Abstract
Atlantic salmon Salmo salar are experiencing widespread population declines, and reductions in growth and survival in the marine environment are contributing factors. Our aims were to estimate marine food consumption of adult salmon and to determine how energetics would be directly affected by the increased ocean temperatures associated with climate change. We tagged previous spawners on outward migration (body size 76–119 cm) with archival tags and used a bioenergetic model to combine in situ temperature recordings with individual data on body growth. Average energy consumption was estimated to be 331–813 kJ per day, which is equivalent to 5–11 prey fish with an average body mass of ca. 15 g. Energy content of prey was the most important factor determining food consumption required to maintain growth. Conversely, the increases in ocean temperatures expected with climate change were predicted to have limited physiological effects on energy budgets and limited impact on the food consumption needed to maintain growth. We conclude that climatic warming will impact Atlantic salmon primarily through changes in prey availability and ecosystem structure rather than the direct effects of temperature on physiological performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temperature increases due to global climate change impact aquatic wildlife (Perry et al., 2004; Moore & Huntington, 2008). For ectothermic fish, which have the same temperature as the surrounding water, these alterations may have direct effects on physiological processes as well as impacts on growth and survival through ecosystem changes (Alfonso et al., 2021; Vollset et al., 2022). Atlantic salmon Salmo salar Linnaeus, 1758 is a diadromous fish species affected by global climate change both in freshwater and marine habitats (Thorstad et al., 2021). Broad-scale thermal shifts in the ocean are associated with ecological regime shifts in marine food webs that have resulted in reduced individual growth and survival (Beaugrand & Reid, 2012; Mills et al., 2013; Vollset et al., 2022). It is likely that changing ocean temperatures will continue to affect Atlantic salmon (Beaugrand & Reid, 2012; Friedland et al., 2014). However, few studies have investigated the direct effects of ocean temperatures on individual energy budgets and growth, and how this mechanistic link will change under future climate scenarios.
In fisheries research, bioenergetic models are frequently used to investigate the direct link between environmental factors and individuals’ energy budgets (Beauchamp et al., 1989; Moss et al., 2009; Smith et al., 2009). This is done by a set of empirical functions that describe key physiological processes (Deslauriers et al., 2017). For ectothermic fish, many of these functions are strongly influenced by water temperature and any discrepancy between the assumed and the actual thermal habitat will result in inaccurate model predictions, regardless of the accuracy of the underlying functions. Detailed information of individuals’ thermal environment would therefore improve estimates of individuals’ energy budgets. This is particularly the case for species such as Atlantic salmon that performs long-term and long-distance migrations across different ocean areas (Strøm et al., 2017; Rikardsen et al., 2021), where accurate assumptions on the thermal habitat are difficult to make.
In this study, we investigate the marine food consumption of repeat ocean migrating Atlantic salmon from a northern population, by using a bioenergetic model that incorporates daily data from temperature-sensing archival tags, and information on individuals’ marine growth based on body length and weight at sea entry and when they returned from the ocean migration. The temperature data used in this study have previously been described by Strøm et al. (2020) who documented that adult ocean migrants spent most of their time in waters with temperatures between 1.4 and 8.6 °C, and consistently inhabiting cold waters (3.4–5.0 °C) during winter in both warmer and colder years. This contrasted the temperatures experienced during the first months at sea, which were higher in warmer than in colder years (Strøm et al., 2020). The main aim of this study was to estimate individual food consumption of the tagged adult Atlantic salmon during their ocean migration based on the recorded water temperatures and growth at sea, and to explore how different temperature scenarios would affect the consumption requirement given consistent realized growth.
Materials and methods
Fish tagging
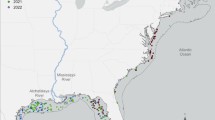
In total, 744 Atlantic salmon were captured, tagged with archival tags, and released in the Alta River, northern Norway (70° N 23.4° E, Fig. 1, Table 1) in May 2008–2015. These were fish that had spawned the previous fall and remained in the river during the winter as kelts, before exiting the river for a new ocean migration in spring and early summer. The fork lengths of the tagged fish ranged from 56 to 121 cm (mean ± SD = 92 ± 9 cm) and body weights from 1.2 to 13.8 kg (mean ± SD = 6.0 ± 1.8 kg). All fish were captured by angling, using spoon lures with barbless treble hooks and anaesthetized in an aqueous solution of 0.5 ml/l 2-phenoxy ethanol prior to tagging. The tags used (DST milli, Star-Oddi, Iceland, or Lat-2810, Lotek Wireless Inc., Canada) recorded temperature and depth and were implanted into the body cavity of the fish. For further details about the tag types and tagging procedures see Strøm et al. (2018, 2020) and Hedger et al. (2017). The tagging of the fish was approved by the Norwegian Animal Research Authority and Norwegian Food Safety Authority (permit reference number 15950).
Map showing the location of the Alta River (yellow diamond) in Fennoscandia, with the main ocean migration range of Atlantic salmon from the Alta River depicted by the shaded area (Chittenden et al., 2013; Strøm et al., 2018; Rikardsen et al., 2021). Inserted map depicts the area surrounding the Alta River (yellow diamond)
Of the 774 tagged Atlantic salmon, 42 individuals were recaptured with functioning tags when returning after spending approximately one year in the ocean. Recaptures were done by sea fishers using bag nets in the Alta fjord or by anglers in the river. Tags from two fish tagged in 2008 failed to log data during the last months at sea because the data memory was full. These two fish were consequently removed from the data set. Furthermore, body length and weight at recapture were not recorded for seven of the recaptured individuals. Hence, the number of individuals with sufficient information for the bioenergetic model was reduced to 33 (Table 1). For the 33 Atlantic salmon included in the analyses, the duration of the ocean migration ranged from 347 to 426 days (mean ± SD = 381 ± 20). Body length at tagging ranged from 76 to 110 cm (mean ± SD = 94 ± 7 cm), and body length at recapture from 93 to 120 cm (mean ± SD = 107 ± 6 cm). Body weight at tagging ranged from 2.9 to 9.9 kg (mean ± SD = 6.2 ± 1.4 kg) and at recapture from 8.9 to 18.4 kg (mean ± SD = 12.3 ± 2.5 kg).
Growth at sea
Scales were sampled from all fish during tagging and from 16 of the 33 individuals at recapture. Paired scale samples were analysed to reconstruct individuals’ somatic growth trajectory during the last ocean migration (i.e. the period of their life examined in this study). Scales of each fish were photographed under a microscope (Leica M60, Wetzlar, Germany) and measured using the Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). The outermost zone in the scales, from the winter annulus laid down before the fish were tagged in the spring, to the scale margin at recapture in the summer about a year after tagging, was used as a proxy for fish growth during the ocean migration, assuming proportionality between scale size and fish body length (Shearer, 1992). The fish body length in the winter was estimated based on the proportion of the outermost scale zone that was formed between tagging and the formation of the last winter annulus in the scale. The last marine winter annulus in the scale was identified by analysis of scale characteristics in combination with counting of circuli and measurements of intercirculi spacing (Todd et al., 2014). We assumed that the winter annulus was formed at winter solstice (i.e. December 21) as suggested by Todd et al. (2014). In the modelling, we assumed a biphasic growth pattern with linear growth in length from sea entry to the winter (first growth period), and linear growth from winter to 14 days prior recapture (second growth period) (Fig. 2). The cessation of growth 14 days prior recapture was set to account for the limited foraging displayed by adult Atlantic salmon from the Alta River during the final phase of the ocean migration (Hedger et al., 2022).
During the first growth period (G1), daily growth increment in body length ranged between 0.23 and 0.66 mm (mean ± SD = 0.48 ± 0.11 mm) and was on average 2.49 times greater than growth during the second growth period (G2), which ranged between 0.13 and 0.38 mm (mean ± SD = 0.22 ± 0.08 mm). This ratio (G1 = 2.49 × G2) was used to estimate the growth trajectories of the fish lacking growth data from scale analyses by using the following equation:
where Linc is the total growth in cm during the marine residency, and N1 and N2 are the number of days in the first and second growth periods. Using this approach to estimate the growth of the Atlantic salmon that lacked paired scale samples ensured that the estimated growth trajectories are independent of the duration of the growth period. Growth in body weight was estimated by using the same growth ratios and followed identical trajectories as growth in body length.
Bioenergetic model
Bioenergetic models can be used to estimate how animals partition consumed energy into metabolism, growth, and waste products (Deslauriers et al., 2017). We estimated the marine consumption of Atlantic salmon based on the energy balance equation:
where C is the consumed energy, G is the energy invested in growth, R is the energetic costs of respiration, F is the energy lost through egestion, E is the energy lost through excretion, and SDA is the specific dynamic action. These physiological processes were formalized by a series of underlying functions of which several depend on water temperature and body size. For the functions that depend on temperature, daily mean water temperatures recorded by the tags were used as the input variable. For the allometric functions, daily estimates of length and weight were used as described above. Due to limited information on certain aspects of Atlantic salmon bioenergetics, particularly for adults, several of the underlying functions used in the simulations were parameterized based on data from other salmonid species (Table 2).
Consumption
Consumption was estimated as the proportion of maximum daily food consumption at a given body size and water temperature (Deslauriers et al., 2017).
where Cmax is the maximum daily food consumption given as g of prey consumed per g of predator, CA is the function’s intercept, w is the weight of the Atlantic salmon (predator) in g, and CB is the weight coefficient. C is the daily consumption rate (g of prey consumed per g of predator), f(T) is the temperature-dependent function, and P is a constant that ranges between 0 and 1 and accounts for ecological constraints on Cmax. Parameters required for estimating Cmax and f(T) were derived from rainbow trout Oncorhynchus mykiss (Walbaum, 1792) (Railsback & Rose, 1999). P was treated as an unknown parameter and estimated individually for each Atlantic salmon using an iterative approach (see Deslauriers et al., 2017). Daily consumption rates were converted from g of prey per g of predator, to energy by multiplying the value with the weight of the Atlantic salmon in g on that given day and the energy density of their prey. When estimating consumption, we assumed that the Atlantic salmon fed on fish with an energy density of 5 kJ/g wet weight while at sea.
Growth
Energy invested in growth is formalized as the increase in energy content of the Atlantic salmon during the ocean migration. The energy density of individual fish was calculated as functions of body length at tagging and recapture, using energy density equations derived from post- and pre-spawned adult Atlantic salmon from Norway (Jonsson et al., 1997).
where GA represents the energy invested in growth in kJ, a1 and a2 are the intercepts before and after the ocean migration, b1 and b2 are the corresponding body length coefficients, and L is the body length in cm.
Respiration
Standard respiration was modelled as a function of body weight and temperature according to an exponential model fitted to metadata from Atlantic salmon (Macnaughton et al., 2019).
where Rs is standard respiration rate in mgO2/h, a is the intercept, w is the body weight in g, b is the weight coefficient, c the temperature coefficient, and T is the water temperature. To include the additional cost of swimming speed, an activity multiplier was incorporated (Grøttum & Sigholt, 1998).
where Rt is the total respiration cost in mgO2/h, ACT is the activity multiplier, and U is the swimming speed in body lengths per second. Respiration was converted to energy using an oxycalorific coefficient of 13.563 J/mgO2 (Beauchamp et al., 1989). Because little is known about the swimming speed of adult Atlantic salmon at sea, parallel simulations were conducted with swimming speeds either fixed to one body length per second or modelled as a positive power function of body weight and temperature (Beauchamp et al., 1989).

where Uopt is the optimal swimming speed in cm/s,  is the intercept for the power function, w is the body weight in g, d is the weight coefficient, f is the temperature-dependent coefficient, and T is the water temperature. For each day, Uopt was converted to body length per second by dividing the estimated value by the length of the fish on that given day. The model for optimal swimming speed was parameterized for sockeye salmon Oncorhynchus nerka (Walbaum, 1792) (Beauchamp et al., 1989).
is the intercept for the power function, w is the body weight in g, d is the weight coefficient, f is the temperature-dependent coefficient, and T is the water temperature. For each day, Uopt was converted to body length per second by dividing the estimated value by the length of the fish on that given day. The model for optimal swimming speed was parameterized for sockeye salmon Oncorhynchus nerka (Walbaum, 1792) (Beauchamp et al., 1989).
Egestion, excretion, and specific dynamic action
Energy lost in faeces (egestion) and through excretion was modelled as functions of water temperature and the ratio between consumption and the daily maximum consumption (Elliott, 1976).
where F is the proportion of consumed energy lost in faeces, E is the proportion of assimilated energy (i.e. consumption–egestion) lost in excretion, Fa and Ea are the intercepts, T is the water temperature, Fb1 and Eb1 are the temperature coefficients, Fb2 and Eb2 are the consumption coefficients, and cr is proportion consumed of the daily maximum consumption (C/Cmax). Models for both egestion and excretion were parametrized for brown trout Salmo trutta Linnaeus, 1758 (Elliott, 1976).
Specific dynamic action, SDA, was set as a fixed proportion of the assimilated energy using a value of 0.17, which corroborates with the previous bioenergetic models for anadromous salmonids (Beauchamp et al., 1989; Smith et al., 2009).
Changes in thermal habitat
To quantify the impacts of increased ocean temperatures on the required food consumption of Atlantic salmon, alternative simulations were run to represent different climate scenarios. The observed growth during the ocean feeding migration was held constant and the recorded temperatures were increased in 5% increments to a maximum of 3 °C during summer (May–October), and by up to 2 °C in winter (November–April). This roughly corresponds to the predicted temperature increase in relevant regions of the Northeast Atlantic Ocean during the next 50 years (Alexander et al., 2018).
Note on model output and estimates in relation to the energy density of prey
The bioenergetic model estimates energy consumption in kJ. This is converted to food consumption in g by dividing the consumed energy on the energy density of prey. This direct link between food consumption and the energy density of prey differs from how the energy density of prey impacts individuals’ energy consumption, which remains constant at all possible energy densities of prey. The reason why energy consumption is not affected by changes in the energy density of prey is that any alterations of prey quality will be absorbed by the ecological constraint of foraging P. The ecological constraint of foraging P is defined as a proportion of the temperature-dependent maximum consumption (see consumption equation) and is inversely related to the energy density of prey. Because we model consumption at a fixed actual growth, this means that a high ecological constraint on foraging (low P) is required at high energy densities of prey, and a low ecological constraint (high P) is required when the prey is of low quality (Supplementary Fig. 1). In summary, this means that a change in the energy density of prey from 4 to 8 kJ/g will halve the food consumption in g, have no effect on the energy consumption in kJ, and increase the ecological constraint of foraging (i.e. lower the P value). Moreover, this means that any changes in the energy consumption caused by alteration of the input temperatures (i.e. the temperatures experienced by the fish) will remain constant across all energy densities of prey.
Results
During the ocean migration, the total increase in body length and weight of the Atlantic salmon (n = 33) ranged from 1 to 20 cm (mean ± SD = 13 ± 4 cm), and from 1.7 to 10.6 kg (mean ± SD = 6.2 ± 1.9 kg). This corresponded to an average increase in length and mass of 15% and 105%, and an absolute growth rate of 4–27 g/day (mean ± SD = 16 ± 5 g/day). Estimated optimal swimming speeds were substantially lower than 1 body length per second (bl/s), with the mean optimal swimming speed of individual fish ranging from 0.37 to 0.44 bl/s (mean ± SD = 0.40 ± 0.02 bl/s).
Food consumption at observed temperatures
The tagged Atlantic salmon experienced daily mean temperatures ranging from − 0.5 to 12.9 ℃ while at sea. Although a clear seasonal trend was present in the thermal habitat, with fish experiencing the coldest water from November till March (see Strøm et al., 2020), this seasonal pattern was absent in the estimated daily food consumption (Fig. 3).
For the tagged Atlantic salmon, mean daily consumption ranged from 331 to 813 kJ/day at optimal swimming speeds and from 397 to 966 kJ/day at a fixed swimming speed of 1 bl/s (Table 3). At the default energy density of prey of 5 kJ/g wet weight, this corresponded to a mean daily food consumption between 66 and 163 g/day at optimal swimming speeds, with individuals consuming between 25,124 and 63,753 g during the entire ocean migration (Table 3). Using a fixed swimming speed of 1 bl/s, mean daily food consumption ranged from 79 to 193 g/day and total prey consumption from 30,076 to 75,720 g (Table 3). Overall growth efficiency (i.e. the total gain in body mass divided by the total food consumption) ranged from 5 to 24% at optimal swimming speeds, and from 4 to 20% at a fixed swimming speed of 1 b1/s. The ecological constraint on maximum daily food consumption P, which can range from 0 to 1, was estimated to range from 0.52 to 0.81 at optimal swimming speeds and from 0.63 to 0.96 at fixed swimming speeds. The correlations between daily mean temperature and consumption ranged between − 0.31 and 0.91 (Spearman correlation, mean ± SD = 0.21 ± 0.34) among individuals.
Food consumption at increased temperatures
When increasing the experienced water temperature, the Atlantic salmon were required to increase their energy consumption to obtain the observed somatic growth at sea (Fig. 4). However, the increase in energy demands was modest even at the most extreme temperature manipulations (i.e. 3 °C increase during summer and 2 °C increase during winter). At optimal swimming speed, the total consumption during the ocean migration was between 10,413 and 22,393 kJ (mean ± SD = 15,312 ± 2951 kJ) greater at the most extreme temperature manipulation than at the recorded temperatures, with an increase in total food consumption between 2083 and 4478 g (mean ± SD = 3062 ± 590 g) for the full ocean migration, assuming an energy density of prey of 5 kJ/g wet weight. This corresponded to an average relative increase in consumption of 7.7% (SD = 0.8%). For comparison, a marginal reduction in energy density of prey, from the default value of 5 kJ/g to 4.64 kJ/g, would at recorded temperatures result in a similar increase in the food consumption.
Discussion
Although Atlantic salmon is one of the most studied fish species, many of the studies have been performed on life stages in freshwater and near-coastal areas, and the knowledge of the ocean part of their life is more restricted (Thorstad et al., 2021). Regarding marine growth, the understanding of how this is impacted by ocean current systems, productivity, and food availability has improved in later years (Renkawitz et al., 2015; Utne et al., 2021, 2022; Vollset et al., 2022), and models of marine growth in relation to water temperature and food consumption have been developed for both wild and farmed Atlantic salmon (Handeland et al., 2008; Smith et al., 2009; Føre et al., 2016). However, little is known on the food consumption of wild fish in terms of how much they must eat during the ocean migration to maintain their growth, and on how increased ocean temperatures with climate change directly impact their energy budgets. By using a bioenergetic model, which couples in situ temperature recordings with information on their actual marine growth, we estimate that repeat ocean migrating Atlantic salmon consumed approximately 200 000 kJ during the ocean migration and that the direct effect of increase ocean temperature on individual food consumption is predicted to be limited.
We based our estimates on the assumption that the Atlantic salmon were eating prey with a mean energy density of 5 kJ/g during the ocean migration. Atlantic salmon are opportunistic feeders that feed on a diversity of prey items including pelagic crustaceans, squid, and fish (e.g. Hansen & Pethon, 1985; Jacobsen & Hanssen, 2001; Dixon et al., 2017, Utne et al., 2021). The energy density of possible prey items for Atlantic salmon varies largely, usually from 3.5 to 7.0 kJ/g, but with values as low as 1.7 kJ/g recorded for some Gammaridae, and as high as 10 kJ/g or more for forage fish in good condition (Pedersen & Hislop, 2001). Despite this possible variability, large-sized Atlantic salmon, as tagged in this study, likely feed on fish during their ocean migration, and for Atlantic salmon returning from the ocean to northern Norway, four fish species comprised the bulk of the diet (Aykanat et al., 2020). These four fish species were sand eel Ammodytes spp., capelin Mallotus villosus (Müller, 1776), herring Clupea harengus (Linnaeus, 1758), and haddock Melanogrammus aeglefinus (Linnaeus, 1758), with sand eel being the most abundant and herring constituting the largest percentage by weight. Furthermore, Hedger et al. (2022) reported that diet of returning Atlantic salmon from the same sample as used here comprised mainly of herring. Notably, the data published by both Aykanat et al. (2020) and Hedger et al. (2022) only reflected foraging during the last part of the ocean migration and it cannot be ruled out that there is variation in prey composition during different stages of the ocean migration and among years. Nevertheless, reported energy contents of herring, haddock, capelin, and sand eel comply with our choice of using a mean energy density of 5 kJ/g (Pedersen & Hislop, 2001; Hedeholm et al., 2011; Renkawitz et al., 2015). In particular, herring during the winter months relevant for our study had an energy density of 4.4–8.8 kJ/g, haddock had an energy density of 3.6–5.5 kJ/g, and sand eel had an energy density of 4.4 kJ/g (Pedersen & Hislop, 2001). Capelin has been reported with energy densities from 4.0 to 4.5 kJ/g (Hedeholm et al., 2011) to 6.5 kJ/g (Renkawitz et al., 2015).
Individual Atlantic salmon tagged in the present study varied considerably in growth during the one-year ocean migration, with an absolute growth rate of 4–27 g per day, corresponding to a total increase in body mass of nearly 2 kg to more than 10 kg. Despite these being large adults tagged after spawning, this is a considerable growth, and according to our estimates, they had to eat on average about 70 to 160 g of prey with an energy density of 5 kJ/g per day. In other studies, Atlantic salmon of similar body sizes had mainly been feeding on fish prey of body lengths 10–20 cm, with a maximum prey size up to about 30–35% of their own body length (Renkawitz et al., 2015; Aykanat et al., 2020). If we, based on the studies referred to above, assume that the Atlantic salmon in our study had been eating prey fish with an individual body size of about 15 g, this would correspond to a daily food intake of about 5 to 11 fish prey per day on average during the course of the ocean migration.
In the bioenergetic model, the Atlantic salmon were set to experience equal daily growth within the two growth periods, which lasted from sea entry to winter and from winter to 14 days prior their return. This is based on the assumption that the Atlantic salmon were feeding during their entire ocean migration, however, to what extent Atlantic salmon feed throughout their ocean residency is uncertain. In a study by Renkawitz et al. (2015), a very low proportion of Atlantic salmon had empty stomachs when sampled West of Greenland in August–October, indicating that the salmon were feeding daily during that period. This contrasted the findings of Jacobsen & Hansen (2001), where only half (53%) of the Atlantic salmon captured in the Norwegian Sea had stomachs containing food in the autumn, while during winter, 78% had stomachs containing food. This pattern was explained by the lower water temperatures during winter, which would lead to lower gastric evacuation rates, and thereby fewer stomachs being empty due to slow digestion. Despite slower gastric evacuation rates at low temperatures, more than 50% of the food content in the stomach is shown to be digested by Atlantic salmon within 24 h even at temperatures down to 6 °C (Sveier et al., 1999; Handeland et al., 2008). Hence, Atlantic salmon seem to feed actively also during the winter months at low temperatures and may even be able to feed daily if prey is available.
The average swimming speed of Atlantic salmon during the entire ocean migration is unknown. We therefore made two parallel estimates of the energy consumption: one based on an average swimming speed of 1 body length per second, and one based on an optimal swimming speed calculated from a model considering body weight and temperature (Beauchamp et al., 1989) resulting in average swimming speeds from 0.37 to 0.44 body lengths per second for individual fish. During feeding, or in cases of predator avoidance, it is likely that Atlantic salmon swim at relatively high speeds, whereas during periods of digesting food, it is reasonable to assume that they swim less actively at lower swimming speeds. Hence, it might be that the most realistic estimates are those based on the optimal swimming speeds. Nevertheless, the estimated energy consumption did not highly depend on whether an average swim speed of 1 body length per second or the optimal swimming speed was used.
Expected increases in ocean temperatures due to climate change did not largely impact the required food consumption to maintain growth of the Atlantic salmon in terms of direct physiological effects on the energy budgets. In the most extreme climate scenario, Atlantic salmon had to increase the food intake during the entire one-year migration by average 3 kg prey to compensate for the increased water temperatures assuming an energy density of prey of 5 kJ/g. This represented a relative increase in energy demand of only 7.7%, which will provide the same effect on the food requirement of Atlantic salmon as a reduction in the energy density of prey from 5 to 4.64 kJ/g. From this, we conclude that the impacts of climate warming on Atlantic salmon growth and survival will likely primarily occur through ecosystem changes that alter prey availability and quality rather than direct physiological effects caused by increased water temperatures per se. This supports recent findings by Vollset et al. (2022) and Utne et al. (2022) who concluded that the reduced growth of Atlantic salmon over large areas of the Northeast Atlantic Ocean around year 2005 was likely due to an ecological regime shift limiting food availability and growth of Atlantic salmon, rather than a direct physiological effect of the observed increase in water temperatures during that period.
Conclusion
Detailed temperature recordings and observed growth of repeat ocean migrating Atlantic salmon, combined with bioenergetic models, enabled us to estimate the required food consumption to maintain body growth in individual fish during the marine feeding migration, and to estimate the energetic impacts of predicted increases in ocean temperatures due to climate change. Expected increases in ocean temperatures due to climate change did not largely impact the required food consumption to maintain growth, indicating that the most pronounced effects of increased ocean temperatures are due to alterations of ecosystems and subsequent changes in prey quality and abundance rather than direct physiological effects of increased water temperatures. These results are likely representative for many northern Atlantic salmon populations in Norway, Denmark, and Canada, which to a large extent seem to migrate to ocean areas with similar prey composition and experience similar waters temperatures throughout large parts of their migration (Strøm et al., 2017; Rikardsen et al., 2021). However, for more southern populations, for instance from Ireland, Iceland, and Spain, the direct consequences of climate change on energy consumption may be more severe, as Atlantic salmon from these regions are already experiencing warmer water temperatures (Guðjónsson et al., 2015; Rikardsen et al., 2021) and likely higher energy requirements compared to their more northern conspecifics.
Data availability
The data and computational codes used in this study are available on reasonable request.
References
Alexander, M. A., J. D. Scott, K. D. Friedland, K. E. Mills, J. A. Nye, A. J. Pershing & A. C. Thomas, 2018. Projected sea surface temperatures over the 21st century: Changes in the mean, variability and extremes for large marine ecosystem regions of Northern Oceans. Elementa: Science of the Anthropocene 6: 9.
Alfonso, S., M. Gesto & B. Sadoul, 2021. Temperature increase and its effects on fish stress physiology in the context of global warming. Journal of Fish Biology 98: 1496–1508.
Aykanat, T., M. Rasmussen, M. Ozerov, E. Niemelä, L. Paulin, J. P. Vähä, K. Hindar, V. Wennevik, T. Pedersen, M. A. Svenning & C. R. Primmer, 2020. Life-history genomic regions explain differences in Atlantic salmon marine diet specialization. Journal of Animal Ecology 89: 2677–2691.
Beauchamp, D. A., D. J. Stewart & G. L. Thomas, 1989. Corroboration of a bioenergetics model for sockeye salmon. Transactions of the American Fisheries Society 118: 597–607.
Beaugrand, F. & P. C. Reid, 2012. Relationships between North Atlantic salmon, plankton, and hydroclimatic change in the Northeast Atlantic. ICES Journal of Marine Science 69: 1549–1562.
Chittenden, C. M., P. Fauchald & A. H. Rikardsen, 2013. Important open-ocean areas for northern Atlantic salmon (Salmo salar)—as estimated using a simple ambient-temperature approach. Canadian Journal of Fisheries and Aquatic Sciences 70: 101–104.
Deslauriers, D., S. R. Chipps, J. E. Breck, J. A. Rice & C. P. Madenjian, 2017. Fish bioenergetics 4.0: an R-based modeling application. Fisheries 42: 586–596.
Dixon, H. J., J. B. Dempson, T. F. Sheehan, M. D. Renkawitz & M. Power, 2017. Assessing the diet of North American Atlantic salmon (Salmo salar L.) off the West Greenland coast using gut content and stable isotope analyses. Fisheries Oceanography 26: 555–568.
Elliott, J. M., 1976. Energy losses in waste products of brown trout (Salmo trutta L.). Journal of Animal Ecology 45: 561–580.
Føre, M., M. Alver, J. A. Alfredsen, G. Marafioti, G. Senneset, J. Birkevold, F. V. Willumsen, G. Lange, Å. Espmark & B. F. Terjesen, 2016. Modelling growth performance and feeding behaviour of Atlantic salmon (Salmo salar L.) in commercial-size aquaculture net pens: model details and validation through full-scale experiments. Aquaculture 464: 268–278.
Friedland, K. D., B. V. Shank, C. D. Todd, P. McGinnity & J. A. Nye, 2014. Differential response of continental stock complexes of Atlantic salmon (Salmo salar) to the Atlantic Multidecadal Oscillation. Journal of Marine Systems 133: 77–87.
Grøttum, J. A. & T. Sigholt, 1998. A model for oxygen consumption of Atlantic salmon (Salmo salar) based on measurements of individual fish in a tunnel respirometer. Aquacultural Engineering 17: 241–251.
Guðjónsson, S., S. M. Einarsson, I. R. Jónsson & J. Guðbrandsson, 2015. Marine feeding areas and vertical movements of Atlantic salmon (Salmo salar) as inferred from recoveries of data storage tags. Canadian Journal of Fisheries and Aquatic Sciences 72: 1087–1098.
Handeland, S. O., A. K. Imsland & S. O. Stefansson, 2008. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 283: 36–42.
Hansen, L. P. & P. Pethon, 1985. The food of Atlatic salmon, Salmo salar L., caught by long-line in northern Norwegian waters. Journal of Fish Biology 26: 553–562.
Hedeholm, R., P. Grønkjær & S. Rysgaard, 2011. Energy content and fecundity of capelin (Mallotus villosus) along a 1,500-km latitudinal gradient. Marine Biology 158: 1319–1330.
Hedger, R. D., A. H. Rikardsen, J. F. Strøm, D. A. Righton, E. B. Thorstad & T. F. Næsje, 2017. Diving behaviour of Atlantic salmon at sea: effects of light regimes and temperature stratification. Marine Ecology Progress Series 574: 127–140.
Hedger, R. D., M. Kjellman, E. B. Thorstad, J. F. Strøm & A. H. Rikardsen, 2022. Diving and feeding of adult Atlantic salmon when migrating through the coastal zone in Norway. Environmental Biology of Fishes 105: 589–604.
Jacobsen, J. A. & L. P. Hansen, 2001. Feeding habits of wild and escaped farmed Atlantic salmon, Salmo salar L., in the Northeast Atlantic. ICES Journal of Marine Science 58: 916–933.
Jonsson, N., B. Jonsson & L. P. Hansen, 1997. Changes in proximate composition and estimates of energetic costs during upstream migration and spawning in Atlantic salmon Salmo salar. Journal of Animal Ecology 66: 425–436.
Macnaughton, C. J., D. Deslauriers, E. L. Ipsen, E. Corey & E. C. Enders, 2019. Using meta-analysis to derive a respiration model for Atlantic salmon (Salmo salar) to assess bioenergetics requirements of juveniles in two Canadian rivers. Canadian Journal of Fisheries and Aquatic Sciences 76: 2225–2234.
Mills, K. E., A. J. Pershing, T. F. Sheehan & D. Mountain, 2013. Climate and ecosystem linkages explain widespread declines in North American Atlantic salmon populations. Global Change Biology 19: 3046–3061.
Moore, S. E. & H. P. Huntington, 2008. Arctic marine mammals and climate change: impacts and resilience. Ecological Applications 18: S157–S165.
Moss, J. H., D. A. Beauchamp, A. D. Cross, E. V. Farley, J. M. Murphy, J. H. Helle, R. V. Walker & K. W. Myers, 2009. Bioenergetic model estimates of interannual and spatial patterns in consumption demand and growth potential of juvenile pink salmon (Oncorhynchus gorbuscha) in the Gulf of Alaska. Deep-Sea Research Part II: Topical Studies in Oceanography 56: 2553–2559.
Pedersen, J. & J. R. G. Hislop, 2001. Seasonal variations in the energy density of fishes in the North Sea. Journal of Fish Biology 59: 380–389.
Perry, A. L., P. J. Low, J. R. Ellis & J. D. Reynolds, 2004. Climate change and distribution shifts in marine fishes. Science 308: 1912–1915.
Railsback, S. F. & K. A. Rose, 1999. Bioenergetics modeling of stream trout growth: temperature and food consumption effects. Transactions of the American Fisheries Society 128: 242–256.
Renkawitz, M. D., T. F. Sheehan, H. J. Dixon & R. Nygaard, 2015. Changing trophic structure and energy dynamics in the Northwest Atlantic: implications for Atlantic salmon feeding at West Greenland. Marine Ecology Progress Series 538: 197–211.
Rikardsen, A. H., D. Righton, J. F. Strøm, E. B. Thorstad, P. Gargan, T. Sheehan, F. Økland, C. M. Chittenden, R. D. Hedger, T. F. Næsje, M. Renkawitz, J. Sturlaugsson, P. Caballero, H. Baktoft, J. G. Davidsen, E. Halttunen, S. Wright, B. Finstad & K. Aarestrup, 2021. Redefining the oceanic distribution of Atlantic salmon. Scientific Reports 11: 12266.
Shearer, W. M., 1992. Atlantic Salmon Scale Reading Guidelines. ICES Cooperative Research Report 188. Copenhagen, Denmark: 46.
Smith, I. P., D. J. Booker & N. C. Wells, 2009. Bioenergetic modelling of the marine phase of Atlantic salmon (Salmo salar L.). Marine Environmental Research 67: 246–258.
Strøm, J. F., E. B. Thorstad, G. Chafe, S. H. Sørbye, D. Righton, A. H. Rikardsen & J. Carr, 2017. Ocean migration of pop-up satellite archival tagged Atlantic salmon from the Miramichi River in Canada. ICES Journal of Marine Science 74: 1356–1370.
Strøm, J. F., E. B. Thorstad, R. D. Hedger & A. H. Rikardsen, 2018. Revealing the full ocean migration of individual Atlantic salmon. Animal Biotelemetry 6: 2.
Strøm, J. F., E. B. Thorstad & A. H. Rikardsen, 2020. Thermal habitat of adult Atlantic salmon Salmo salar in a warming ocean. Journal of Fish Biology 96: 327–336.
Sveier, H., E. Wathne & E. Lied, 1999. Growth, feed and nutrient utilisation and gastrointestinal evacuation time in Atlantic salmon (Salmo salar L.): the effect of dietary fish meal particle size and protein concentration. Aquaculture 180: 265–282.
Thorstad, E. B., D. Bliss, C. Breau, K. Damon-Randall, L. E. Sundt-Hansen, E. M. C. Hatfield, G. Horsburgh, H. Hansen, N. Maoiléidigh, T. Sheehan & S. G. Sutton, 2021. Atlantic salmon in a rapidly changing environment—facing the challenges of reduced marine survival and climate change. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 2654–2665.
Todd, C. D., B. D. M. Whyte, J. C. Maclean, C. W. Revie, M. E. Lonergan & N. N. Hanson, 2014. A simple method of dating marine growth circuli on scales of wild one sea-winter and two sea-winter Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 71: 645–655.
Utne, K. R., B. D. Pauli, M. Haugland, J. A. Jacobsen, N. Maoileidigh, W. Melle, C. T. Broms, L. Nøttestad, M. Holm, K. Thomas & V. Wennevik, 2021. Poor feeding opportunities and reduced condition factor for salmon post-smolts in the Northeast Atlantic Ocean. ICES Journal of Marine Science 78: 2844–2857.
Utne, K. R., Ø. Skagseth, V. Wennevik, C. T. Broms, W. Melle & E. B. Thorstad, 2022. Impacts of a changing ecosystem on the feeding and feeding conditions for Atlantic salmon during the first months at sea. Frontiers in Marine Science 9: 824614.
Vollset, K. W., K. Urdal, K. Utne, E. B. Thorstad, H. Sægrov, A. Raunsgard, Ø. Skagseth, R. J. Lennox, G. M. Østborg, O. Ugedal, A. J. Jensen, G. H. Bolstad & P. Fiske, 2022. Ecological regime shift in the Northeast Atlantic Ocean revealed from the unprecedented reduction in marine growth of Atlantic salmon. Science Advanced 8: eabk2542.
Acknowledgements
The authors thank the Alta Laksefiskeri Interessentskap and its members for assistance in conducting the study. We also thank students and staff of the Freshwater ecology group at UiT The Arctic University of Norway for assistance during the fieldwork, and Gunnel Østborg at the Norwegian Institute for Nature Research for reading the Atlantic salmon scales.
Funding
Open access funding provided by Institute Of Marine Research. The study was funded by the Research Council of Norway (Project 221400/E40 Salmotrack and 280308 SeaSalar) and Alta Laksefiskeri Interessentskap.
Author information
Authors and Affiliations
Contributions
AHR designed and arranged the data sampling. JFS, OU, and EBT conceived the idea for the manuscript. JFS constructed the model and analysed the data, with contribution from OU and EBT. JFS led the writing of the manuscript, with contribution from EBT, OU and AHR. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors have no conflict of interests to declare.
Additional information
Handling editor: Michael Power
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Strøm, J.F., Ugedal, O., Rikardsen, A.H. et al. Marine food consumption by adult Atlantic salmon and energetic impacts of increased ocean temperatures caused by climate change. Hydrobiologia 850, 3077–3089 (2023). https://doi.org/10.1007/s10750-023-05234-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05234-2