Abstract
Morphological analysis of organelles is one of the important clues for understanding the cellular conditions and mechanisms occurring in cells. In particular, nanoscale information within crowded intracellular organelles of tissues provide more direct implications when compared to analyses of cells in culture or isolation. However, there are some difficulties in detecting individual shape using light microscopy, including super-resolution microscopy. Transmission electron microscopy (TEM), wherein the ultrastructure can be imaged at the membrane level, cannot determine the whole structure, and analyze it quantitatively. Volume EM, such as focused ion beam/scanning electron microscopy (FIB/SEM), can be a powerful tool to explore the details of three-dimensional ultrastructures even within a certain volume, and to measure several parameters from them. In this review, the advantages of FIB/SEM analysis in organelle studies are highlighted along with the introduction of mitochondrial analysis in injured motor neurons. This would aid in understanding the morphological details of mitochondria, especially those distributed in the cell bodies as well as in the axon initial segment (AIS) in mouse tissues. These regions have not been explored thus far due to the difficulties encountered in accessing their images by conditional microscopies. Some mechanisms of nerve regeneration have also been discussed with reference to the obtained findings. Finally, future perspectives on FIB/SEM are introduced. The combination of biochemical and genetic understanding of organelle structures and a nanoscale understanding of their three-dimensional distribution and morphology will help to match achievements in genomics and structural biology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
3D morphological analysis for organelle in injured motor neurons
When cells encounter stress, the organelles respond drastically and alter their shapes to maintain cellular homeostasis. Mitochondria, which are particularly important in the nervous system for energy production, Ca2+ regulation, and maintenance of plasma membrane potential, have been studied extensively in neurological and nerve injury models (Knott et al. 2008a; Berman et al. 2009; Bilsland et al. 2010; Sheng and Cai 2012; Sleigh et al. 2019; Han et al. 2020; Licht-Mayer et al. 2020; Collier et al. 2023).
Mitochondrial dynamics, that undergo a continuous cycle of fission and fusion, maintain their own homeostasis, and regulate cellular homeostasis as well. Regulatory proteins are now well characterized and their ablation leads to neurodegenerative diseases (Giacomello et al. 2020; Cheng et al. 2022). In particular, mitochondrial fission with dynamin-related protein 1 (Drp1) is one of the representative factors for mitochondria to be transported effectively and accelerate nerve regeneration after injury (Kiryu-Seo et al. 2016; Pozo Devoto et al. 2022).
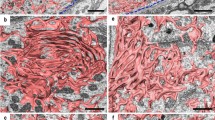
Sciatic nerve and hypoglossal nerve transection models are frequently used as regenerative models after injury (Nakagomi et al. 2003). Since the condition without isolated cells and cultured cells is relatively close to in vivo situation, studying the response in this model might help explain some regenerative mechanisms. However, the mitochondria within neuronal cell bodies display a rich distribution and possess innumerable tiny structures. These make their individual detection almost impossible due to the limited resolution of light microscopy (Fig. 1a). Each mitochondrion can be detected using transmission electron microscopy (TEM), but it is still difficult to understand the whole structure and analyse it quantitatively (Fig. 1b). Then, Focused ion beam/scanning electron microscopy (FIB/SEM) is a powerful tool to solve this problem.
The difficulty in observations of mitochondria in cell bodies with conditional microscopy a The immunohistochemistry with cytochrome c (CytC) for the neuronal cell bodies in mouse hypoglossal nucleus stained the whole cytoplasm and it was impossible to detect each mitochondrion because of their sizes and richness. bThe transmission electron microscopy showed each mitochondrion, but the whole structure couldn’t be understood. Scale bar a 10 µm; b 2 µm (Images from Tamada et al. (2017) J Comp Neurol)
The FIB/SEM is a novel EM technique for obtaining volume EM images (Knott et al. 2008b; Merchán-Pérez et al. 2009; Ohta et al. 2012; Narayan and Subramaniam 2015). In addition to FIB/SEM, serial block face-scanning electron microscopy (SBF-SEM) (Denk and Horstmann 2004; Wilke et al. 2013) and automated tape-collecting ultramicrotome SEM (ATUM-SEM) (Hayworth et al. 2014; Morgan et al. 2016) are used. Under these methods, the x, y, z-axis images can be obtained by acquiring tremendous number of serial EM images and stacking them (Fig. 2a). This makes it possible to know three-dimensional ultrastructure even for very tine organelle in cells (Fig. 2b). They possess different advantages depending on the process of creating the observing surfaces. One of the key advantages of FIB/SEM is that very small z-pitches (~ 10 nm) intervals are obtained by milling gallium ion beams, which is not possible with other methods that uses diamond knives. This allows the observation of small organelles which tend to be distributed close to each other and their membrane-contact sites.
FIB/SEM images in soma a The representative x, y, z EM images could be obtained with serial images from FIB/SEM. b The three-dimensional ultrastructures of organelle in neurons could be understood with the FIB/SEM images. N: nucleus, ER: endoplasmic reticulum, G: Golgi body, M: Mitochondria, arrows: cell membrane Scale bar 5 µm
3D ultrastructure of mitochondria in cell bodies
The FIB/SEM analysis revealed a high degree of morphological variability of mitochondria in the soma, that is, length, width, and roundness (Fig. 3a) (Tamada et al. 2017). One of the unexpected findings in the soma after injury was that the mitochondrial sizes did not change drastically (Fig. 3b), although it has been well established that mitochondrial fission is accelerated after injury and transported into the axon tips to provide ATP sources for nerve regeneration (Cho et al. 2009; Wang and Schwarz 2009; Kiryu-Seo et al. 2010; Chamberlain and Sheng 2019). It is possible that the morphological response of mitochondria in axons is not necessarily the same as that in soma, because axonal mitochondria exist in unique environments and possess different properties than somatic mitochondria (Hollenbeck and Saxton 2005; Pathak et al. 2013). In addition, since the mitochondrial transport speed is not very fast and it is assumed to take several days to convey mitochondria from the soma to the distal axon part (Sheng 2017), mitochondrial fission regulation may occur not only in the soma but also in the distal axonal part.
FIB/SEM analysis of mitochondria in soma and AIS a Each mitochondrion in soma of healthy motor neurons was reconstructed in different colours. Tremendous number of mitochondria showing round and tubular shapes were distributed. b Reconstructed mitochondria in soma of motor neuron at one week after injury showed almost the same features as that in healthy ones. c The AIS in healthy motor neurons didn’t show any mitochondria distribution, although the cell bodies, the axon hillock and the myelinated region did. (blue: cell membrane including AIS region, green: myelin sheath) d At one week after injury, mitochondria distribution could be observed even in the AIS. Their shapes and sizes of mitochondria here varied as the same as that distributed in the soma, the axon hillock, and the myelinated region. (red: cell membrane including AIS region, green: myelin sheath) Scale bar 5 µm [Images from Tamada et al. (2017) J Comp Neurol and Tamada et al. (2021) J Comp Neurol]
As described above, mitochondria repeat fission and fusion for maintaining their homeostasis and cellular homeostasis. The regulating proteins have been explored well and dynamin-related protein 1 (Drp1) is one of the crucial proteins for fission (Ishihara et al. 2009; Kageyama et al. 2011). The analysis of injury-induced Drp1 knockout (KO) mice with FIB/SEM demonstrated alternation processes from healthy shapes to degenerative structures (Tamada et al. 2017). In a previous study, the mitochondrial fission protein Drp1 was found to be crucial for nerve regeneration, and giant mitochondria were detected after injury in the same Drp1 KO mouse (Kiryu-Seo et al. 2016). However, the question of how these structures were formed still remains unsolved. The FIB/SEM analysis in Drp1 KO mice showed spherical mitochondria together with single or multiple long and thin processes at one week after injury (Supp Fig. a) (Tamada et al. 2017). In some cases, one or more spherical mitochondria are connected by tubular mitochondria. Furthermore, the tubular processes of mitochondria disappeared, showing an extremely large round shape, two weeks after injury (Supp Fig. b). This indicated that the mitochondria did not merely swell uniformly, but initially formed long tubules by connecting nearby mitochondria and subsequently enlarged to form a balloon structure. Based on quantitative analysis, their volume was 10-fold more than their volume in non-injured motor neurons (3.0 × 108 nm3 in non-injured vs 1.0 × 109 nm3 in KO mice). This means that under normal conditions, less than 10% of the cytoplasmic volume was occupied by mitochondria, but 50% was occupied at two weeks after injury in the Drp1 inhibited model.
With FIB/SEM, details of the inside of the mitochondria and cristae structures can also be observed. These extremely large spheroid mitochondria were finally degraded with the collapse of the interior and mitophagy-like processes accompanied by lysosomes (Supp Fig. c, d) (Tamada et al. 2017). The degradation of mitochondria from the inside indicated that not only the inhibition of mitochondrial transport to axonal tips but also the collapse of mitochondrial quality control occurred in this Drp1 KO injury model. Usually, mitochondrial homeostasis is maintained by the clearance of damaged mitochondria. In this process, Drp1-dependent fragmentation is crucial (Giacomello et al. 2020). In the Drp1 KO injury model, natural mitophagy accompanied by healthy fission did not occur, and the whole degenerated spherical mitochondria were wrapped with isolation membranes (Tamada et al. 2017).
FIB/SEM analysis provides information about mitochondrial membrane dynamics, which are linked to several common diseases (Giacomello et al. 2020). These results would also accelerate the understanding of mitochondrial pathology and explore their therapies.
3D ultrastructure of mitochondria in AIS
The axon initial segment (AIS), between the end of the axon hillock and the beginning of the first myelin sheath, is an anatomically and physiologically crucial point when mitochondria are transported from somata to axons. Since AIS is the axonal domain responsible for action potential initiation, internal structures and membrane circumstances are characteristic (Nelson and Jenkins 2017; Leterrier 2018). The distribution of mitochondria in AIS is obscure compared to that in axons (Zhang et al 2010; Ohno et al 2011; Cheng et al 2022). This may be because it is extremely difficult to access the AIS, which are small and short regions embedded in neuronal tissues, rendering it impossible to isolate them clearly. Next, we demonstrated the mitochondrial distribution in AIS using FIB/SEM.
Surprisingly, normal AIS possessed almost no mitochondrial distribution, although a large number of mitochondria could be detected in the cell bodies, axon hillock, and myelinated axons (Fig. 3c, Fig, 4) (Tamada et al. 2021). At present, although it is unclear why mitochondria are not distributed only in the AIS region, we speculate two possibilities: (1) the transport velocity along the AIS is so rapid that they cannot be captured, (2) only the motile mitochondria are distributed in the AIS, although some mitochondria move persistently in axons and some are anchored or stationary (Morris and Hollenbeck 1993; Pilling et al. 2006; Ohno et al. 2011).
The details of mitochondria distribution in the healthy AIS. In addition to the AIS shown in Fig. 3c, the other AIS and long myelinated areas are shown. Again, no mitochondria were observed in the AIS region. Scale bar 5 µm
Frequently, the AIS and the nodes of Ranvier are described as similar structures because they share a common ion channel and a common cytoskeleton comprised of βIV spectrin and ankyrin G (AnkG) (Kordeli et al. 1995; Berghs et al. 2000; Chang and Rasband 2013; Zollinger et al. 2015). According to some studies, there is a variation in the distribution of mitochondria within the node of Ranvier. For example, some nodes do not possess mitochondria or some have a few mitochondria (Berthold et al. 1993; Fabricius et al. 1993; Edgar et al. 2008; Ohno et al. 2011; Perkins & Ellisman 2011). Even in the case of mitochondria existences, their size indicated motile mitochondria with short shapes, although the stationary mitochondria with long shapes were not distributed (Ohno et al. 2011). These results are consistent with the hypothesis that mitochondria does not accumulate in the normal AIS because the stationary mitochondria are not likely to exist. Although the results of nodes of Ranvier tend to vary depending on the areas that come across due to their short lengths, the continuous observation of whole AIS structures by FIB/SEM could show more obvious trends.
Meanwhile, after axon injury, a tremendous number and varying length of mitochondria could be observed even in the AIS, myelinated area, axon hillock, and somata (Figs. 3d, 5c, d) (Tamada et al. 2021). At the same time, microglia adhesion around AIS was also explored in the same adhesion manner as that around cell bodies (Fig. 5a, b). These results indicated that the intracellular and extracellular conditions of AIS and somata are likely to become homogenous. However, mitochondrial influx in AIS could also be detected when the crush mild injury model was adopted to maintain AnkG expression (Tamada et al. 2021), which is the main scaffolding protein for AIS assembly and maintenance (Buffington and Rasband 2011). The finding indicates that this phenomenon of mitochondria is not dependent on cytoskeletal depletion.
3D reconstructed images of microglia around AIS a 3D reconstructed images show the details of the surrounding microglia. (Red: neurons with AIS, green: myelin sheath, yellow: microglia, blue and orange: the other neurons which the same microglia attaches). b Representative one-slice SEM images for Fig. 5a showing one AIS (red), two neurons (green and pink), and one microglia (yellow) attached to them. There are no other structures between microglia and other elements, indicating that microglia make direct membrane contact with AIS and neuronal cell bodies. c and d Representative serial SEM images around AIS of Fig. 5a, b. AIS is surrounded in red line and each colours in the AIS are showing mitochondria. Scale bar a 5 µm (Images from Tamada et al. (2021) J Comp Neurol)
Thus, the interaction between microglia and mitochondrial localisation could be considered. Although microglial contacts to the AIS have been reported during development and for maintaining axon conditions such as in multiple sclerosis, traumatic brain injury, and acute neuroinflammatory conditions (Baalman et al. 2015; Clark et al. 2016; Benusa et al. 2017; Benusa and Lafrenaye 2020; Gallo et al. 2022), the functional details and consistent understanding have not yet been explored. Benusa et al. (2017) suggested that the expression of microglia around the AIS increases the intracellular Ca2+ concentration in the AIS and results in the disruption of AnkG expression secondary to calpain, which is regulated by Ca2+ (Schafer et al. 2009; Benned-Jensen et al. 2016). Mitochondria possess a Ca2+ regulatory system, and the mitochondrial transport is attenuated in the axon when cytosolic Ca2+ levels are elevated (Macaskill et al. 2009; Wang and Schwarz 2009; Zhang et al. 2010; Ohno et al. 2011; Saxton and Hollenbeck 2012; Kontou et al. 2021; Kole et al. 2022). With all things considered, some regulatory systems coordinated with microglia, mitochondria, and Ca2+ might occur in AIS in this injury model. Although the details of the function of microglia in AIS have not yet been explored, the microglial attachment does not seem to have a strong and direct influence on AIS collapse with AnkG disruption (Clark et al. 2016; Benusa et al. 2017). Instead, they might mildly maintain AIS circumstances.
Recently, AIS-related mechanisms for nerve regeneration and neurodegenerative disorders have been studied (Kiryu-Seo et al. 2022; Teliska et al. 2022; Tjiang and Zempel 2022). Parallel analysis with FIB/SEM for intra- and extracellular milieu in three dimensions can provide a new comprehensive research target and lead to a new therapy.
Future issues of organelle analysis with FIB/SEM
This review focuses on mitochondrial structures. Furthermore, the endoplasmic reticulum (ER) is also an important factor in regulating cell homeostasis (Westrate et al. 2015; Hetz and Saxena 2017; Marciniak et al. 2022) and the membrane attachment between mitochondria and ER (mitochondria-associated membrane: MAM) (Kornmann et al. 2009; Csordás et al. 2010; Friedman et al. 2011; Prinz et al. 2020) is also a hot topic in neurological diseases (Hedskog et al. 2013; Paillusson et al. 2016; Watanabe et al. 2016; Area-Gomez et al. 2018; Kim et al. 2022). Apart from MAM structures, mitochondria possess a variety of signalling pathways in cells, such as mitochondria-derived vesicles (MDV) (Sugiura et al. 2014; Matheoud et al. 2016; König et al. 2021). For these tiny structures, FIB/SEM analysis is required. FIB/SEM allows us to approach these structures in mammalian tissues, although these studies have been well-explored in yeast and Drosophila so far. Recently, it was reported that the fission point, where Drp1 also accumulates, determines the mitochondrial fate and functions after fission (Kleele et al. 2021; Ul Fatima and Ananthanarayanan 2023). It is also possible to determine the details of the location and attachment manner of the MAM structure in mitochondria with FIB/SEM. As such, the FIB/SEM analysis contributes to providing novel findings and exploring new study fields to understand the physiological meanings by showing organelle structures and interactions with surrounding elements at the ultrastructural level, which has never been seen before.
However, there are some technical challenges to FIB/SEM analysis. First, to perform integral analysis spatially and temporally, some attempts for new volume correlative light-electron microscopy (CLEM) have been challenged (Hayashi et al. 2023) with improved accuracy, throughput, and accessibility. These include new genetically labelling systems such as GFP and APEX (a soybean ascorbate peroxidase) (Martell et al. 2012; Okayama et al. 2014; Lam et al. 2015; Thomas et al. 2019), with arrangements of natural fiducial marks (Maclachlan et al. 2018) or artificial ones (Maco et al. 2014), developing the workflow of image acquisition by devices and application modification (Loginov et al 2022), and so on. Although the CLEM itself is the classic attempt to observe the same structures detected with light microscopy and electron microscopy, which could integrate physiological and ultrastructural data (Armer et al. 2009; Lidke and Lidke 2012; Maco et al. 2014; Blazquez-Llorca et al. 2015; Karreman et al 2016), the state-of-the-art CLEM is used to visualise the nanoscale relationship of specific proteins in the context of the global cellular ultrastructure (Hoffman et al. 2020), to link live-imaging data to high-resolution ultrastructural detail in three dimensions (Loginov et al. 2022) along with the development of other advanced technologies.
Second, sample preparation is also a topic to be discussed (Korogod et al. 2015; Tamada et al. 2020). The simulation model (Tønnesen et al. 2014) and computation geometry analysis (Kashiwagi et al. 2019) of spines with actual values of morphological data have been performed well, accompanied by the development of super-resolution microscopy. A variety of spines also affect their functional properties (Ofer et al. 2021) and consequently affect brain functions such as synaptic plasticity, learning, and memory. Therefore, more precise observations of native structures are required to obtain correct interpretations, and FIB/SEM analysis can be a suitable strategy for this analysis. However, since long ago, EM samples have been called into question because strong aldehydes are used for sample preparation that might convert native structures (Karlsson and Schultz 1965). Therefore, high-pressure cryo-fixation without aldehyde fixatives, which has been known also as the freeze-substitution (van Harreveld et al 1965), was performed in mouse brain tissues to maintain a more native structure (Tamada et al 2020). In the fixation, through the low-temperature embedding process after freezing, the sample in epoxy resin can be obtained as normal EM sample. The study suggested that some tiny structures, such as spine necks, tended to be swollen by conditional chemical fixation (Tamada et al. 2020). Because spine shape critically depends on the arrangement of actin, which is easily influenced by exposure to aldehydes (Honkura et al. 2008), this might contribute to the differences between cryo- and chemical-fixed tissue. The value of FIB/SEM required in the future will depend on the extent to which it reveals the native ultrastructure and whether these cells are representative of the physiological state (Hoffman et al. 2020). Furthermore, also for the study field of organelle, the influence of sample preparation might be one of the discussion points (Möbius et al. 2010). In this situation, more accurate methods, such as cryo-FIB/SEM (Schertel et al. 2013; Zachs et al. 2020), may be required to remove any possible structural changes. Because the present cryo-fixation has some limits technically, including difficulties for obtaining high-quality samples constantly which is strongly depending on quickness of preparation (Ohno et al. 2010) and limitations of effective sample depth, more technical improvement is required to obtain volume FIB/SEM images like the whole brain.
Third, the development of an auto-segmentation system using image processing and deep learning (DL) is a noteworthy field of study. The image segmentation is required to reconstruct the three-dimensional images from serial EM images. At present, the manual segmentation is broadly performed and is a very time- and labour-consuming task. Thus, the automated segmentation method is in high demand accompanied by an improved ability to acquire larger datasets by FIB/SEM, which exceeds the capacity of manual annotation. The main strategy for auto-segmentation is a three-dimensional convolutional neural network (CNN) architecture based on a three-dimensional U-Net (Çiçek et al. 2016; Falk et al. 2019). Some developing tools have been released by an open source, such as a repository providing large quantities of reliable data, codes, and trained models (Heinrich et al. 2021), a new pipeline to train a CNN effectively (Gallusser et al. 2023), improved DL platforms (Suga et al. 2021), and so on. However, more challenges remain in an ongoing effort to reduce human labour for the generation of training data, proofreading predictions, and reducing computation costs. Because innovation in this field has largely been driven by connectomics focusing on segmentation of cell and synaptic junctions (Kreshuk et al. 2011; Dorkenwald et al. 2017; Januszewski et al. 2018), further studies, especially on organelles, are awaited hereafter.
Conclusion
With the arrival of new EM methods, we were able to gather three-dimensional ultrastructures even in tissue samples. This could accelerate a more direct approach for understanding biological questions. In particular, the ability of FIB/SEM to image cells and tissues at several-nanometre resolution over volumes as large as several tens of micrometers is an ideal tool. By solving some technical issues and combining several microscopic technologies, novel findings can be understood in the future.
Data availability
All the relevant data used in this study can be accessed upon reasonable request from the corresponding author.
References
Area-Gomez E, de Groof A, Bonilla E, Montesinos J, Tanji K, Boldogh I, Pon L, Schon EA (2018) A key role for MAM in mediating mitochondrial dysfunction in alzheimer disease. Cell Death Dis 9:335
Armer HE, Mariggi G, Png KM, Genoud C, Monteith AG, Bushby AJ, Gerhardt H, Collinson LM (2009) Imaging transient blood vessel fusion events in zebrafish by correlative volume electron microhscopy. PLoS ONE 4:e7716
Baalman K, Marin MA, Ho TS, Godoy M, Cherian L, Robertson C, Rasband MN (2015) Axon initial segment-associated microglia. J Neurosci 35:2283–2292
Benned-Jensen T, Christensen RK, Denti F, Perrier JF, Rasmussen HB, Olesen SP (2016) Live imaging of Kv7.2/7.3 cell surface dynamics at the axon initial segment: high steady-state stability and calpain-dependent excitotoxic downregulation revealed. J Neurosci 36:2261–2266
Benusa SD, Lafrenaye AD (2020) Microglial process convergence on axonal segments in health and disease. Neuroimmunol Neuroinflamm 7:23–39
Benusa SD, George NM, Sword BA, DeVries GH, Dupree JL (2017) Acute neuroinflammation induces AIS structural plasticity in a NOX2-dependent manner. J Neuroinflammation 14:116
Berghs S, Aggujaro D, Dirkx R Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M (2000) betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol 151:985–1002
Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB 3rd, Goebbels S, Nave KA, Arnold BA, Jonas EA, Pineda FJ, Hardwick JM (2009) Bcl-x l increases mitochondrial fission fusion, and biomass in neurons. J Cell Bio 184:707–719
Bilsland LG, Sahai E, Kelly G, Golding M, Greensmith L, Schiavo G (2010) Deficits in axonal transport precede ALS symptoms in vivo. Proc Natl Acad Sci U S A 107:20523–20528
Blazquez-Llorca L, Hummel E, Zimmerman H, Zou C, Burgold S, Rietdorf J, Herms J (2015) Correlation of two-photon in vivo imaging and FIB/SEM microscopy. J Microsc 259:129–136
Buffington SA, Rasband MN (2011) The axon initial segment in nervous system disease and injury. Eur J Neurosci 34:1609–1619
Chamberlain KA, Sheng ZH (2019) Mechanisms for the maintenance and regulation of axonal energy supply. J Neurosci Res 97:897–913
Chang KJ, Rasband MN (2013) Excitable domains of myelinated nerves: axon initial segments and nodes of Ranvier. Curr Top Membr 72:159–92
Cheng XT, Huang N, Sheng ZH (2022) Programming axonal mitochondrial maintenance and bioenergetics in neurodegeneration and regeneration. Neuron 110:1899–1923
Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA (2009) S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324:102–105
Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O (2016) 3D U-Net: learning dense volumetric segmentation from sparse annotation. In: Ourselin S, Joskowicz L, Sabuncu M, Unal G, Wells W (eds) Medical image computing and computer-assisted intervention – MICCAI 2016. MICCAI 2016. Lecture notes in computer science, vol 9901. Springer, Cham. https://doi.org/10.1007/978-3-319-46723-8_49
Clark KC, Josephson A, Benusa SD, Hartley RK, Baer M, Thummala S, Joslyn M, Sword BA, Elford H, Oh U, Dilsizoglu-Senol A, Lubetzki C, Davenne M, DeVries GH, Dupree JL (2016) Compromised axon initial segment integrity in EAE is preceded by microglial reactivity and contact. Glia 64:1190–1209
Collier JJ, Oláhová M, McWilliams TG, Taylor RW (2023) Mitochondrial signalling and homeostasis: from cell biology to neurological disease. Trends Neurosci 46:137–152
Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG, Balla T, Hajnóczky G (2010) Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39:121–132
Denk W, Horstmann H (2004) Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol 2:e329
Dorkenwald S, Schubert PJ, Killinger MF, Urban G, Mikula S, Svara F, Kornfeld J (2017) Automated synaptic connectivity inference for volume electron microscopy. Nat Methods 14:435–442
Edgar JM, McCulloch MC, Thomson CE, Griffiths IR (2008) Distribution of mitochondria along small-diameter myelinated central nervous system axons. J Neurosci Res 86:2250–2257
Falk T, Mai D, Bensch R, Çiçek Ö, Abdulkadir A, Marrakchi Y, Böhm A, Deubner J, Jäckel Z, Seiwald K, Dovzhenko A, Tietz O, Dal Bosco C, Walsh S, Saltukoglu D, Tay TL, Prinz M, Palme K, Simons M, Diester I, Brox T, Ronneberger O (2019) U-Net: deep learning for cell counting, detection, and morphometry. Nat Methods 16:67–70
Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK (2011) ER tubules mark sites of mitochondrial division. Science 334:358–362
Gallo NB, Berisha A, Van Aelst L (2022) Microglia regulate chandelier cell axo-axonic synaptogenesis. Proc Natl Acad Sci USA 119:e2114476119
Gallusser B, Maltese G, Di Caprio G, Vadakkan TJ, Sanyal A, Somerville E, Sahasrabudhe M, O’Connor J, Weigert M, Kirchhausen T (2023) Deep neural network automated segmentation of cellular structures in volume electron microscopy. J Cell Biol 222:e202208005
Giacomello M, Pyakurel A, Glytsou C, Scorrano L (2020) The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol 21:204–224
Han Q, Xie Y, Ordaz JD, Huh AJ, Huang N, Wu W, Liu N, Chamberlain KA, Sheng ZH, Xu XM (2020) Restoring cellular energetics promotes axonal regeneration and functional recovery after spinal cord injury. Cell Metab 31:623–641
Hayashi S, Ohno N, Knott G, Molnár Z (2023) Correlative light and volume electron microscopy to study brain development. Microscopy (oxf) 9:dfad002
Hayworth KJ, Morgan JL, Schalek R, Berger DR, Hildebrand DG, Lichtman JW (2014) Imaging ATUM ultrathin section libraries with wafermapper: a multi-scale approach to EM reconstruction of neural circuits. Front Neural Circuits 8:68
Hedskog L, Pinho CM, Filadi R, Rönnbäck A, Hertwig L, Wiehager B, Larssen P, Gellhaar S, Sandebring A, Westerlund M, Graff C, Winblad B, Galter D, Behbahani H, Pizzo P, Glaser E, Ankarcrona M (2013) Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci U S A 110:7916–7921
Heinrich L, Bennett D, Ackerman D, Park W, Bogovic J, Eckstein N, Petruncio A, Clements J, Pang S, Xu CS, Funke J, Korff W, Hess HF, Lippincott-Schwartz J, Saalfeld S, Weigel AV (2021) COSEM project team whole-cell organelle segmentation in volume electron microscopy. Nature 599:141–146
Hetz C, Saxena S (2017) ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13:477–491
Hoffman DP, Shtengel G, Xu CS, Campbell KR, Freeman M, Wang L, Milkie DE, Pasolli HA, Iyer N, Bogovic JA, Stabley DR, Shirinifard A, Pang S, Peale D, Schaefer K, Pomp W, Chang CL, Lippincott-Schwartz J, Kirchhausen T, Solecki DJ, Betzig E, Hess HF (2020) Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells. Science.
Hollenbeck PJ, Saxton WM (2005) The axonal transport of mitochondria. J Cell Sci 118:5411–5419
Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC, Kasai H (2008) The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron 57:719–729
Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K (2009) Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11:958–966
Januszewski M, Kornfeld J, Li PH, Pope A, Blakely T, Lindsey L, Maitin-Shepard J, Tyka M, Denk W, Jain V (2018) High-precision automated reconstruction of neurons with flood-filling networks. Nat Methods 15:605–610
Kageyama Y, Zhang Z, Sesaki H (2011) Mitochondrial division: molecular machinery and physiological functions. Curr Opin Cell Biol 23:427–434
Karlsson U, Schultz RL (1965) Fixation of the central nervous system for electron microscopy by aldehyde perfusion: I. preservation with aldehyde perfusates versus direct perfusion with osmium tetroxide with special reference to membranes and the extracellular space. J Ultrastruct Res 12:160–186
Karreman MA, Hyenne V, Schwab Y, Goetz JG (2016) Intravital correlative microscopy: imaging life at the nanoscale. Trends Cell Biol 26:848–863
Kashiwagi Y, Higashi T, Obashi K, Sato Y, Komiyama NH, Grant SGN, Okabe S (2019) Computational geometry analysis of dendritic spines by structured illumination microscopy. Nat Commun 10:1285
Kim S, Coukos R, Gao F, Krainc D (2022) Dysregulation of organelle membrane contact sites in neurological diseases. Neuron 110:2386–2408
Kiryu-Seo S, Ohno N, Kidd GJ, Komuro H, Trapp BD (2010) Demyelination increases axonal stationary mitochondrial size and the speed of axonal mitochondrial transport. J Neurosci 30:6658–6666
Kiryu-Seo S, Tamada H, Kato Y, Yasuda K, Ishihara N, Nomura M, Mihara K, Kiyama H (2016) Mitochondrial fission is an acute and adaptive response in injured motor neurons. Sci Rep 6:28331
Kiryu-Seo S, Matsushita R, Tashiro Y, Yoshimura T, Iguchi Y, Katsuno M, Takahashi R, Kiyama H (2022) Impaired disassembly of the axon initial segment restricts mitochondrial entry into damaged axons. EMBO J 41:e110486
Kleele T, Rey T, Winter J, Zaganelli S, Mahecic D, Perreten Lambert H, Ruberto FP, Nemir M, Wai T, Pedrazzini T, Manley S (2021) Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593:435–439
Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008a) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9(7):505–518
Knott G, Marchman H, Wall D, Lich B (2008b) Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. J Neurosci 28:2959–2964
Kole K, Voesenek BJB, Brinia ME, Petersen N, Kole MHP (2022) Parvalbumin basket cell myelination accumulates axonal mitochondria to internodes. Nat Commun 13:7598
König T, Nolte H, Aaltonen MJ, Tatsuta T, Krols M, Stroh T, Langer T, McBride HM (2021) MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control. Nat Cell Biol 23:1271–1286
Kontou G, Antonoudiou P, Podpolny M, Szulc BR, Arancibia-Carcamo IL, Higgs NF, Lopez-Domenech G, Salinas PC, Mann EO, Kittler JT (2021) Miro1-dependent mitochondrial dynamics in parvalbumin interneurons. Elife 10:e65215
Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P (2009) An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325:477–481
Korogod N, Petersen CC, Knott GW (2015) Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. Elife 4:e05793
Kreshuk A, Straehle CN, Sommer C, Koethe U, Cantoni M, Knott G, Hamprecht FA (2011) Automated detection and segmentation of synaptic contacts in nearly isotropic serial electron microscopy images. PLoS ONE 6:e24899
Lam SS, Martell JD, Kamer KJ, Deerinck TJ, Ellisman MH, Mootha VK, Ting AY (2015) Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat Methods 12:51–54
Leterrier C (2018) The axon initial segment: an updated viewpoint. J Neurosci 38:2135–2145
Licht-Mayer S, Campbell GR, Canizares M, Mehta AR, Gane AB, McGill K, Ghosh A, Fullerton A, Menezes N, Dean J, Dunham J, Al-Azki S, Pryce G, Zandee S, Zhao C, Kipp M, Smith KJ, Baker D, Altmann D, Anderton SM, Kap YS, Laman JD, Hart BA, Rodriguez M, Watzlawick R, Schwab JM, Carter R, Morton N, Zagnoni M, Franklin RJM, Mitchell R, Fleetwood-Walker S, Lyons DA, Chandran S, Lassmann H, Trapp BD, Mahad DJ (2020) Enhanced axonal response of mitochondria to demyelination offers neuroprotection: implications for multiple sclerosis. Acta Neuropathol 140:143–167
Lidke DS, Lidke KA (2012) Advances in high-resolution imaging–techniques for three-dimensional imaging of cellular structures. J Cell Sci 125:2571–2580
Loginov SV, Fermie J, Fokkema J, Agronskaia AV, De Heus C, Blab GA, Klumperman J, Gerritsen HC, Liv N (2022) Correlative organelle microscopy: fluorescence guided volume electron microscopy of intracellular processes. Front Cell Dev Biol 10:829545
Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT (2009) Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61:541–555
Maclachlan C, Sahlender DA, Hayashi S, Molnár Z, Knott G (2018) Block face scanning electron microscopy of fluorescently labeled axons without using near infra-red branding. Front Neuroanat 12:88
Maco B, Holtmaat A, Jorstad A, Fua P, Knott GW (2014) Correlative in vivo 2-photon imaging and focused ion beam scanning electron microscopy: 3D analysis of neuronal ultrastructure. Methods Cell Biol 124:339–361
Marciniak SJ, Chambers JE, Ron D (2022) Pharmacological targeting of endoplasmic reticulum stress in disease. Nat Rev Drug Discov 21:115–140
Martell JD, Deerinck TJ, Sancak Y, Poulos TL, Mootha VK, Sosinsky GE, Ellisman MH, Ting AY (2012) Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol 30:1143–1148
Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, Fazel A, Bergeron JJ, Trudeau LE, Burelle Y, Gagnon E, McBride HM, Desjardins M (2016) Parkinson’s disease-related proteins pink1 and parkin repress mitochondrial antigen presentation. Cell 166:314–327
Merchán-Pérez A, Rodriguez JR, Alonso-Nanclares L, Schertel A, Defelipe J (2009) Counting synapses using FIB/SEM microscopy: a true revolution for ultrastructural volume reconstruction. Front Neuroanat 3:18
Möbius W, Cooper B, Kaufmann WA, Imig C, Ruhwedel T, Snaidero N, Saab AS, Varoqueaux F (2010) Electron microscopy of the mouse central nervous system. Methods Cell Biol 96:475–512
Morgan JL, Berger DR, Wetzel AW, Lichtman JW (2016) The fuzzy logic of network connectivity in mouse visual thalamus. Cell 165:192–206
Morris RL, Hollenbeck PJ (1993) The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci 104:917–927
Nakagomi S, Suzuki Y, Namikawa K, Kiryu-Seo S, Kiyama H (2003) Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. J Neurosci 23:5187–5196
Narayan K, Subramaniam S (2015) Focused ion beams in biology. Nat Methods 12:1021–1031
Nelson AD, Jenkins PM (2017) Axonal membranes and their domains: assembly and function of the axon initial segment and node of ranvier. Front Cell Neurosci 11:136
Ofer N, Berger DR, Kasthuri N, Lichtman JW, Yuste R (2021) Ultrastructural analysis of dendritic spine necks reveals a continuum of spine morphologies. Dev Neurobiol 81:746–757
Ohno S, Terada N, Ohno N, Saitoh S, Saitoh Y, Fujii Y (2010) Significance of “in vivo cryotechnique” for morphofunctional analyses of living animal organs. J Electron Microsc (tokyo) 59:395–408
Ohno N, Kidd GJ, Mahad D, Kiryu-Seo S, Avishai A, Komuro H, Trapp BD (2011) Myelination and axonal electrical activity modulate the distribution and motility of mitochondria at CNS nodes of Ranvier. J Neurosci 31:7249–7258
Ohta K, Sadayama S, Togo A, Higashi R, Tanoue R, Nakamura K (2012) Beam deceleration for block-face scanning electron microscopy of embedded biological tissue. Micron 43:612–620
Okayama S, Ohta K, Higashi R, Nakamura K (2014) Correlative light and electron microscopic observation of mitochondrial DNA in mammalian cells by using focused-ion beam scanning electron microscopy. Microscopy 63:i35
Paillusson S, Stoica R, Gomez-Suaga P, Lau DHW, Mueller S, Miller T, Miller CCJ (2016) There’s something wrong with my MAM; the ER-mitochondria axis and neurodegenerative diseases. Trends Neurosci 39:146–157
Pathak D, Berthet A, Nakamura K (2013) Energy failure: does it contribute to neurodegeneration? Ann Neurol 74:506–516
Perkins GA, Ellisman MH (2011) Mitochondrial configurations in peripheral nerve suggest differential ATP production. J Struct Bio 173:117–127
Pilling AD, Horiuchi D, Lively CM, Saxton WM (2006) Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in drosophila motor axons. Mol Biol Cell 17:2057–2068
Pozo Devoto VM, Onyango IG, Stokin GB (2022) Mitochondrial behavior when things go wrong in the axon. Front Cell Neurosci 16:959598
Prinz WA, Toulmay A, Balla T (2020) The functional universe of membrane contact sites. Nat Rev Mol Cell Biol 21:7–24
Saxton WM, Hollenbeck PJ (2012) The axonal transport of mitochondria. J Cell Sci 125:2095–2104
Schafer DP, Jha S, Liu F, Akella T, McCullough LD, Rasband MN (2009) Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J Neurosci 29:13242–13254
Schertel A, Snaidero N, Han HM, Ruhwedel T, Laue M, Grabenbauer M, Möbius W (2013) Cryo FIB-SEM: volume imaging of cellular ultrastructure in native frozen specimens. J Struct Biol 184:355–360
Sheng ZH (2017) The interplay of axonal energy homeostasis and mitochondrial trafficking and anchoring. Trends Cell Biol 27:403–416
Sheng ZH, Cai Q (2012) Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci 13:77–93
Sleigh JN, Rossor AM, Fellows AD, Tosolini AP, Schiavo G (2019) Axonal transport and neurological disease. Nat Rev Neurol 15:691–703
Suga S, Nakamura K, Humbel BM, Kawai H, Hirabayashi Y 2021 An interactive deep learning-based approach reveals mitochondrial cristae topologies bioRxiv
Sugiura A, McLelland GL, Fon EA, McBride HM (2014) A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J 33:2142–2156
Tamada H, Kiryu-Seo S, Hosokawa H, Ohta K, Ishihara N, Nomura M, Mihara K, Nakamura KI, Kiyama H (2017) Three-dimensional analysis of somatic mitochondrial dynamics in fission-deficient injured motor neurons using FIB/SEM. J Comp Neurol 525:2535–2548
Tamada H, Blanc J, Korogod N, Petersen CC, Knott GW (2020) Ultrastructural comparison of dendritic spine morphology preserved with cryo and chemical fixation. Elife 9:e56384
Tamada H, Kiryu-Seo S, Sawada S, Kiyama H (2021) Axonal injury alters the extracellular glial environment of the axon initial segment and allows substantial mitochondrial influx into axon initial segment. J Comp Neurol 529:3621–3632
Teliska LH, Dalla Costa I, Sert O, Twiss JL, Rasband MN (2022) Axon initial segments are required for efficient motor neuron axon regeneration and functional recovery of synapses. J Neurosci 42:8054–8065
Thomas CI, Keine C, Okayama S, Satterfield R, Musgrove M, Guerrero-Given D, Kamasawa N, Young SM Jr (2019) Presynaptic mitochondria volume and abundance increase during development of a high-fidelity synapse. J Neurosci 39:7994–8012
Tjiang N, Zempel H (2022) A mitochondria cluster at the proximal axon initial segment controls axodendritic TAU trafficking in rodent primary and human iPSC-derived neurons. Cell Mol Life Sci 79:120
Tønnesen J, Katona G, Rózsa B, Nägerl UV (2014) Spine neck plasticity regulates compartmentalization of synapses. Nat Neurosci 17:678–685
Ul Fatima N, Ananthanarayanan V (2023) Mitochondrial movers and shapers: recent insights into regulators of fission, fusion and transport. Curr Opin Cell Biol 80:102150
Van Harreveld A, Crowell J, Malhotra SK (1965) A study of extracellular space in central nervous tissue by freeze-substitution. J Cell Biol 25:117–137
Wang X, Schwarz TL (2009) The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 136:163–174
Watanabe S, Ilieva H, Tamada H, Nomura H, Komine O, Endo F, Jin S, Mancias P, Kiyama H, Yamanaka K (2016) Mitochondria-associated membrane collapse is a common pathomechanism in SIGMAR1- and SOD1-linked ALS. EMBO Mol Med 8:1421–1437
Westrate LM, Lee JE, Prinz WA, Voeltz GK (2015) Form follows function: the importance of endoplasmic reticulum shape. Annu Rev Biochem 84:791–811
Wilke SA, Antonios JK, Bushong EA, Badkoobehi A, Malek E, Hwang M, Terada M, Ellisman MH, Ghosh A (2013) Deconstructing complexity: serial block-face electron microscopic analysis of the hippocampal mossy fiber synapse. J Neurosci 33:507–522
Zachs T, Schertel A, Medeiros J, Weiss GL, Hugener J, Matos J, Pilhofer M (2020) Fully automated, sequential focused ion beam milling for cryo-electron tomography. Elife 9:e52286
Zhang CL, Ho PL, Kintner DB, Sun D, Chiu SY (2010) Activity-dependent regulation of mitochondrial motility by calcium and Na/K-ATPase at nodes of Ranvier of myelinated nerves. J Neurosci 30:3555–3566
Zollinger DR, Baalman KL, Rasband MN (2015) The ins and outs of polarized axonal domains. Annu Rev Cell Dev Biol 31:647–667
Acknowledgements
This work was partly supported by KAKENHI 20K08355 (to H.T.). HT wrote and edited the manuscript. The author would like to thank Prof. Hiroshi Kiyama for valuable comments and Editage (www.editage.com) for English language editing.
Funding
Open access funding provided by University of Fukui.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12565_2023_720_MOESM1_ESM.tif
FIB/SEM analysis of mitochondrial in injured neurons of Drp1 KO mouse (a) Each mitochondrion in soma of injured motor neurons was reconstructed in different colours. At one week after injury, some swollen mitochondria with processes are observed. (b) At two weeks after injury, extremely large round mitochondria without processes are detected. (c) Mitophagy-like structures with lysosomes are observed at two weeks after injury (green: mitochondria, pink: isolation membranes, purple: lysosome). (d) The representative SEM image for (c). The inner structures of mitochondria are also collapsed. Scale bar 5 µm (Images from Tamada et al. (2017) J Comp Neurol) (TIF 25514 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tamada, H. Three-dimensional ultrastructure analysis of organelles in injured motor neuron. Anat Sci Int 98, 360–369 (2023). https://doi.org/10.1007/s12565-023-00720-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-023-00720-y