Abstract
Offshore migration of Pacific salmon Oncorhynchus spp. is partly triggered by increasing body size and high motility in the early stages of life. The survival of juvenile salmon may depend on their growth rate during the first few months in the sea, and this factor partly regulates the dynamics of adult populations. Here, we assessed the effects of water temperature and food availability on the growth of juvenile chum salmon O. keta. In addition, by combining the measurements of metabolic performance for growth and activity (Absolute Aerobic Scope: AAS) with a bioenergetics model, we estimated the energy allocation for different activities in the juveniles. Under high temperatures (14 °C), juveniles reared at low food levels (1% body weight) allocated less than half their energy for growth than those reared at high food levels (4% body weight). These findings suggest that high temperature and low food level constrain the growth of juveniles, providing an insight into the effect of the recent increase in warm and low-nutrient water masses on survival of juveniles and catches of adult chum salmon on the Pacific side of Honshu Island, Japan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pacific salmonids Oncorhynchus spp. change their habitats partly depending on body size and physiological development (Beamish 2018). They have relatively high mortality rates during the first few months at sea (Parker 1968), with this high mortality potentially being correlated to body size (Pearcy 1992). The second phase of major mortality potentially occurs during the first fall and winter at sea in juvenile salmon, particularly if individuals have not attained a critical size. This phenomenon is termed the “Critical size and period hypothesis” (Beamish and Mahnken 2001). However, the early growth of juvenile salmon in marine habitats often varies temporally, owing to the fluctuations in certain environmental factors, including sea surface temperature (SST) and/or food abundance in coastal areas (Farley and Trudel 2009).
Chum salmon O. keta is a highly migratory Pacific salmonid and is strongly influenced by fluctuating environmental conditions during the early life stages. This species migrates downstream to the sea from 1 day to 1 month after fry emerge from gravel beds in rivers (Beacham and Murray 1986; Iwata and Komatsu 1984). The coastal areas of Japan represent the southernmost regions of the Asian population of chum salmon (Augerot 2005), where juveniles aggregate in waters of 9–13 °C SST (maximum 14 °C) (Irie 1990). After a period of coastal residence, the juvenile fish move to offshore habitats, including the Okhotsk Sea and the western Bering Sea (Urawa et al. 2009). This shift might be triggered by increased coastal SST and decreased prey availability, in conjunction with the weakening of the Oyashio Current, which is western boundary current of subarctic gyres in the North Pacific Ocean (Seki 2005). These environmental fluctuations vary annually, and potentially drive annual differences in the growth of juvenile salmon, affecting the population dynamics of future adult cohorts. On the Pacific side of Honshu Island, Japan (Fig. 1a), the return rates of adults have been mainly linked with coastal areas that have suitable SST range (5–13 °C) during the coastal residence of juveniles (Saito and Nagasawa 2009). Moreover, recent increases in the amount of warm water, such as from the Kuroshio Extension, might have caused lower growth and survival of juveniles and, consequently, lower return rates of adults (Wagawa et al. 2016). These previous studies suggest that juvenile chum salmon inhabit areas with SSTs that facilitate faster growth during coastal residence and offshore migration, allowing them to escape size-dependent predation by predatory fishes and diving sea birds (Parker 1971; Tucker et al. 2016). However, knowledge remains limited on the relationship between hydrographic features and juvenile body condition, which strongly affect the early survival of juveniles.
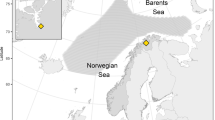
Map of the study area. a Sanriku coastal area of Japan where fish were obtained for this study. b Map of the Katagishi River in Japan where adults were captured. Adults from the Katagishi River were used for collecting eggs. The arrowhead shows the location of the set net. The diamond shows where eggs were artificially fertilized. Rearing and respirometry measurements of juveniles were conducted at the Iwate Fisheries Technology Center, Japan
For an individual fish, the energy available for somatic growth is assumed to equal the difference between the energy provided by food consumption and the energy cost through respiration, excretion, and egestion (Railsback and Rose 1999). Given these physiological processes, high metabolic loss could constrain the energy available for growth. The “critical size and period hypothesis” suggests that Pacific salmon must reach a sufficient body size by the end of the first summer in the marine environment to survive a period of energy deficit in late fall and winter (Beamish and Mahnken 2001). Chum salmon juveniles frequenting Japan should also reach a large body size enough to have high swimming performance for escape from predators during the first few months at sea, particularly those originating from the Pacific side of Honshu (Honda et al. 2017). Thus, juvenile salmon in Japan should allocate surplus energy toward growth during the first few months to survive. The oxygen capacity of fish is generally quantified by measuring the absolute aerobic scope (AAS), which is the difference between resting metabolic rate (RMR) and maximum (aerobic) metabolic rate (MMR). This measure has been linked to swimming performance and growth rates (Auer et al. 2015; Eliason and Farrell 2016). AAS are required during food digestion processes including pre-absorptive (e.g., ingestion), absorptive (e.g., digestion, absorption), and post-absorptive (biosynthesis, nutrient turnover, and assimilation) components (McCue 2006). Thus, aerobic metabolic rates are important factors in the growth of juvenile fish. However, juveniles with a high RMR and AAS might not always have high growth because high RMR may have a downside under restricted food availability (Burton et al. 2011). It has been known that the amount of food available influences AAS. For instance, juvenile brown trout Salmo trutta with a high AAS exhibited high growth at ad libitum food levels, while high AAS was not linked to high growth at low food levels (Auer et al. 2015). Water temperature also strongly influences the AAS of Oncorhynchus spp. juveniles, including Chinook salmon O. tshawytscha and rainbow trout O. mykiss (Verhille et al. 2016; Poletto et al. 2017). The temperature range (17.8–24.6 °C) over which the AAS of rainbow trout remained above 95% of the maximum AAS was strongly correlated with the local river temperature range (12–26 °C), optimizing fitness-related performance (e.g., growth, reproduction, and locomotion) (Verhille et al. 2016). However, the effects of both temperature and food level on metabolic traits have only been examined in a few fish species, such as common carp Cyprinus carpio (Zeng et al. 2018), snapper Chrysophrys auratus, and yellow-eyed mullet Aldrichetta forsteri (Flikac et al. 2020), but not in Pacific salmon juveniles. Moreover, the linkages between AAS (which has been known to be affected by food and temperature) and the life history of fish species are not fully understood.
While the metabolic traits (including AAS) of chum salmon adults returning to rivers in Japan have been measured (Abe et al. 2019), those of juveniles have not. Accurate measures of metabolic loss during respiration can be combined with bioenergetics models to examine how the energy budgets of fish species are affected (Beauchamp 2009). Such insights could provide information on the physiological responses of juvenile salmon to thermal regimes and changing food levels. Here, we used a swim tunnel respirometer to clarify how the AAS and growth rate of juvenile chum salmon varies when individuals are exposed to two food levels and four temperatures. Respiration cost acquired from the respirometry experiment was used to construct a bioenergetics model to estimate the amount of energy allocated to growth and activity. We applied this information to infer the thermal sensitivity and energy dynamics of chum salmon juveniles at different food levels in the context of migration ecology.
Materials and methods
Fish rearing
Chum salmon O. keta eggs were collected from spawning adults at Katagishi Hatchery, Iwate, Japan (Fig. 1b), where the adults return from mid-November to late January and the adults do not differ genetically throughout the season (Tsukagoshi et al. 2021). For rearing the same-sized juveniles on day 1 at the feeding study, two batches of eggs fertilized in different months were used. The eggs were collected on November 10, 2017 for rearing at the high food level and on December 11–12, 2017 for rearing at the low food level. The body sizes and ages of the adults did not differ between the two samplings (Online Resource Table S1). The eggs from the first group were artificially fertilized, and the juveniles were reared in a tank at the research station of Iwate Fisheries Technology Center. The fish were reared at 11.4–13.6 °C in natural freshwater and were fed artificial pellets (EPC; Marubeni Nisshin Feed Co. Ltd, Tokyo, Japan) representing 2.5–3.0% body weight (BW) until February 16, 2018. Juveniles in the second group were reared at 10.4–12.6 °C in natural fresh water and were fed with the same feeding condition as the first group’s juveniles until April 14, 2018. Juveniles in the first group were subsequently placed in four 200-l tanks (85.0-cm diameter, 57.5 cm deep, 100 individuals per tank) on February 17, and those in the second group were placed in four 100–l tanks (67.5 cm diameter, 45.0 cm deep, 100 individuals per tank) on April 15 at the Iwate Fisheries Technology Center. The fish were acclimated in the tank supplied with filtered natural seawater at 10 °C for 2 days. The top of the tanks was covered with a black film except during the feeding time, so that daylight had little effect on these juveniles. Natural mortality rates in the first group were 1–4%, and those in the second group were 0–1% during the feeding study.
Feeding study
Juveniles in the high food level group were reared at four temperatures (6, 10, 12, and 14 °C) with ± 0.5 °C variation between February and March. The monthly averaged air temperature was 0.7 °C (data collected by Japan Meteorological Agency, available at https://www.data.jma.go.jp/obd/stats/etrn/index.php) in February. To avoid the sea water temperature in the rearing tanks to be below 6 °C, a titanium heater (SHI-G, RSD-20A, IWAKI Co., Ltd., Tokyo, Japan) and temperature controller probe (TC-101, IWAKI Co., Ltd., Tokyo, Japan) were placed in each of four tanks. In addition, the rearing tanks were wrapped in glass wool mats to be insulated from heat and to keep the experimental water temperatures constant. The fish were fed artificial pellets representing 4% BW (EPC) twice a day for 15 days (“high food level”). Food levels were determined based on the study by (Leitritz and Lewis 1976). Approximately 1 h after feeding the juveniles in the high food level group, some pellets sank to the bottom of the tank under all temperature conditions. Hence, juveniles were considered to have fed to almost satiation. On day 13, the temperature probe of the 6 °C tank temporarily broke down, and the temperature rose to 12 °C. After the temperature probe was fixed and natural seawater newly added, the temperature immediately decreased to 8.6 °C. From the 14th day, the air temperature quickly increased because of the drastic weather fluctuations in the Sanriku coastal area in spring (daily mean temperature on the 14th day, i.e., March 4: 11.2 °C; daily mean temperature a week ago: − 0.1 °C; data collected by Japan Meteorological Agency, available at https://www.data.jma.go.jp/obd/stats/etrn/index.php), hence the water temperature of the 6 °C tank could have been cooled down to 7.3–8.1 °C.
Juveniles in the low food level group were reared at four temperatures (8, 10, 12, and 14 °C) with ± 0.5 °C variation between April and May. In April, the temperature of natural seawater was above 6 °C, so the custom-made cooling water system which consists of the four rearing tanks (in which the same heater and temperature probe used in the high food level were placed), 200-l water bath (85.0 cm in diameter, 57.5 cm deep) wrapped in glass wool mats, and PVC pipes with which connected the bath and the seawater faucet. The rearing tanks were floated on the water bath with ambient seawater. The fish were fed 1% BW (“low food level”) twice a day for 15 days.
Juveniles were randomly selected for fork length (FL, mm) and BW (g) measurements on days 1, 8, and 15 (n = 8 per tank). This information was used to adjust feeding rations in relation to changes in body size. Fecal matter and water were siphoned from each tank each day after feeding to maintain water quality. During the experiments, oxygenated seawater was supplied to each tank, with dissolved oxygen (DO) being ≥ 8.28 mg l−1. There were no replicate tanks and a single trial was conducted for each temperature and feeding regime.
The apparent daily growth rate (mm day−1) over the 2-week period was calculated as the difference between mean FL (n = 8 for each experimental condition) on day 1 and 15. The specific growth rate (SGR) over the 2-week period was calculated as 100 × [ln (final mean BW)–ln (initial mean BW)]/14 days. All experimental procedures followed the guidelines of the Animal Ethics Committee of the University of Tokyo, Japan. The study protocols were approved by the same committee (P16-7).
Respirometry
On day 15, the rate of oxygen uptake \(\left( {\dot{M}{\text{o}}_{{2}} } \right)\) by individual fish was measured using a 280-ml Blazka-type swim tunnel respirometer (#SW10000, Loligo Systems, Viborg, Denmark) with a working section of 2.5-cm inner diameter and was 16.5 cm long. DO in the chamber was measured using a dipping probe oxygen mini sensor (Loligo Systems, Viborg, Denmark). A flush pump (RSD-20A, IWAKI Co., Ltd., Tokyo, Japan) was attached via a one-way outflow valve to the swim tunnel to maintain DO above 80% air saturation every time the respirometer was closed to make measurements.
Juveniles in the high food level (total n = 10; median FL [interquartile ranges]: 62.4 [59.5–63.9] mm; median BW [interquartile ranges]: 1.63 [1.55–1.81] g) and low food level groups (total n = 9; median FL [interquartile ranges]: 57.7 [56.0–59.6] mm; median BW [interquartile ranges]: 1.20 [1.11–1.47] g) were used for respirometry measurements. To calculate the RMR and MMR, \(\dot{M}{\text{O}}_{{2}}\) was measured for individual fish from the high food level group on March 6–9, and from the low food level group on May 1–4 at the four test temperatures. Juveniles reared at high food levels were assigned to 8.4–8.8, 10, 12, and 14 °C (n = 2–3 per temperature), while those reared at low food levels were assigned to 8, 10, 12, and 14 °C (n = 2–3 per temperature). It was very difficult to lower the water temperature in the swim tunnel respirometer to 6 °C because of high air temperature on the 14th day. Therefore, fish in the 6 °C tank were assigned to 8.4–8.8 °C. Specific dynamic action, which is the energy cost of digestion, elevates metabolic rates (Secor 2009); thus, fish were not fed 24 h before measurements. The total period used to determine \(\dot{M}{\text{o}}_{{2}}\) was 15 min. At the start of this period, the respirometer was flushed with ambient water for 5 min and closed for the next 10 min. \(\dot{M}{\text{o}}_{{2}}\) was determined during the measurement period (Δt) as the decline in \(P{\text{o}}_{2}\) (Δ|O2|) in the swim tunnel (Abe et al. 2019). \(\dot{M}{\text{o}}_{{2}}\) (μg O2 min−1) was calculated as follows:
where [O2] is DO (measured in μg O2 l−1), t is time (in min), Vch is the volume of the swim chamber (in l), and Vf is the volume of fish (in l). This was calculated from body mass assuming the density of the fish was 1000 g l−1. The precision of \(\dot{M}{\text{o}}_{{2}}\) determination was demonstrated by the regression coefficient of the slope.
\(\dot{M}{\text{o}}_{{2}}\) is a function of body weight (BW, in g) and water temperature (T, in °C), based on a previously described formula (Dewar et al. 1994):
where \(a\) is the intercept of the allometric mass function for the oxygen consumption rate of 1 g fish at rest at 0 °C, \(b\) is the slope of the allometric mass function for \(\dot{M}{\text{o}}_{{2}} ,\) and \(c\) is a coefficient relating water temperature to \(\dot{M}{\text{o}}_{{2}}\).
Fish were placed in respirometry chambers in the afternoon, and \(\dot{M}{\text{o}}_{{2}}\) was measured at least 11 cycle times for all fish at a water velocity of 4.0 cm s−1. The \(\dot{M}{\text{o}}_{{2}}\) for almost all individuals stabilized (defined by Guo et al. (2021) as estimated \(\dot{M}{\text{o}}_{{2}}\) that did not vary more than 10% from the mean over three consecutive 15 min measurement cycles) at 1.5 h after acclimation was initiated (> 7 measurement cycle times; Online Resource Fig. S1). The \(\dot{M}{\text{o}}_{{2}}\) of fish during acclimation \(\left( {\dot{M}{\text{o}}_{{2}} ,\,{\text{rest}}} \right)\) was calculated as the mean of the three measurements of \(\dot{M}{\text{o}}_{{2}}\) with a regression coefficient of > 0.95 obtained 2.15–3.0 h after the acclimation was started (Schurmann and Steffensen 1997). After measuring \(\dot{M}{\text{o}}_{2} ,\,{\text{rest}},\) a constant acceleration test was used following a previously described protocol (Hammenstig et al. 2014) to measure post-exercise \(\dot{M}{\text{o}}_{{2}}\). In brief, flow was increased to 5.0 cm s−1, and fish were allowed to swim for 1 min. After each 1 min period, water flow was increased by an additional 1.5 cm s−1 until fish were not able to swim against the current, were pushed to the downstream screen, and remained there for more than 30 s. As soon as a fish showed signs of fatigue (e.g., failing to maintain their position in the swim tunnel), the measurement of \(\dot{M}{\text{o}}_{{2}}\) was initiated. Maximum \(\dot{M}{\text{o}}_{{2}}\) \((\dot{M}{\text{o}}_{2} ,\,\max )\) was determined as the highest value obtained during a 3-min period, after which the fish were exhausted (Norin and Clark 2016). Following \(\dot{M}{\text{o}}_{{2}}\) measurements, the fish were removed from the swim tunnel, deeply anesthetized in FA100 (4-allyl-2-methoxyphenol known as eugenol, 107 mg ml−1, DS Pharma Animal Health Co., Ltd.) for euthanasia, and FL and BW were measured. Resting metabolic rates (RMR) and maximum metabolic rates (MMR) were calculated by dividing \(\dot{M}{\text{o}}_{2} ,\,{\text{rest}}\) and \(\dot{M}{\text{o}}_{2} ,\,\max\) by the BWb of each individual, respectively. The AAS for each fish was calculated as the difference between MMR and RMR (n = 2–3 fish per temperature per food level).
Thermal sensitivity of individuals was quantified by calculating Q10 values. In general, Q10 values are calculated by comparing the measured values of a rate (r) at two different temperatures (T1 and T2):
In this study, \(\dot{M}{\text{o}}_{{2}}\) was measured at more than two temperatures. The Q10 relationship, which is a non-linear function that describes the increase in a rate, was fitted across a broader range of temperature values.
Logarithm metabolic traits (RMR and AAS) were expressed as a linear function of temperature by substituting 0 for T1, based on the equation in Rangel and Johnson (2019), and taking the logarithm of Eq. (3):
where metabolic trait0 describes the metabolic trait (RMR or AAS) as temperatures approach zero. The slope can be used to estimate log10 (Q10).
Bioenergetics models
The bioenergetics model used for immature chum salmon (age > 1 +) (Kamezawa et al. 2007; Kishi et al. 2010) was based on the model for Atlantic herring (Clupea harengus) proposed by Rudstam (1988). The growth rate of individual juvenile chum salmon was represented by the SGR over 14 days, calculated as [ln (final mean BW) –ln (initial mean BW)]/14 days. The energy budget of the juvenile was defined as:
where C is consumption (g prey g fish−1 day−1), R is loss through resting metabolism (g prey g fish−1 day−1), A is loss from energy spent on active metabolism or digesting food (g prey g fish−1 day−1), F is egestion or loss from feces (g prey g fish−1 day−1), E is the excretion or loss of nitrogenous excretory wastes (g prey g fish−1 day−1), G is SGR (day−1), and CALap and CALf are caloric equivalents of artificial pellets (cal g prey−1) and fish (cal g fish−1), respectively.
The bioenergetics model was run during the rearing experiments in 2018. The model for fish in the high food level group was run until day 12, and the model in the low food level group was run for 15 days. The percentage partition of dietary energy was obtained by dividing each component by C. The partition rate for growth and feed conversion ratio were calculated using Eqs. (6), (7), respectively:
Consumption, feces, and excretion
The feeding rates during the 2018 experiments (0.04 g prey g fish−1 day−1 in high food level group; 0.01 g prey g fish−1 day−1 in low food level group) were used to calculate C values. In the high food level group reared at 6.0 and 10 °C, some leftover pellets were confirmed. Feeding rates of juvenile chum salmon reared at 6.0 and 10 °C were previously documented as 2.4 and 3.12% body mass, respectively (Koshiishi 1980; Nogawa and Yagisawa 2011). Consequently, the feeding rates in the 6 and 10 °C tanks in the current study were 0.024 and 0.0312 g prey g fish−1 day−1, respectively. F and E were calculated using the following formula (Ito et al. 2004):
where the energy partitioning rate of feces was 20% and that for non-fecal matter was 7% for sockeye salmon (energy derived from C) (Brett and Groves 1979). Therefore, aF was assumed to be 0.20, and the excreted aE was calculated by substituting the partitioning rate of non-fecal matter in Eqs. (8), (9):
Therefore, aE was considered as 0.088.
Respiration, activity, and specific dynamic action
\(\dot{M}{\text{o}}_{2} ,\,{\text{rest}},\) (μg O2 min−1) values of the juveniles measured on day 15 in 2018 (see “Respirometry”) were converted to R (g O2 g fish−1 day−1). R is a function of water temperature (T, °C):
where \(a_{R}\) is the intercept of the allometric mass function for the oxygen consumption rate of a 1-g fish at rest at 0 °C, and \(c_{R}\) is a coefficient relating water temperature to R. A coefficient of 0.738 was used to convert R from g O2 g fish−1 day−1 to g prey g fish−1 day−1 using the conversion:
It was not possible to measure the oxygen consumption rates of juveniles when they were voluntary swimming because the fish were forced to swim in a swim tunnel respirometer. The voluntary swimming speed of Atlantic salmon Salmo salar has been measured by using un underwater camera (Hvas et al. 2017). However, the rearing tanks were too narrow to be observed by swim. Therefore, A in Eq. (5) including the energy loss during the voluntary swimming was calculated by subtracting F, E, R, and \(G \cdot \frac{{{\text{CAL}}_{f} }}{{{\text{CAL}}_{ap} }}\) from C.
Caloric values of fish and prey
Juveniles in the high food level (n = 8 for each temperature condition, in total: n = 32) and the low food level groups (n = 32) were moved to − 20 °C conditions on day 15 in the freezer. The fish were weighed (0.001 g), and their gills and guts were removed for minimizing the effect of pellets retained in the stomachs and other guts on the caloric values of the juveniles. Then, the fish were freeze-dried at − 80 °C for 16 h (Freeze dryer FDU-2200; Tokyo Rikakikai Co., Ltd, Tokyo, Japan) and dry weight was measured to determine water content after freeze-drying. Juveniles in the high food level group were crushed separately (Wonder Crusher WC-3; Osaka Chemical Co., Ltd, Osaka, Japan) and burned in a bomb calorimeter (C6000, IKA, Staufen, Germany) to measure the caloric value (J g−1). The wet weight of fish from the low food level group was too low to allow for separate measurement of the energy content of individuals. Therefore, three freeze-dried fish per temperature condition were pooled. The caloric values of some fish could not be obtained because of machine error (n = 1 in the high food level; n = 20 in the low food level). Seven to eight caloric values per temperature condition in the high food level and one caloric value per temperature condition in the low food level were obtained. The energy content was converted from J g−1 to cal g−1 using an equivalent 4.184 J cal−1.
Data analysis
All statistical analyses were conducted using the R 3.6.3 software (R Core Team 2020). First, the individual effects of body size and temperature on \(\dot{M}{\text{o}}_{2} ,\,{\text{rest}}\) and \(\dot{M}{\text{o}}_{2} ,\,\max\) were analyzed using a generalized linear model (GLM). The model included ln-transformed\(\dot{M}{\text{o}}_{2},\,{\text{rest}}\) or \(M{\text{o}}_{2},\,\max\)as dependent variables and temperature and ln-transformed BW as continuous predictors. Second, the effects of temperature and food levels on body size (FL and BW) were determined using GLM. The model related to body size included the FL or BW as dependent variables and two predictors (food level: a categorical variable; temperature: a continuous variable; their interaction). The model of the metabolic traits included the RMR, MMR, or AAS as dependent variables, and two predictors (food level: a categorical variable; temperature (T): a continuous variable). The metabolic traits were fitted with three types of functions (linear, quadratic, and exponential). Akaike’s information criterion corrected for small sample size (AICc) was calculated by the MuMIN package (Barton 2019) to compare the models. The model with the lowest AICc was considered the most parsimonious. In addition, the likelihood ratio test was also performed. Third, the individual effects of temperature and food levels on R was analyzed using GLM to estimate the parameters of the bioenergetics model. The GLM included ln-transformed R as dependent variables, and temperature as a continuous predictor and food levels as a categorical effect. Unless otherwise indicated, all statistical analyses used a significance level p < 0.05. The standard error (SE) was also calculated when the mean or estimate values were calculated.
Results
Growth trajectories
Median FL in the high food level group on day 15 (60.8 mm, n = 32) was 1.2 times greater than that on day 1 (49.8 mm, n = 32; Fig. 2a). Median BW in the high food level group on day 15 (1.68 g, n = 32) also greater than that on day 1 (0.91 g, n = 32). In comparison, median FL and BW in the low food level on day 15 (FL: 51.1 mm, n = 32; BW: 1.11 g, n = 32) did not differ much from that on day 1 (FL: 52.2 mm, n = 32; BW: 1.01 g, n = 3 2; Fig. 2b). The GLM indicated that on day 15, there was an interactive effect of food level and temperature on body size (Table 1).
Fork length (FL: upper) and body weight (BW: lower) of juvenile chum salmon Oncorhynchus keta at four temperatures in the high (a) and the low (b) food level groups. The thick line in the middle of the box represents the median. Upper and lower boundaries of the box show the 75th and 25th percentile lines, respectively. Data points set outside these limits are plotted
The apparent daily growth rates in the high food level groups were 0.65, 0.86, 0.83, and 1.02 mm day−1 at 6.0–8.1, 10, 12, and 14 °C, respectively. The apparent growth rates in the low food level groups were 0.14, − 0.04, − 0.08, and − 0.05 mm day−1 at 8.0, 10, 12, and 14 °C, respectively. The specific BW growth rate in the high food level group was higher at higher temperatures. In comparison, this rate in the low food level group was lower at higher temperatures (Fig. 3).
Metabolic mass-scaling exponent
Ln-transformed \(\dot{M}{\text{o}}_{2} ,\,{\text{rest}}\) and \(\dot{M}{\text{o}}_{2} ,\,\max\)increased with ln-transformed BW (Online Resource Fig. S2). The generalized linear model indicated that the \(b\) metabolic mass-scaling exponents of the allometric mass function for \(\dot{M}{\text{o}}_{2} ,\,{\text{rest}}\) and \(\dot{M}{\text{o}}_{2} ,\,\max\) (in Eq. 2) were estimated as 1.06 ± 0.26SE and 1.08 ± 0.38SE, respectively. These b values are considered almost 1.0 and have high variation. Because of the narrow range of BW, it was difficult to estimate the exact mass-scaling exponent. Therefore, in this study, the b value was considered to be 1.0 for \(\dot{M}{\text{o}}_{2} ,\,{\text{rest}}\) and \(\dot{M}{\text{o}}_{2} ,\,\max\).
Metabolic rate at different temperatures and food levels and estimated AAS
RMR and MMR were calculated by dividing \(\dot{M}{\text{o}}_{2} ,\,{\text{rest}}\) and \(\dot{M}{\text{o}}_{2} ,\,\max\) by the BW1.0 of each individual, respectively. RMRs at 14 °C exceeded those at 10 °C by almost 1.2- to twofold. From AICc model selection, this thermal response was expressed as a quadratic function of temperature (Fig. 4, Online Resource Table S2). The quadratic functions indicated that RMRs tended to increase with increasing water temperature, regardless of food level (Fig. 4). The most parsimonious model of thermal response of MMR was a linear function of temperature (Online Resource Table S2). However, the temperature effect on MMR was not significant regardless of food level (MMR = a T + b; a = 0.63 ± 0.37SE, t = 1.71, p > 0.05; b = 3.92 ± 4.10SE, t = 0.96, p > 0.05. The parameter of food level was not significant (t = 0.88, p > 0.05). Consequently, the effect of temperature on AAS was not observed regardless of food levels (Fig. 5). AAS ranged from 2.81 to 11.22 μg O2 g−1 min−1 in the high food level group and 2.72–13.38 μg O2 g−1 min−1 at the low food level (Fig. 5). Q10 of RMR was 2.54 in the high food level (t = 3.64, p < 0.01). In comparison, Q10 of RMR in the low food level was 1.24, but was not significantly different from 1.0 (t = 0.63, p = 0.55). Q10 of AAS in the high food level was 4.18, and that in the low food level was 0.84. However, the Q10 values of AAS were not significant (the high food level: t = 1.95, p = 0.09; the low food level: t = − 0.24, p = 0.82).
Comparison of metabolic traits between the two food levels. The dotted curve indicates the quadratic function of temperature (T in °C) and resting metabolic rate (RMR in μg O2 g−1 min−1): RMR = a T2 + b T + c; a = 0.15 ± 0.05SE, t = 2.95, p < 0.05; b = − 3.11 ± 1.16SE, t = − 2.67, p < 0.05; c = 19.27 ± 6.22, t = 3.10, p < 0.01. The parameter of food level was not significant (t = 1.86, p = 0.08). RMR (open triangles) and maximum metabolic rate (MMR; open squares) at the high (a) and the low (b) food levels
Comparison of absolute aerobic scope (AAS = MMR—RMR) between the two food levels. AAS at the high food level (filled circles) and the low food level (open circles). The parameters of temperature and food level were not significant (temperature: t = 0.96, p = 0.35; the low food level: food level: t = 0.45, p = 0.65)
As mentioned in the “Metabolic mass-scaling exponent”, it was difficult to estimate the exact mass-scaling exponent. If we assume that the b value was 0.80, which is considered the scaling exponent for standard metabolic rates in teleost fishes (Clarke and Johnston 1999), the effect of temperature and food levels on the metabolic traits (RMR, MMR, and AAS; Online Resource Table S3, Fig. S3 and S4) did not differ greatly from the results in Online Resource Table S2, Figs. 4, 5. We therefore assumed the b was 1.0.
Parameter estimation in the bioenergetics model
The respiration-related parameters \(a_{R}\), and \(c_{R}\) for juveniles were 0.0037 g O2 g fish−1 day−1 and 0.055 °C−1, respectively (t = − 22.28 and t = 2.41, p < 0.05). The parameter of food levels showed no significant relationship with ln R (t = 1.08, p > 0.05). Thus, Eq. (11) became R = 0.0037e(0.055 T).
Caloric values of fish and prey
The caloric value in the high food level group was 1050 ± 23 SE to 1157 ± 24 SE cal g fish−1 and exceeded that in the low food level group (829–898 cal g fish−1) by 1.3-fold (Table 2). The caloric values of prey (artificial pellets) were 4389 ± 50 SE cal g prey−1 (wet weight).
Allocation of dietary energy
In the high food level group, the bioenergetics model estimated that 32–47% of the energy consumed by fish would be allocated to daily growth. The percentage partitioning of energy for activity and specific dynamic action amounted to 10–27 and 3–13% in the high and low food level groups, respectively (Fig. 6). Juveniles in the low food level group at 8 °C were estimated to allocate − 16% energy to activity and specific dynamic action. This value might be attributed to overestimating the percent partitioning energy for egestion and excretion (F, E in Eq. (5)).
Energy budget of juvenile chum salmon Oncorhynchus keta at the high (a) and the low (b) food levels. Energetic property abbreviations follow Eq. (5); G is the specific growth rate (day−1); CALap and CALf are caloric equivalents of artificial pellets (cal g prey−1) and fish (cal g fish−1), respectively; A is the loss due to energy costs of active metabolism or digesting food (g prey g fish −1 day−1); R is loss through resting metabolism (g prey g fish −1 day−1); F is egestion or loss because of feces (g prey g fish −1 day−1); and E is the excretion or loss of nitrogenous excretory wastes (g prey g fish −1 day−1)
The feed conversion ratios of fish in the high food level groups were 1.79, 1.54, 1.25, and 1.45 at 6, 10, 12, and 14 °C, respectively. Those of the low food level groups were 2.29, 1.00, 0.37, and 0.58 at 8, 10, 12, and 14 °C, respectively.
Discussion
Effects of temperature and food levels on bioenergetics and growth
The growth rate was higher at higher temperatures at the high food level in the current study (Fig. 3). Juvenile chum salmon consumes more food at higher temperatures (Koshiishi 1980). Accordingly, juvenile chum salmon reserves more surplus energy at higher temperatures, and allocates more energy to growth (Fig. 6a), and therefore, have higher growth rates. In comparison, the growth rate was lower at higher temperatures at the low food level (Fig. 3). Even at the coolest temperature, the energy for respiration (R) accounted for 50% of intake energy (Fig. 6b). Juveniles allocate more energy for R at higher temperatures because RMRs tend to increase with increasing water temperature (Fig. 4b), resulting in less energy being allocated to somatic growth (Fig. 6b), and lower growth rates. These results suggest that the effect of temperature on bioenergetics and growth vary depending on food level.
The bioenergetics modeling quantified the energy partitioning rates for respiration and growth, but the rate for egestion and excretion (F, E in Eq. (5)) might have been overestimated. Consequently, the partitioning rate for activity and specific dynamic action could have been miscalculated (the value of A was − 16%) (Fig. 6b). Although the partitioning rates F and E were not estimated correctly, the sum of F, E, and A at 8 °C with the low food level (Fig. 6b) was much lower than that at 6.0–8.1 °C with the high food level (Fig. 6a). This finding indicates that juveniles at lower temperatures and low food levels allocated low amounts of energy to activity and showed low growth. We first estimated parameters related to metabolism based on direct measurements of oxygen consumption rates in juvenile chum salmon. However, when estimating percent partitioning energy for egestion and excretion (F, E in Eq. (5)), bioenergetic parameters of sockeye salmon were used (Brett and Groves 1979), on the premise that these parameters are constant regardless of temperature. However, the gut oxygen uptake rates of rainbow trout differ depending on temperature (Brijs et al. 2018); therefore, the energy for egestion and excretion is probably not constant. Differences among species or life history stages could lead to the under- or overestimation of the bioenergetics of juvenile salmon (Litz et al. 2019). Hence, direct assessments of the interactive effects of temperature and food levels on energy allocation for food digestion are needed.
The effect of temperature on AAS and growth
The growth rate was expected to be highest at the water temperature where the AAS would be highest (ToptAAS). However, contrary to our expectation, ToptAAS was not clearly observed across the temperature range of 6–14 °C (Fig. 5). One possible reason is that chum salmon juveniles can show constant levels of AAS at wider temperature ranges compared to eggs and larvae. In teleost fish, the juveniles show high aerobic capacity at wider temperature ranges compared to eggs and larvae (Dahlke et al. 2020). The water temperature during the coastal residence of Japanese chum salmon can largely fluctuate year to year (Nagata 2016). This means that chum salmon juveniles have relatively constant levels of AAS and foraging ability in habitats with large temperature variability. Even with a high AAS, the fish cannot grow fast without food as indicated from the results of bioenergetics in the low food levels (Fig. 6b).
Intrinsic factors driving habitat shifts and the period of coastal residency of chum juveniles
When chum salmon juveniles reside in the coastal waters of Sanriku, the effect of the Oyashio Current carrying cold subarctic waters weakens and the effect of the Tsugaru Warm Current carrying warm water increases from May to June (Wagawa et al. 2016). Subsequently, the SST gradually increases from 6 to 13 °C (Seki 2005). The composition of zooplankton, the main prey of the juveniles, also changes from cold-water zooplankton transported by the Oyashio Current to warm-water zooplankton transported by the Tsugaru Warm Current (Seki 2005; Yamada et al. 2019). The warm-water plankton, such as decapod zoea and gammarids, are less nutritious than those in cold-water plankton (Davis et al. 1998). In other words, the warm current could carry warmer water and lower-quality food compared to those environmental conditions soon after seaward migration. Irie (1990) observed that chum salmon juveniles inhabiting the Japanese coastal and offshore waters mainly forage on zooplankton such as copepods and hyperiid amphipods. Chum salmon juveniles are planktivorous both in coastal and offshore habitats, whereas Chinook salmon juveniles are piscivorous in coastal habitats (Irie 1990; Duguid et al. 2021). Therefore, it is possible that the quality and quantity of zooplankton in the coastal area influence the survival of chum salmon juveniles compared to other salmonids. The current study showed that juvenile growth rates were low at high temperatures and low food availability, with the feed conversion ratio noticeably dropping above 10 °C. Only after SST exceeded 13 °C, juveniles could begin to allocate energy to activity. Such juveniles might exhibit higher swimming performance and start offshore migration earlier than their counterparts in cooler waters. Further study on the energy allocation strategies is needed to reveal the factors regulating the offshore migration timing of juvenile chum salmon.
The effect of juvenile’s growth period on recent adult catch fluctuations along the Sanriku coast
Coastal conditions have been hypothesized to affect the survival of Pacific salmonid juveniles (Mueter et al. 2005). The current study supports this hypothesis, showing that juveniles grew well when food availability was high across the temperature range of 6–14 °C. These conditions might be optimum for the growth of juvenile chum salmon. This finding raises the question of what areas do juveniles inhabit the wild. In the Sanriku costal area, juvenile chum salmon reside in coastal areas for about 2 months (Irie 1990; Minegishi et al. 2019), because littoral and neritic areas with < 12 °C SST occur in April and June (Seki 2005; Yamada et al. 2019). The rates of water temperature (averaged from surface to 20 m depth) increase have been frequently higher after 2000 (Kawashima et al. 2018). In addition, periods of coastal residency in the Sanriku coastal area could be further shortened owing to recent increases in the amount of warm water originating from the Kuroshio Extension (Wagawa et al. 2016). This could lead the caloric value and growth rate of the juveniles to decrease in the Sanriku coastal area.
What would happen to wild juvenile chum salmon if caloric value and growth rate decline? The caloric value reflects the amount of lipid stores in somatic tissue (Heintz et al. 2013). Therefore, when food availability is low, juveniles store less energy, leading to reduced growth. Juveniles reared at low food levels grew much slower (− 0.08 to –0.14 mm day−1) than wild juvenile chum originating from the rivers along the Sanriku coast on the east Pacific side of Hokkaido during summer (median: 0.68 mm day−1; Honda et al. 2017). In comparison, juveniles reared at high food levels grew at similar or higher rates than their wild counterparts. As suggested by the “critical size, and critical period hypothesis” (Beamish and Mahnken 2001), smaller-sized fish with lower energy stores and higher metabolic rates are more vulnerable than larger-sized fish. This phenomenon has been repeatedly supported by existing studies on Pacific salmon juveniles (reviewed in (Farley et al. 2007)). Our results indicate that small chum salmon juveniles with low energy stores could experience high mortality during their first summer in the Sanriku coastal area. The low food level and high temperature conditions demonstrated by the current study of this Pacific region could drive the low survival of chum salmon juveniles in the Sanriku and the part of Pacific side of Hokkaido. This suggestion supports the fact that catches of adult chum salmon in the Iwate prefecture along the Sanriku coast has decreased since the 2000s (Wagawa et al. 2016).
Here, by combining the measurements of metabolic performance with a bioenergetics model, we found the less energy allocation for growth and activity regardless of temperature at the low food level. Although the long-term rearing experiments must confirm whether the temperature and food level affect the growth of juvenile chum salmon, short growth periods and low food levels might lead to smaller body sizes before offshore migration, a decline of swimming performance and, consequently, lower survival, leading to fewer adult chum salmon returning to breed. Besides, application of the bioenergetics model to Pacific salmonids’ juveniles would remarkably enhance our understanding of how the juveniles will respond to changes in various abiotic factors in the near future.
Change history
27 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12562-023-01685-7
References
Abe TK, Kitagawa T, Makiguchi Y, Sato K (2019) Chum salmon migrating upriver adjust to environmental temperatures through metabolic compensation. J Exp Biol. https://doi.org/10.1242/jeb.186189
Auer SK, Salin K, Rudolf AM, Anderson GJ, Metcalfe NB (2015) The optimal combination of standard metabolic rate and aerobic scope for somatic growth depends on food availability. Funct Ecol 29:479–486
Augerot X (2005) Atlas of Pacific salmon. University of California Press, Berkeley
Barton K (2019) MuMIn: multi-model inference (Version 1.43.6). Retrieved from https://CRAN.R-project.org/package=MuMIn. Accessed 1 Jun 2020
Beacham TD, Murray CB (1986) Comparative developmental biology of chum salmon (Oncorhynchus keta) from the Fraser River, British Columbia. Can J Fish Aquat Sci 43:252–262
Beamish RJ (2018) Ocean ecology of pacific salmon and trout. American Fisheries Society, Maryland
Beamish RJ, Mahnken C (2001) A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change. Prog Oceanogr 49:423–437
Beauchamp DA (2009) Bioenergetic ontogeny: linking climate and mass-specific feeding to life-cycle growth and survival of salmon. Am Fish Soc Symp 70:1–19. https://doi.org/10.1116/1.588730
Brett JR, Groves TDD (1979) Physiological energetics. In: Hoar H et al (eds) Fish physiology, vol 8. Academic Press, New York, pp 279–352
Brijs J, Gräns A, Hjelmstedt P, Sandblom E, van Nuland N, Berg C, Axelsson M (2018) In vivo aerobic metabolism of the rainbow trout gut and the effects of an acute temperature increase and stress event. J Exp Biol. https://doi.org/10.1242/jeb.180703
Burton T, Killen SS, Armstrong JD, Metcalfe NB (2011) What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc Biol Sci 278:3465–3473
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905. https://doi.org/10.1046/j.1365-2656.1999.00337.x
Dahlke FT, Wohlrab S, Butzin M, Pӧrtner HO (2020) Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369:65–70
Davis ND, Myers KW, Ishida Y (1998) Caloric value of high-seas salmon prey organisms and simulated salmon ocean growth and prey consumption. N Pac Anadr Fish Comm Bull 1:146–162
Dewar H, Graham J, Brill R (1994) Studies of tropical tuna swimming performance in a large water tunnel-thermoregulation. J Exp Biol 192:33–44
Duguid WDP, Iwanicki TW, Qualley J, Juanes F (2021) Fine-scale spatiotemporal variation in juvenile Chinook salmon distribution, diet and growth in an oceanographically heterogeneous region. Prog Oceanogra 193:102512
Eliason EJ, Farrell AP (2016) Oxygen uptake in Pacific salmon Oncorhynchus spp.: when ecology and physiology meet. J Fish Biol 88:359–388
Farley EV, Trudel M (2009) Growth rate potential of juvenile sockeye salmon in warmer and cooler years on the eastern Bering Sea shelf. J Mar Biol. https://doi.org/10.1155/2009/640215
Farley EV, Moss JH, Beamish RJ (2007) A review of the critical size, critical period hypothesis for juvenile Pacific salmon. N Pac Anadr Fish Comm Bull 4:311–317
Flikac T, Cook DG, Davison W (2020) The effect of temperature and meal size on the aerobic scope and specific dynamic action of two temperate New Zealand finfish Chrysophrys auratus and Aldrichetta forsteri. J Comp Physiol B 190:169–183
Guo C, Ito SI, Yoneda M, Kitano H, Kaneko H, Enomoto M, Aono T, Nakamura M, Kitagawa T, Wegner NC, Dorval E (2021) Fish specialize their metabolic performance to maximize bioenergetic efficiency in their local environment: conspecific comparison between two stocks of pacific chub mackerel (Scomber japonicus). Front Mar Sci 8:1–13
Hammenstig D, Sandblom E, Axelsson M, Johnsson JI (2014) Effects of rearing density and dietary fat content on burst-swim performance and oxygen transport capacity in juvenile Atlantic salmon Salmo salar. J Fish Biol 85:1177–1191
Heintz RA, Siddon EC, Farley EV, Napp JM (2013) Correlation between recruitment and fall condition of age-0 pollock (Theragra chalcogramma) from the eastern Bering Sea under varying climate conditions. Deep Sea Res Part 2 Top Stud Oceanogr 94:150–156
Honda K, Kawakami T, Suzuki K, Watanabe K, Saito T (2017) Growth rate characteristics of juvenile chum salmon Oncorhynchus keta originating from the Pacific coast of Japan and reaching Konbumori, eastern Hokkaido. Fish Sci 83:987–996
Hvas M, Folkedal O, Solstorm D, Vågseth T, Fosse JO, Gansel LC, Oppedal F (2017) Assessing swimming capacity and schooling behaviour in farmed Atlantic salmon Salmo salar with experimental push-cages. Aquaculture 473:423–429
Irie T (1990) Ecological studies on the migration of juvenile chum salmon, Oncorhynchus keta, during early ocean life. Bullet Seikai National Fish Res Inst 68:1–142
Ito S, Kishi MJ, Kurita Y, Oozeki Y, Yamanaka Y, Megrey BA, Werner FE (2004) Initial design for a fish bioenergetics model of Pacific saury coupled to a lower trophic ecosystem model. Fish Oceanogr 13:111–124
Iwata M, Komatsu S (1984) Importance of estuarine residence for adaptation of chum salmon (Oncorhynchus keta) fry to seawater. Can J Fish Aquat Sci 41:744–749
Kamezawa Y, Azumaya T, Nagasawa T (2007) A fish bioenergetics model of Japanese chum salmon (Oncorhynchus keta) for studying the influence of environmental factor changes. Bull Jap Soc Fish Oceanogr 71:87–95 (In Japanese with English abstract)
Kawashima T, Shimizu Y, Ota K, Yamane K (2018) Abundance and habitats of juvenile chum salmon and their adult returns in the Sanriku coast. Aquabiology 40:342–345 (In Japanese with English abstract)
Kishi MJ, Kaeriyama M, Ueno H, Kamezawa Y (2010) The effect of climate change on the growth of Japanese chum salmon (Oncorhynchus keta) using a bioenergetics model coupled with a three-dimensional lower trophic ecosystem model (NEMURO). Deep Sea Res Part 2 Top Stud Oceanogr 57:1257–1265
Koshiishi Y (1980) Growth of juvenile chum salmon Oncorhynchus keta, reared in tanks supplied sea water in relation to food consumption, salinity and dietary protein content. Bullet of Japan Sea Reg Fish Res Lab 31:41–55 (In Japanese with English abstract)
Leitritz E, Lewis RC (1976) Trout and salmon culture (Hatchery methods). Fish Bull 164:1–197
Litz MNC, Miller JA, Brodeur RD, Daly EA, Weitkamp LA, Hansen AG, Claiborne AM (2019) Energy dynamics of subyearling Chinook salmon reveal the importance of piscivory to short-term growth during early marine residence. Fish Oceanogr 28:273–290
McCue MD (2006) Specific dynamic action: A century of investigation. Comp Biochem Physiol A Mol Integr Physiol 144:381–394
Minegishi Y, Wong MKS, Kanbe T, Araki H, Kashiwabara T, Ijichi M, Kogure K, Hyodo S (2019) Spatiotemporal distribution of juvenile chum salmon in Otsuchi Bay, Iwate, Japan, inferred from environmental DNA. PLoS One. https://doi.org/10.1371/journal.pone.0222052
Mueter FJ, Pyper BJ, Peterman RM (2005) Relationship between coastal ocean conditions and survival rates and northeast Pacific salmon at multiple lags. Trans Am Fish Soc 134 105–119
Nagata M, Miyakoshi Y, Fujiwara M, Kasugai K, Ando D, Torao M, Saneyoshi H, Irvine JR (2016) Adapting Hokkaido hatchery strategies to regional ocean conditions can improve chum salmon survival and reduce variability. N Pac Anadr Fish Comm Bull 6:73–85
Nogawa H, Yagisawa I (2011) Development of techniques for rearing juvenile chum salmon in artificial propagation in Japan. J Fish Tech 3:67–89 (In Japanese with English abstract)
Norin T, Clark TD (2016) Measurement and relevance of maximum metabolic rate in fishes. J Fish Biol 88:122–151
Parker RR (1968) Marine mortality schedules of pink salmon of the Bella Coola River, central British Columbia. J Fish Res Board Can 25:757–794. https://doi.org/10.1139/f68-068
Parker RR (1971) Size selective predation among juvenile Salmonid fishes in a British Columbia inlet. J Fish Res Board Can 28:1503–1510
Pearcy WG (1992) Ocean ecology of North Pacific salmonids. University of Washington Press, Seattle, USA
Poletto JB, Cocherell DE, Baird SE, Nguyen TX, Cabrera-Stagno V, Farrell AP, Fangue NA (2017) Unusual aerobic performance at high temperatures in juvenile Chinook salmon, Oncorhynchus tshawytscha. Conserv Physiol 5:1–13
R Core Team (2020). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https ://www.r-proje ct.org/. Accessed 7 Jan 2022
Railsback SF, Rose KA (1999) Bioenergetics modeling of stream trout growth: temperature and food consumption effects. Trans Am Fish Soc 128:241–256
Rangel RE, Johnson DW (2019) Variation in metabolic rate and a test of differential sensitivity to temperature in populations of woolly sculpin (Clinocottus analis). J Exp Mar Bio Ecol 511:68–74
Rudstam LG (1988) Exploring the dynamics of herring consumption in the Baltic—applications of an energetic model of fish growth. Kieler Meeresforsch Sonderh 6:312–322
Saito T, Nagasawa K (2009) Regional synchrony in return rates of chum salmon (Oncorhynchus keta) in Japan in relation to coastal temperature and size at release. Fish Res 95:14–27
Schurmann H, Steffensen JF (1997) Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J Fish Biol 50:1166–1180
Secor SM (2009) Specific dynamic action: a review of the postprandial metabolic response. J Comp Physiol B. https://doi.org/10.1007/s00360-008-0283-7
Seki J (2005) Study of characteristic of feeding habitat of juvenile chum salmon and their food environment in the Pacific coastal waters, central part of Hokkaido. Bull Natl Salmon Res Center 7:1–104
Tsukagoshi H, Terui S, Ogawa G, Sato S, Abe S. (2021) Genetic population structure of chum salmon in the Sanriku-region, Japan. Abstract booklet of the third NPAFC-IYS Workshop. Available from https://npafc.org/workshop21-home/. Accessed 10 Jan 2022
Tucker S, Hipfner JM, Trudel M (2016) Size- and condition-dependent predation: a seabird disproportionately targets substandard individual juvenile salmon. Ecology 97:461–471
Urawa S, Sato S, Crane PA, Agler B, Josephson R, Azumaya T (2009) Stock-specific ocean distribution and migration of chum salmon in the Bering Sea and North Pacific Ocean. N Pac Anadr Fish Comm Bull 5:131–146
Verhille CE, English KK, Cocherell DE, Farrell AP, Fangue NA (2016) High thermal tolerance of a rainbow trout population near its southern range limit suggests local thermal adjustment. Conserv Physiol. https://doi.org/10.1093/conphys/cow057
Wagawa T, Tamate T, Kuroda H, Ito SI, Kakehi S, Yamanome T, Kodama T (2016) Relationship between coastal water properties and adult return of chum salmon (Oncorhynchus keta) along the Sanriku coast, Japan. Fish Oceanogr 25:598–609
Yamada Y, Sasaki K, Yamane K, Yatsuya M, Shimizu Y, Nagakura Y, Kurokawa T, Nikaido H (2019) The utilization of cold-water zooplankton as prey for chum salmon fry (Oncorhynchus keta) in Yamada Bay, Iwate, Pacific coast of northern Japan. Reg Stud Mar Sci. https://doi.org/10.1016/j.rsma.2019.100633
Zeng LQ, Fu C, Fu SJ (2018) The effects of temperature and food availability on growth, flexibility in metabolic rates and their relationships in juvenile common carp. Comp Biochem Physiol A Mol Integr Physiol 217:26–34
Acknowledgements
We thank the fishermen of the Fisheries Cooperative Association of Toni Bay, Japan, for providing us with chum salmon eggs collected from returning adults in Katagishi River, Japan. We thank Kaoru Nagaoka and Shino Sawashita at Iwate Fisheries Technology Center for providing us with supporting experimental conditions. This study was financially supported by the Ministry of Education, Culture, Sports, Science and Technology in Japan for the Tohoku Ecosystem-Associated Marine Science, and the Japan Society for the Promotion of Science KAKENHI Grant Numbers JP16H04968, JP19H04927 and JP20H004. We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised for retrospective open access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Iino, Y., Kitagawa, T., Abe, T.K. et al. Effect of food amount and temperature on growth rate and aerobic scope of juvenile chum salmon. Fish Sci 88, 397–409 (2022). https://doi.org/10.1007/s12562-022-01599-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-022-01599-w