1093/jn/134.10.2579. PMID: 15465751.
Arimond, M., Wiesmann, D., Becquey, E., Carriquiry, A., Daniels, M. C., Deitchler, M., Fanou-Fogny, N., Joseph, M. L., Kennedy, G., Martin-Prevel, Y. & Torheim, L. E. (2010). Simple food group diversity indicators predict micronutrient adequacy of women's diets in 5 diverse, resource-poor settings. Journal of Nutrition, 140(11), 2059S–69S. https://doi.org/10.3945/jn.110.123414. Epub 2010 Sep 29. PMID: 20881077; PMCID: PMC2955880.
Bachewe, F., Minten, B., & Yimer, F. (2017). The rising costs of animalsource foods in Ethiopia: evidence and implications. Working Paper 108. IFPRI Ethiopia Strategy Support Program and Ethiopian Development Research Institute.
Baye, K., Guyot, J. P., Icard-Vernière, C., & Mouquet-Rivier, C. (2012). Nutrient intakes from complementary foods consumed by young children (aged 12–23 months) from North Wollo, northern Ethiopia: The need for agro-ecologically adapted interventions. Public Health Nutrition, 16, 10.
Behrman, J. R., & Deolalikar, A. B. (1988). Health and Nutrition. In C. Hollis & T. N. Srinivasan (Eds.), Handbook of Development Economics (pp. 631–711). Amsterdam: North-Holland.
Belayneh, M., Loha, E., & Lindtjørn, B. (2021). Seasonal variation of household food insecurity and household dietary diversity on wasting and stunting among young children in a drought prone area in South Ethiopia: A cohort study. Ecology of Food and Nutrition, 60(1), 44–69. https://doi.org/10.1080/03670244.2020.1789865
Bellemare, M. F., Masaki, T., & Pepinsky, T. B. (2017). Lagged Explanatory Variables and the Estimation of Causal Effects. The Journal of Politics, 79(3), 949–963. https://doi.org/10.1086/690946
Black, R. E., Victora, C. G., Walker, S. P., Bhutta, Z. A., Christian, P., de Onis, M., Ezzati, M., Grantham-McGregor, S., Katz, J., Martorell, R., & Uauy, R. (2013). Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet, 382(9890), 427–451. https://doi.org/10.1016/S0140-6736(13)60937-X
Central Statistics Agency of Ethiopia (CSA) & the World Bank. (2020). Ethiopia Socioeconomic Survey (ESS) 2018/19. Survey Report. Central Statistics Agency of Ethiopia | World Bank.
Coates, J., Rogers, B. L., Blau, A., Lauer, J., & Rob, A. (2017). Filling a dietary data gap? Validation of the adult male equivalent method of estimating individual nutrient intakes from household-level data in Ethiopia and Bangladesh. Food Policy, 72, 27–42.
Coates, J., Patenaude, B. N., Rogers, B. L., Roba, A. C., Woldetensay, Y. K., Tilahun, A. F., & Spielman, K. L. (2018). Intra-household nutrient inequity in rural Ethiopia. Food Policy, 81, 82–94. https://doi.org/10.1016/j.foodpol.2018.10.006
Correia, S. (2014). REGHDFE: Stata module to perform linear or instrumental-variable regression absorbing any number of high-dimensional fixed effects, Statistical Software Components S457874. Boston College Department of Economics, revised 18 Nov 2019.
Correia, S. (2015). Singletons, cluster-robust standard errors and fixed effects: A bad mix.
Daba, A. K., Murimi, M., Abegaz, K., & Hailu, D. (2021). Determinants and constraints to household-level animal source food consumption in rural communities of Ethiopia. Journal of Nutritional Science, 10, e58. https://doi.org/10.1017/jns.2021.52
Desalegn, B. B., Lambert, C., Riedel, S., Negese, T., & Biesalski, H. K. (2019). Feeding practices and undernutrition in 6–23-month-old children of orthodox christian mothers in rural Tigray, Ethiopia: Longitudinal study. Nutrients, 11, 138. https://doi.org/10.3390/nu11010138
Dror, D. K., & Allen, L. H. (2011). The importance of milk and other animal-source foods for children in low-income countries. Food and Nutrition Bulletin, 2011(32), 227–243.
Ethiopian Public Health Institute (EPHI). (2013). Ethiopian national food consumption survey. Ethiopia: Addis Ababa.
Ethiopian Public Health Institute (EPHI). (2016). Ethiopian national micronutrient survey report. Ethiopia: Addis Ababa.
Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF. (2021). Ethiopia Mini Demographic and Health Survey 2019: Final Report. Rockville, Maryland, USA: EPHI and ICF.
FAO, IFAD, UNICEF, WFP and WHO. (2023). The State of Food Security and Nutrition in the World 2023. Rome, FAO: Urbanization, agrifood systems transformation and healthy diets across the rural–urban continuum. https://doi.org/10.4060/cc3017en
Federal Democratic Republic of Ethiopia (FDRE). (2016). “Seqota” Declaration. Implementation Plan (2016 – 2030). Summary Programme Approach Document.
Feyisa, B. B., Tefera, G. M., Endris, B. S., Asayehu, T. T., & Gebreyesus, S. H. (2020). Feeding practice, energy, and nutrient intake adequacy among children aged 6–23 months in Southern Ethiopia: a community based cross-sectional study. Food Science and Nutrition, 8(12), 6680–6690. https://doi.org/10.1002/fsn3.1962
Gebreayohanes, M., & Dessie, A. (2022). Prevalence of stunting and its associated factors among children 6–59 months of age in pastoralist community, Northeast Ethiopia: A community-based cross-sectional study. Plos One, 17(2), e0256722. https://doi.org/10.1371/journal.pone.0256722
Gibson, R. S., Abebe, Y., Hambidge, K. M., Arbide, I., Teshome, A., & Stoecker, B. J. (2009). Inadequate feeding practices and impaired growth among children from subsistence farming households in Sidama, Southern Ethiopia. Maternal and Child Nutrition, 5(3), 260–275. https://doi.org/10.1111/j.1740-8709.2008.00179.x
Grillenberger, M., Neumann, C. G., Murphy, S. P., Bwibo, N. O., Weiss, R. E., Jiang, L., Hautvast, J. G. A. J., & West, C. E. (2006). Intake of micronutrients high in animal-source foods is associated with better growth in Rural Kenyan School Children. British Journal of Nutrition, 95(2), 379–390. https://doi.org/10.1079/bjn20051641
Haile, D., Azage, M., Mola, T., & Rainey, R. (2016). Exploring spatial variations and factors associated with childhood stunting in Ethiopia: Spatial and multilevel analysis. BMC Pediatrics, 16, 49. https://doi.org/10.1186/s12887-016-0587-9
Haileselassie, M., Redae, G., Berhe, G., Henry, C. J., Nickerson, M. T., Tyler, B., & Mulugeta, A. (2020). Why are animal source foods rarely consumed by 6–23 months old children in rural communities of Northern Ethiopia? A qualitative study. PLoS One, 15(3), e0230527. https://doi.org/10.1371/journal.pone.0225707
Haileselassie, M., Redae, G., Berhe, G., Henry, C. J., Nickerson, M. T., & Mulugeta, A. (2022). The influence of fasting on energy and nutrient intake and their corresponding food sources among 6–23 months old children in rural communities with high burden of stunting from Northern Ethiopia. Nutrition Journal, 2022(21), 4. https://doi.org/10.1186/s12937-022-00759-z
Harris-Fry, H., Shrestha, N., Costello, A., & Saville, N. M. (2017). Determinants of intra-household food allocation between adults in South Asia – A systematic review. International Journal for Equity in Health, 16(1), 107. https://doi.org/10.1186/s12939-017-0603-1
Headey, D., Hirvonen, K., & Hoddinott, J. (2018). Animal Sourced Foods and Child Stunting. American Journal of Agricultural Economics, 100(5), 1302–1319. https://doi.org/10.1093/ajae/aay053
Hoddinott, J., Headey, D., & Dereje, M. (2015). Cows, missing milk markets, and nutrition in rural Ethiopia. Journal of Development Studies, 51(8), 958–975.
Iannotti, L. L., Chapnick, M., Nicholas, J., Gallegos-Riofrio, C. A., Moreno, P., Douglas, K., Habif, D., Cui, Y., Stewart, C., Lutter, C. K., & Waters, W. F. (2019). Egg intervention effect on linear growth no longer present after two years. Maternal and Child Nutrition. https://doi.org/10.1111/mcn.12925
Iannotti, L. L., Lutter, C. K., Stewart, C. P., Gallegos-Riofrio, C. A., Malo, C., Reinhart, G., et al. (2017). Eggs in early complementary feeding and child growth: A randomized controlled trial. Pediatrics, 140(1), e20163459. https://doi.org/10.1542/peds.2016-3459
Institute of Medicine (IOM). (2006). In J. J. Otten, J. P. Hellwig, & L. Meyers (Eds.), Dietary reference intakes: The essential guide to nutrient requirements. Washington, D.C.: National Academies Press.
Langenhoven, M. L., Conradie, P. J., Wolmarans, P., & Faber, M. (1991). MRC food quantities manual (Second edition: Parow (Valley). South African Medical Research Council.
Mekonnen, D. A., Talsma, E. F., Trijsburg, L., et al. (2020). Can household dietary diversity inform about nutrient adequacy? Lessons from a food systems analysis in Ethiopia. Food Security, 12, 1367–1383. https://doi.org/10.1007/s12571-020-01056-5
Mekonnen, T. C., Tadesse, S. E., Dawed, Y. A., Cherie, N., Abebe, H., Shumye, G., Mohammed, F., & Hussien, A. (2022). The role of nutrition-sensitive agriculture combined with behavioral interventions in childhood growth in Ethiopia: An adequacy evaluation study. Health Science Reports. https://doi.org/10.1002/hsr2.524
Melesse, M., & van den Berg, M. (2020). Consumer Nutrition Knowledge and Dietary Behavior in Urban Ethiopia: A Comprehensive Study. Ecology of Food and Nutrition, 60(2), 244–256. https://doi.org/10.1080/03670244.2020.1835655
Mengistu, G., Moges, T., Samuel, A., & Baye, K. (2017). Energy and nutrient intake of infants and young children in pastoralist communities of Ethiopia. Nutrition, 41, 1–6. https://doi.org/10.1016/j.nut.2017.02.012
Ministry of Health-Ethiopia (MOH). (2023). National Food and Nutrition Strategy Baseline Survey Report – Key Findings Preliminary Report.
Mohammed, S. T., Habtewold, T. D., Tegegne, B. S., Birhanu, M. M., Sissay, T. A., Larijani, B., & Esmaillzadeh, A. (2019). Dietary and non-dietary determinants of linear growth status of infants and young children in Ethiopia: Hierarchical regression analysis. PLoS One, 14(1), e0209220. https://doi.org/10.1371/journal.pone.0209220
Motbainor, A., Worku, A., & Kumie, A. (2015). Stunting is associated with food diversity while wasting with food insecurity among under five children in East and West Gojjam Zones of Amhara Region, Ethiopia. PLoS One, 10(8), e0133542. https://doi.org/10.1371/journal.pone.0133542
Murphy, S. P., & Allen, L. H. (2003). Nutritional importance of animal source foods. https://doi.org/10.1093/jn/133.11.3932S
Neumann, C., Harrisa, D. M., & Rogers, L. M. (2002). Contribution of animal source foods in improving diet quality and function in children in the developing world. Nutrition Research, 22(1–2), 193–220. https://doi.org/10.1016/S0271-5317(01)00374-8
Omer, A., Hailu, D., & Whiting, S. J. (2022). Effect of a child-owned poultry intervention providing eggs on nutrition status and motor skills of young children in southern Ethiopia: A cluster randomized and controlled community trial. International Journal of Environmental Research and Public Health, 19(22), 15305. https://doi.org/10.3390/ijerph192215305
Omer, A., Mulualem, D., Classen, H., Vatanparast, H., & Whiting, S. J. (2019). Promotion of egg and eggshell powder consumption on the nutritional status of young children in Ethiopia. International Journal of Food Sciences and Nutrition Research, 1(1), 1004. https://doi.org/10.31546/IJFSNR.1004
Oosting, S., van der Lee, J., Verdegem, M., de Vries, M., et al. (2022). Farmed animal production in tropical circular food systems. Food Security, 14, 273–292. https://doi.org/10.1007/s12571-021-01205-4
Parikh, P., Semba, R., Manary, M., Swaminathan, S., Udomkesmalee, E., Bos, R., Poh, B. K., Rojroongwasinkul, N., Geurts, J., Sekartini, R., & Nga, T. T. (2021). Animal source foods, rich in essential amino acids, are important for linear growth and development of young children in low- and middle-income countries. Maternal and Child Nutrition, 18(1), e13264. https://doi.org/10.1111/mcn.13264
Parlasca, M. C., & Qaim, M. (2022). Meat Consumption and Sustainability. Annual Review of Resource Economics, 14, 17–41. https://doi.org/10.1146/annurev-resource-111820-032340
Puentes, E., Wang, F., Behrman, J. R., Cunha, F., Hoddinott, J., Maluccio, J. A., Adair, L. S., Borja, J. B., Martorell, R., & Stein, A. D. (2016). Early life height and weight production functions with endogenous energy and protein inputs. Economics and Human Biology, 22, 65–81. https://doi.org/10.1016/j.ehb.2016.03.002
Ramakrishnan, U., Nguyen, P., & Martorell, R. (2009). Effects of micronutrients on growth of children under 5 y of age: Meta-analyses of single and multiple nutrient interventions. American Journal of Clinical Nutrition, 2009(89), 191–203.
Ruel, M. (2003). Operationalizing dietary diversity: A review of measurement issues and research priorities. The Journal of Nutrition, 133, 3911S–3926S.
Sanin, K. I., Islam, M. M., Mahfuz, M., Ahmed, A. M. S., Mondal, D., Haque, R., & Ahmed, T. (2018). Micronutrient adequacy is poor, but not associated with stunting between 12–24 months of age: A cohort study findings from a slum area of Bangladesh. PLoS One, 13(3), e0195072. https://doi.org/10.1371/journal.pone.0195072
Sununtnasuk, C., & Fiedler, J. L. (2017). Can household-based food consumption surveys be used to make inferences about nutrient intakes and inadequacies? A Bangladesh case study. Food Policy, 72, 121–131.
Tasic, H., Akseer, N., Gebreyesus, S. H., Ataullahjan, A., Brar, S., Confreda, E., Conway, K., Endris, B. S., Islam, M., Keats, E., Mohammedsanni, A., Wigle, J., & Bhutta, Z. A. (2020). Drivers of stunting reduction in Ethiopia: A country case study. American Journal of Clinical Nutrition, 112(Suppl 2), 875S–893S. https://doi.org/10.1093/ajcn/nqaa163
United Nations Children’s Fund (UNICEF). (2021). UNICEF Conceptual Framework on the Determinants of Maternal and Child Nutrition. Nutrition and Child Development Section, Programme Group, 3 United Nations Plaza, New York, NY 10017, USA: A framework for the prevention of malnutrition in all its forms.
US Department of Agriculture, Agricultural Research Service (USDA). (2016). Nutrient data laboratory. USDA national nutrient database for standard reference, Release 28 (Slightly revised). Version Current: May 2016.
Victora, C. G., de Onis, M., Curi Hallal, P., Blossner, M., & Shrimpton, R. (2010). Worldwide Timing of Growth Faltering: Revisiting Implications for Interventions. Pediatrics, 125, 473–480.
Victoria, C. G., Adair, L., Fall, C., Hallal, P. C., Martorell, R., Richter, L., Sachdev, H. S., et al. (2008). Maternal and child undernutrition: Consequences for adult health and human capital. Lancet, 2008(371), 340–357.
von Braun, J., Afsana, K., Fresco, L. O., & Hassa, M. (2021). Food systems: seven priorities to end hunger and protect the planet. Nature, 597(7874), 28–30. https://doi.org/10.1038/d41586-021-02331-x. PMID: 34462598.
Voortman, T., Kiefte-de Jong, J. C., Ikram, M. A., Stricker, B. H., van Rooij, F. J. A., Lahousse, L., Tiemeier, H., et al. (2017). Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam study. European Journal of Epidemiology, 32(11), 993–1005.
West, C. E., Pepping, F., Scholte, I., Jansen, W., & Albers, H. F. (1989). Tanzania food composition table for energy and eight important nutrients in foods commonly eaten in East Africa. Technical Centre for Agriculture and Rural Cooperation (CTA) of ACP/ECP.
Wiesmann, D., Arimond, M., & Loechl, C. (2009). Dietary diversity as a measure of the micronutrient adequacy of women’s diets: Results from rural Mozambique site. Washington, DC: Food and Nutrition Technical Assistance II Project, FHI 360.
Willet, W., Rockström, J., Loken, B., Springmann, M., Lang, T., Vermeulen, S., Garnett, T., Tilman, D., DeClerck, F., Wood, A., et al. (2019). Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. The Lancet Commissions, 393(10170), 447–492. https://doi.org/10.1016/S0140-6736(18)31788-4
Wirth, J. P., Rohner, F., Petry, N., Onyango, A. W., Matji, J., Bailes, A., de Onis, M., & Woodruff, B. A. (2017). Assessment of the WHO Stunting Framework using Ethiopia as a case study. Maternal & Child Nutrition, 2017(13), e12310.
World Health Organization (WHO). (2006). WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. World Health Organization.
World Health Organization (WHO). (2007). WHO child growth standards: Head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: Methods and development. World Health Organization.
World Health Organization (WHO). (2010). Indicators for assessing infant and young child feeding practices part 3: country profiles. World Health Organization.
World Health Organization (WHO), Food and Agriculture Organization (FAO). (2004). Vitamin and mineral requirements in human nutrition (2nd ed.). Geneva: WHO.
Zaharia, S., Ghosh, S., Shrestha, R., Manohar, S., Thorne-Lyman, A. L., Bashaasha, B., Kabunga, N., Gurung, S., Namirembe, G., Appel, K. H., et al. (2021). Sustained intake of animal-sourced foods is associated with less stunting in young children. Nature Food, 2, 246–254. https://doi.org/10.1038/s43016-021-00259-z
Acknowledgements
The author thanks Kalle Hirvonen (IFPRI) and Michiel van Dijk (WUR) for their valuable feedback on the earlier draft of this paper.
Funding
This study was supported by the CGIAR Research Initiative on Sustainable Healthy Diets through Food Systems Transformation (SHiFT) and Wageningen University and Research (WUR). Any opinions stated herein are those of the author and not necessarily representative of or endorsed by CGIAR or WUR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author has no competing interests to declare that are relevant to the content of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mekonnen, D.A. Does household’s food and nutrient acquisition capacity predict linear growth in children? Analysis of longitudinal data from rural and small towns in Ethiopia. Food Sec. 16, 533–550 (2024). https://doi.org/10.1007/s12571-024-01430-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12571-024-01430-7