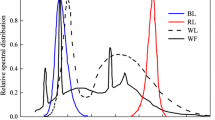
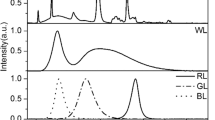
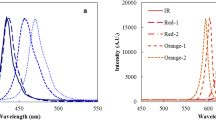
Abstract
Efficient production of beneficial metabolites by microalgae using light-emitting diodes (LED) is attracting more interest. In batch cultures, metabolic changes that occur with nutrient deficiency over time must be monitored to estimate the metabolite content. Our previous study showed that nutrient deficiency in Nannochloropsis oculata can be briefly evaluated by measuring the absorption ratio at 490 and 680 nm (Abs490/Abs680). However, little is known about the relation with this ratio and metabolic change, particularly in LED light-conditioned cultures. In this study, we investigate if Abs490/Abs680 can be an indicator of the biochemical status of N. oculata cultured under various LED color regimes. Three LED treatments were used: Blue (B), Blue + Red (BR), and Red (R). In all LED treatments, the Abs490/Abs680 decreased for several days and then increased when phosphate was limited or non-existent. The trends in the C16:0 and C20:5ɷ3 proportions and chlorophyll a content commonly changed at the turning points of the Abs490/Abs680. Furthermore, significant correlations were found among the metabolic state and absorption ratio, except in treatment R. We suggest that spectrophotometry can be used for monitoring metabolic changes in N. oculata cultures under various LED irradiance, unless there is excessive 630 nm-red light exposure.





Similar content being viewed by others
References
Andrews T, Lorimer GH (2003) Manipulating ribulose bisphosphate carboxylase/oxygenase in the chloroplasts of higher plants. Arch Biochem Biophys 414:159–169
Atta M, Idris A, Bukhari A, Wahidin S (2013) Intensity of blue LED light: a potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour Technol 148:373–378
Berner T, Dubinsky Z, Wyman K, Falkowski PG (1989) Photoadaptation and the package effect in Dunaliella tertiolecta (Chlorophyceae). J Phycol 25:70–78
Blair MF, Kokabian B, Gude VG (2014) Light and growth medium effect on Chlorella vulgaris biomass production. J Environ Chem Eng 2:665–674
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brown MR, Dunstan GA, Norwood SJ, Miller KA (1996) Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. J Phycol 32:64–73
Carvalho AP, Silva SO, Baptista JM, Malcata FX (2011) Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl Microbiol Technol 89:1275–1288
Chen C-Y, Chen Y-C, Huang H-C, Ho S-H, Chang J-S (2015) Enhancing the production of eicosapentaenoic acid (EPA) from Nannochloropsis oceanica CY2 using innovative photobioreactors with optimal light source arrangements. Bioresour Technol 191:407–413
Connor WE (2000) Importance of n-3 fatty acids in health and disease. Am J Clin Nutr 71:171–175
Das P, Lei W, Aziz SS, Obbard JP (2011) Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Bioresour Technol 102:3883–3887
Falkowski PG, Owens TG (1980) Light-shade adaptation. Two strategies in marine phytoplankton. Plant Physiol 66:592–595
Fidalgo JP, Cid A, Torres E, Sukenik A, Herrero C (1998) Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture 166:105–116
Glemser M, Heining M, Schmidt J, Becker A, Garbe D, Buchholz R, Brück T (2016) Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: current state and perspectives. Appl Microbiol Biotechnol 100:1077–1088
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Guillard RRL, Sieracki MS (2005) Counting cells in cultures with the light microscope. In: Anderson RA (ed) Algal culturing techniques. Elsevier, New York, pp 239–252
Harrison PJ, Thompson PA, Calderwood GS (1990) Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J Appl Phycol 2:45–56
Hodgson PA, Henderson RJ, Sargent JR, Leftley JW (1991) Patterns of variation in the lipid class and fatty acid composition of Nannochloropsis oculata (Eustigmatophyceae) during batch culture. J Appl Phycol 3:169–181
Hultberg M, Jönsson HL, Bergstrand K-J, Carlsson AS (2014) Impact of light quality on biomass production and fatty acid content in the microalga Chlorella vulgaris. Bioresour Technol 159:465–467
Hyka P, Lickova S, Přibyl P, Melzoch K, Kovar K (2013) Flow cytometry for the development of biotechnological processes with microalgae. Biotechnol Adv 31:2–16
Ishikawa T, Isowa K (2012) Cultivation of microalgae for live food under white light-emitting diodes (LEDs). J Fish Technol 4:51–55 (in Japanese with English abstract)
Juaneda P, Rocquelin G (1985) Rapid and convenient separation of phospholipids and non phosphorus lipids from rat heart using silica cartridges. Lipids 20:40–41
Kandilian R, Lee E, Pilon L (2013) Radiation and optical properties of Nannochloropsis oculata grown under different irradiances and spectra. Bioresour Technol 137:63–73
Kawamura G (2006) Application of LED to fisheries field. In: Association of Agricultural Electrification (ed) Application of LED to agriculture, forestry and fisheries. Association of Agricultural Electrification, Tokyo, pp 51–62
Kim S-K, Ravichandran YD, Khan SB, Kim YT (2008) Prospective of the cosmeceuticals derived from marine organisms. Biotechnol Bioprocess Eng 13:511–523
Kim T-H, Lee Y, Han S-H, Hwang S-J (2013) The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour Technol 130:75–80
Kim DG, Lee C, Park S-M, Choi Y-E (2014) Manipulation of light wavelength at appropriate growth stage to enhance biomass productivity and fatty acid methyl ester yield using Chlorella vulgaris. Bioresour Technol 159:240–248
Korbee N, Figueroa FL, Aguilera J (2005) Effect of light quality on the accumulation of photosynthetic pigments, proteins and mycosporine-like amino acids in the red alga Porphyra leucosticta (Bangiales, Rhodophyta). J Photochem Photobiol, B 80:71–78
Lacour T, Sciandra A, Talec A, Mayzaud P, Bernard O (2012) Diel variations of carbohydrates and neutral lipids in nitrogen-sufficient and nitrogen-starved cyclostat cultures of Isochrysis sp. J Phycol 48:966–975
Lee TH, Chang JS, Wang HY (2013) Current developments in high-throughput analysis for microalgae cellular contents. Biotechnol J 8:1301–1314
Ley AC, Mauzerall DC (1982) Absolute absorption cross-sections for photosystem II and the minimum quantum requirement for photosynthesis in Chlorella vulgaris. Biochim Biophys Acta 680:95–106
Malapascua J, Jerez C, Sergejevová M, Figueroa F, Masojídek J (2014) Photosynthesis monitoring to optimize growth of microalgal mass cultures: application of chlorophyll fluorescence techniques. Aquat Biol 22:123–140
Maruyama I, Nakamura T, Matsubayashi T, Ando Y, Maeda T (1986) Identification of the alga known as ‘marine Chlorella’ as a member of the Eustigmatophyceae. Jpn J Phycol 34:319–325
Matsui H, Okawa R, Anraku K, Kotani T (2017) Application of spectrophotometry to estimate the optimum culture conditions for Nannochloropsis oculata as a diet for zooplankton. Aquacult Sci 65:209–219
Mayers JJ, Flynn KJ, Shields RJ (2014) Influence of the N: P supply ratio on biomass productivity and time-resolved changes in elemental and bulk biochemical composition of Nannochloropsis sp. Bioresour Technol 169:588–595
Meng Y, Jiang J, Wang H, Cao X, Xue S, Yang Q, Wang W (2015) The characteristics of TAG and EPA accumulation in Nannochloropsis oceanica IMET1 under different nitrogen supply regimes. Bioresour Technol 179:483–489
Merzlyak MN, Chivkunova OB, Gorelova OA, Reshetnikova IV, Solovchenko AE, Khozin-Goldberg I, Cohen Z (2007) Effect of nitrogen starvation on optical properties, pigments, and arachidonic acid content of the unicellular green alga Parietochloris incisa (Trebouxiophyceae, Chlorophyta). J Phycol 43:833–843
Mohsenpour SF, Richards B, Willoughby N (2012) Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresour Technol 125:75–81
Muller-Feuga A, Moal J, Kaas R (2003) The micro algae of aquacutlrue. In: Støttrup JG, Mc Ecoy LA (eds) Live feeds in marine aquaculture. Blackwell, Oxford, pp 206–252
Okauchi M (2012) Changes in nutritive value of Pavlova sp. collected from different population growth phases in batch style culture assessed from cell ultra-structure. Algal Resour 8:11–22
Okauchi M (2013) III-1. Energy conservation culture of microalgae as live feed using an energy-saving illuminator. Nippon Suisan Gakkaishi 79:886 (in Japanese)
Okauchi M, Wen-Jian Z, Wan-Hong Z, Kunihiko F, Kanazawa A (1990) Difference in nutritive value of a microalga Nannochloropsis oculata at various growth phases. Nippon Suisan Gakkaishi 56:1293–1298
Okumura H (2008) Study of the efficiental cultivation of marine microalgae on mass algae cultivation system for mariculture breeding. Sci Rep Hokkaido Fish Exp Stn 73:9–29 (in Japanese with English abstract)
Okumura H, Nakajima K, Masuda A, Takahashi M, Kosaka S, Horaguchi K, Matsuyama K, Murakami K (2001) Experimental mass culturing of Pavlova lutheri using step-style light control. J Illum Engng Inst Jpn 85:571–575 (in Japanese with English abstract)
Parsons TR, Maita Y, Lalli CM (1984) Nutrients. In: Parsons TR, Maita Y, Lalli CM (eds) A manual of chemical and biological methods for seawater analysis. Pergamon, Oxford, pp 3–36
Petkov G, Garcia G (2007) Which are fatty acids of the green alga Chlorella? Biochem Syst Ecol 35:281–285
Priyadarshani I, Rath B (2012) Commercial and industrial applications of micro algae: a review. J Algal Biomass Util 3:89–100
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–221
Reichardt TA, Collins AM, Garcia OF, Ruffing AM, Jones HDT, Timlin JA (2012) Spectroradiometric monitoring of Nannochloropsis salina growth. Algal Res 1:22–31
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J Phycol 30:972–979
Richardson K, Beardall J, Raven JA (1983) Adaptation of unicellular algae to irradiance: an analysis of strategies. New Phytol 93:157–191
Roscher E, Zetsche K (1986) The effects of light quality and intensity on the synthesis of ribulose-1,5-bisphosphate carboxylase and its mRNAs in the green alga Chlorogonium elongatum. Planta 167:582–586
Santos-Ballardo DU, Rossi S, Hernández V, Vázquez-Gómez R, Rendón-Unceta MC, Caro-Corrales J, Valdez-Ortiz A (2015) A simple spectrophotometric method for biomass measurement of important microalgae species in aquaculture. Aquaculture 448:87–92
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Skjånes K, Rebours C, Lindblad P (2013) Potential for green microalgae to produce hydrogen, pharmaceuticals and other high value products in a combined process. Crit Rev Biotechnol 33:172–215
Smith VH, Sturm BSM, DeNoyelles FJ, Billings SA (2010) The ecology of algal biodiesel production. Trends Ecol Evol 25:301–309
Solovchenko A, Khozin-Goldberg I, Recht L, Boussiba S (2011) Stress-induced changes in optical properties, pigment and fatty acid content of Nannochloropsis sp.: implications for non-destructive assay of total fatty acids. Mar Biotechnol 13:527–535
Stansell GR, Gray VM, Sym SD (2012) Microalgal fatty acid composition: implications for biodiesel quality. J Appl Phycol 24:791–801
Sukenik A (1991) Ecophysiological considerations in the optimization of eicosapentaenoic acid production by Nannochloropsis sp. (Eustigmatophyceae). Bioresour Technol 35:263–269
Teo CL, Atta M, Bukhari A, Taisir M, Yusuf AM, Idris A (2014a) Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Bioresour Technol 162:38–44
Teo CL, Idris A, Zain NAM, Taisir M (2014b) Synergistic effect of optimizing light-emitting diode illumination quality and intensity to manipulate composition of fatty acid methyl esters from Nannochloropsis sp. Bioresour Technol 173:284–290
Ueno J (2003) Cultivation of microalgae using light-emitting diodes (LED). Aquanet 6:48–52 (in Japanese)
Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D (2015) Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res 8:121–127
Welschmeyer NA (1994) Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol Oceanogr 39:1985–1992
White S, Anandraj A, Bux F (2011) PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresour Technol 102:1675–1682
Yeh N, Chung J-P (2009) High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Renew Sustain Energy Rev 13:2175–2180
You T, Barnett SM (2004) Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem Eng J 19:251–258
Acknowledgements
We would like to thank Professor Shunsuke Koshio and Manabu Ishikawa and assistant professor Saichiro Yokoyama for lending us the gas chromatography. We would like to thank associate professor Hikaru Endo for the review and discussion. We are grateful to Iris Ann Borlongan and Viliame Pita Waqalevu for some advice on how to brush up the paper. We would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsui, H., Anraku, K. & Kotani, T. Spectrophotometry can monitor changes in algal metabolism triggered by nutrient deficiency in Nannochloropsis oculata cultured under various light-emitting diode light regimes. Fish Sci 85, 167–176 (2019). https://doi.org/10.1007/s12562-018-1261-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-018-1261-y