Abstract
In the Mediterranean Sea, the symbiosis between the gorgonian Paramuricea clavata (Risso, 1826) and the polychaete Haplosyllis chamaeleon Laubier, 1960 (Annelida, Syllidae, Syllinae) has only been documented from the western basin. Our findings extend its geographic distribution to the north-central basin and represent the first record of H. chamaeleon in Italy and Croatia. Periodic observations from the Ligurian Sea allowed establishing that the symbiont occurs on P. clavata almost throughout the year, showing a reproductive period longer than previously reported. Morphometric comparisons of three Mediterranean populations, from Portofino Promontory (Ligurian Sea), Cape of Creus (Catalan Sea) and Chafarinas Archipelago (Alboran Sea) proved that there were no significant differences in body measurements, whilst the observed differences in dorsal cirri length pattern could be consider intra-specific. Our behavioural observations confirm that the species had (i) a kleptoparasitic behaviour, (ii) did not cause injuries to the host and (iii) did not induce the host to generate any malformation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Symbiosis is common in marine ecosystems, especially in complex habitats like tropical and temperate bioconstructions, where competitive and cooperative interactions are highly frequent. A particular type of bioconstructions are the submarine animal forests formed by gorgonians (Cerrano et al. 2010). Their three-dimensional structures may affect edaphic conditions (Valisano et al. 2016; Ponti et al. 2018) and enhance local biodiversity, especially in Mediterranean coralligenous habitats (Ponti et al. 2014, 2016, 2018). Anthozoans, including sea-fans, often host organisms seeking for shelter (Molodtsova et al. 2016), food and/or reproductive success (Goh et al. 1999). Amongst them (Patton 1972; Goh et al. 1999; Dias et al. 2015), there are either sessile species like sponges (Calcinai et al. 2013) and hydroids (Puce et al. 2008; Seveso et al. 2016; Pica et al. 2017) or vagile species like crustaceans (Ponti et al. 2016; Valisano et al. 2016) and polychaetes (Martin and Britayev 1998, 2018; Glasby and Watson 2001; Barnich et al. 2013; Britayev et al. 2014; Carvalho et al. 2014; Cúrdia et al. 2015; Martin et al. 2002; Nygren and Pleijel 2010).
As much as 26% of the polychaetes living in association with cnidarians are Syllidae (Molodtsova et al. 2016). Syllids have complex taxonomy (Aguado et al. 2012) and their life strategies often include associations with sponges, cnidarians, molluscs, crustaceans, echinoderms and other polychaetes (Martin and Britayev 1998, 2018). However, only a few have octocoral hosts; amongst them Imajimaea draculai (San Martín and López, 2002) is a parasite of the pennatulacean Funiculina quadrangularis (Pallas, 1766) (Nygren and Pleijel 2010), whilst Haplosyllis villogorgicola Martin et al., 2002, associated to the gorgonian Villogorgia bebrycoides (Koch, 1887) from the Canary Islands, and Haplosyllis anthogorgicola Utinomi, 1956, a symbiont of the gorgonian Acanthogorgia bocki Aurivillius, 1931, from Japan, are known to be kleptocommensals (Martin et al. 2002). However, probably the best-known species is Haplosyllis chamaeleon Laubier, 1960, which lives in association with the gorgonian Paramuricea clavata (Risso, 1826).
The species of Paramuricea Kölliker, 1865, were reported to host the hydroid Ectopleura sp. (Bo et al. 2011), the solenogaster Anamenia gorgonophila (Kowalevsky, 1880) (Mifsud et al. 2008), the pycnogonid Callipallene spectrum (Dohrn, 1881), the nudibranch Marionia blainvillea (Risso, 1818) (Ponti et al. 2016), the decapod Balssia gasti (Balss, 1921) (Mori et al. 1995) and the syllid Haplosyllis chamaeleon Laubier, 1960 (López et al. 1996, Martin et al. 2002). In particular, the purple gorgonian P. clavata is an ecosystem engineer in Mediterranean coralligenous environments (Linares et al. 2008a; Ponti et al. 2014), which recently suffered widespread mass mortality events (Cerrano et al. 2000; Garrabou et al. 2009; Huete-Stauffer et al. 2011). Whilst its ecology, connectivity and reproduction are in-depth investigated (Coma et al. 1995; Cerrano et al. 2005; Linares et al. 2008a, b; Arizmendi-Mejía et al. 2015; Ponti et al. 2016, 2018), there is scant information on its interspecific relationships. In fact, the ectoparasitic association of P. clavata with H. chamaeleon was first described from Banyuls-sur-Mer (France) and later reported from the Chafarinas Archipelago (Spain) (Laubier 1960; López et al. 1996), being considered as having a restricted Mediterranean distribution (Martin and Britayev 1998), until Lattig and Martin (2009) reported a population living in association with Paramuricea grayi (Johnson, 1861) along the north-eastern Atlantic coast of the Iberian Peninsula. Overall, the morphology, ecology and reproductive strategy of H. chamaeleon and the characteristics of its association with the gorgonians are well documented (Martin et al. 2002; Lattig and Martin 2009), whilst its distribution and behaviour warrant further investigation.
The EU project Marine Ecosystem Restoration in Changing European Seas (MERCES) seeks to support decision-making aimed at conserving and restoring key European habitats and particularly emphasised the importance of life history in species affected by restoration activities, including the ecosystem engineer P. clavata. Within this frame, our study aimed (i) to review and update the geographic distribution of the symbiotic populations of H. chamaeleon, (ii) to describe the host/symbiont interactions based on populations sampled in the Ligurian Sea, (iii) to describe reproductive traits of the symbiont and (iv) to compare the main morphometric traits of the symbiont from the Ligurian Sea, the Cap de Creus (Catalan Sea) and the Chafarinas Archipelago (Alboran Sea).
Materials and methods
To analyse the infestation characteristics, we collected apical branches of both of yellow and red chromotypes of P. clavata (about 15 cm long) by scuba diving (Table 1). For the temporal monitoring, we collected 192 apical branches of P. clavata at about 40 m depth along the Portofino Promontory (Ligurian Sea, Italy) in September and November 2016, from May to October 2017, in January and February 2018 and in May and June 2018 (Table 2). In all sites, we randomly cut the branches with sharp scissors and enclosed them in individual plastic zip-bags to prevent faunal loss. Detailed collection information for the specimens collected in the Cap of Creus and the Chafarinas Archipelago can be found in Martin et al. (2002). The Italian polychaetes were identified according to Laubier (1960), López et al. (1996), Martin et al. (2002), and Lattig and Martin (2009). All records of H. chamaeleon and the related information were included in a datasheet following Di Camillo et al. (2018a) (Electronic Supplementary Material 1) to be analysed.
In all sampling sites, the frequency of the association was estimated as the number of branches hosting polychaetes versus the total number of collected branches (expressed as percentage). Infestation rates were estimated as the total number of polychaetes found in a given branch divided by the total linear branch length (in centimetre and measured from pictures using the “ImageJ 1.46r” software) and extrapolated to the number of symbionts per host linear metre. The possible changes in infestation through time were assessed on non-transformed data by the non-parametric Kruskal–Wallis test (Sokal and Rohlf 1981) as data were not normally distributed (Shapiro–Wilk’s test). Analyses were performed using PAST for Windows version 4.0 (Hammer et al. 2001).
To describe the location and behaviour of the symbionts, gorgonian fragments were placed on Petri dishes with a base covered by graph paper, and examined in vivo under a stereomicroscope. In addition to direct observations, we obtained photographs and videos using a Canon PowerShot G16. After keeping samples at 4 °C overnight, to relax them before fixation, the samples were preserved (separately, the gorgonians and the syllids) in 70% ethanol, except those used for observations in vivo, which were fixed either in 96% ethanol for light microscope observations or rinsed in distilled water, dehydrated through a graded ethanol series (20%, 50%, 70%, 90%, 100%), dried with hexamethyldisilazane 98%, and after complete evaporation, attached to stubs, coated with gold-platinum in a Balzer Union evaporator, and examined under a Philips XL20 scanning electron microscope (SEM). The specimens collected in July 2017 and May and June 2018 were set aside for a parallel chemical analysis (not included in the present paper).
According to Lattig and Martin (2009), the main morphological traits were measured on well-preserved, complete polychaete specimens (from Portofino, Cap of Creus and the Chafarinas Archipelago); 36 specimens for body measurements and six specimens for dorsal cirri length pattern (as number of antennae and cirri articles) were placed on slides with glycerine and measured using a micrometric scale. The inter-population morphometric differences were analysed for size-independent data (by dividing all individual measurements by their respective body width) according to Martin et al. (2017) and Meca et al. (2019). All data were normalised prior to the analyses. Data ordinations were performed by principal component analysis (PCA) and the significance of the obtained clusters was assessed by one-way analyses of similarity (ANOSIM) based on Euclidean distance resemblance matrices, both for the main body measurements and for the dorsal cirri length pattern. The morphometric characters most contributing to the average inter-population differences were estimated and shown as percentages according to Martin et al. (2017) and Meca et al. (2019). PCA and ANOSIM were executed with PRIMER, version 6.1.11, copyright by PRIMER-E Ltd. 2008 (Clarke and Warwick 2001; Clarke and Gorley 2006).
To describe the reproductive characteristics, we estimated the number of chaetigers in all still attached and already detached stolons (except in those from May and June 2018, kept apart for the chemical study, as indicated above). Sex was determined by the type of gametes present, and, when these were not detectable, we considered the number of stolon chaetigers (excluding the single stolon collected in February 2018) according to Martin et al. (2002). Some stolons were prepared for SEM observations. One 27-chaetiger stolon from September 2016 and the posterior portion of a ripe adult from November 2016 were previously fixed in glutaraldehyde (2.5%) for histological analyses, then dehydrated through a graded ethanol series (20%, 50%, 70%, 90%, 100%), embedded in resin (Technovit 8100), and attached to plastic stubs, cut in 5 μm in thick slices with a Histo-line MRS3500 microtome, stained with toluidine blue for 30 s, dried and mounted in Eukitt.
Results
Geographical, temporal, infestation and reproductive variability
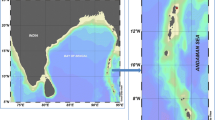
The two host chromotypes occurred separately in Costacuti Shoals (yellow) and Portofino, Santa Teresa di Gallura and Molunat (purple) (Fig. 1; Table 1) hosted slightly different symbiont chromotypes yellow, with purple dorsal marks on the yellow hosts and transparent, with a strong purple pigmentation in purple hosts (Fig. 2a, c). All polychaetes (i) showed the typical simple unidentate chaetae of the species, with those in anterior parapodia with slightly bidentate chaetae and those from posterior parapodia being more hooded and more clearly bidentate (Fig. 2b), (ii) were present in all studied months, except January 2018, and (iii) occurred in 65 out of the 192 (i.e. 34%) apical branches of P. clavata collected in Italy and Croatia (Table 1, Electronic Supplementary Material 1).
Haplosyllis chamaeleon. Data geo-referenced with QGISS 2.4.0 (https://www.qgis.org/it/site/)
Haplosyllis chamaeleon. a Living specimen on a purple gorgonian branch (scale bar = 1 cm); b posterior bidentate chaetae (scale bar = 10 μm); c living male stolon (scale bar, 1 mm); d histological section of a male stolon showing sperm cysts (scale bar = 100 μm); e histological section of a female stolon showing oocytes (scale bar = 100 μm)
The temporal trends of the infestation in the Portofino Promontory were characterised by average infections of 0.84 ± 0.27 ind/m in spring, 1.7 ± 0.37 ind/m in summer, 0.25 ± 0.03 ind/m autumn and 0.03 ± 0.03 ind/m in winter (Fig. 3). However, only summer infestations were significantly different from those in winter (Kruskal–Wallis, H = 9.08; p < 0.05). The highest number of specimens were also collected in June 2017, whilst the largest (on average and in terms of number of chaetigers, body length and width) were found in February 2018 (Table 3). In turn, the specimens with the longest pharynx (on average) occurred in august 2017 and those with the longest proventriculum in February 2018.
Mature adults showed intra-coelomic gametes and had attached growing stolons with 22–24 chaetigers, whilst free stolons showed more than 17 to 30 chaetigers, except one with 14 chaetigers from February 2018 (Tables 2 and 4). The spermatic cysts from a free stolon measured 211.5 ± 44.34 μm in diameter, whereas the oocytes from a developing stolon of a mature polychaete measured 50.75 ± 4.94 μm in diameter (Fig. 2d, e). Considering the number of the chaetigers of the stolons, nearly all ripe specimens were identified as males (Table 4).
Morphometry
The PCA based on morphometric data of the three populations of H. chamaeleon revealed a different trend when analysing the body measurements and the dorsal cirri length pattern (Fig. 4). Despite the population from Portofino were more numerous and thus showed a more disperse distribution in terms of body measurements, they were partially intermixed with those from Cape of Creus and Chafarinas (Fig. 4a), leading to the absence of significant differences amongst them (ANOSIM, Global R = 0.066; p = 0.175). Conversely, the dorsal cirri length patterns (in number of articles) were clearly different (Fig. 4b), thus showing overall significant differences (ANOSIM, Global R = −0.458; p = 0.001). In this case, however, the pairwise tests revealed that the only significant differences occurred between the Portofino population and those of Cape of Creus and Chafarinas Archipelago (Table 5). A detailed analysis of the morphometric measurements revealed that those from the Portofino Promontory population were always smaller than those from Cape of Creus and Chafarinas Archipelago; posterior dorsal cirri of Portofino specimens are shorter than those of the other populations, according to Table 6.
Graphical representation of the PCAs based on body measurements (a) and dorsal cirri length pattern as number of articles; (b). BL, body length; Phl, pharynx length; Prl, proventriculum length; NC, number of chaetigers; 1 to 10, segments of dorsal cirri. P, Portofino; CC, Cape of Creus; CH, Chafarinas Archipelago
Behaviour
Most polychaetes were paced directly on the surface of the host gorgonian, usually (but not always) amongst the polyps. However, juveniles polychaetes were occasionally observed to directly enter the coelenteron through the polyp pharynx (Fig. 5) and disappear completely inside, without inducing any host reactions or visible tissue injuries.
Discussion
The association of H. chamaeleon with P. clavata was chiefly recorded along the Mediterranean coast of Spain (Martin et al. 2002; Musco and Giangrande 2005), as well as in the Alboran Sea (Baratech and San Martín 1987; López et al. 1996; Martin et al. 2002). Two additional reports, from the coasts of Egypt (Abdelnaby 2014) and from the Arafura Sea (Australia) (Wilson 2006), have to be considered doubtful. Particularly, the latter, likely corresponds to Haplosyllis depressa Augener, 1913, currently accepted as Trypanobia depressa (Augener, 1913) (Aguado et al. 2015). Therefore, our findings represent the first record for Italian and Croatian waters and extend the geographic range of H. chamaeleon to the Tyrrhenian and Adriatic Seas (Castelli et al. 2008; Micac and Musco 2010; Micac 2015). Except for the finding of the polychaete in association with P. grayi in the Iberian Atlantic coasts, our results contribute to confirm the probable overlapping distribution of H. chamaeleon with those of the purple and yellow host chromotypes (Di Camillo et al. 2018b; Pica et al. 2018), which are widely but exclusively distributed in the Mediterranean Sea (Ponti et al. 2019). In Portofino, however, only part of the studied colonies of P. clavata seemed to host H. chamaeleon (although we cannot discard that, in a given individual, the worms could be present in unsampled branches) and the polychaetes were present all year round, with a particularly high infestation in summer. The infestation rates were overall very low, which agrees with previously published data on other known populations (Martin et al. 2002; Lattig and Martin 2009).
Morphometrically, the Portofino population were non-significantly distinguishable from those of Cape of Creus and the Chafarinas Archipelago in terms of body measurements, whilst they had significantly smaller dorsal cirri. Nevertheless, the explained percentage of difference (expressed by the ANOSIM Global R), is lower than 50%, so that we do not consider this as significant at the species level. More likely this seemed to be caused by the different population characteristics, with that from Portofino being collected during several periods of the year, whilst Iberian ones were collected during more restricted time-ranges (or even in a single season). Therefore, we suggest that the observed inter-populations differences must be considered as intra-specific, likely being caused by the presence of different phases of the life cycle in the three populations under study.
Characteristically, all studied populations showed the presence of ripe adults with attached stolons, with the Portofino population allowing us to confirm for the first time its presence in different periods of the year. Conversely, free stolons were more rarely found on the gorgonian hosts, probably because they leave the host towards the water column for reproductive purposes. Some syllids may have short reproductive periods (Giangrande 1989; Franke 1999), whilst Syllis prolifera (Krohn, 1852) reproduces monthly under an endogenous control and according to a semi-lunar cycle (Franke 1986, 1999), but shows a longer stolonisation period during the coldest months of the year. In H. chamaeleon, male and female gametes develop within the anterior part (parent stock), as it happens in Haplosyllis spongicola (Wissocq, 1966), and are subsequently relocated to the stolons, where they are stocked in different stage of maturation and for variable time before their release. The fact that the adults started to regenerate the posterior segments after releasing the stolon confirmed that the species had life-spans longer than a year, although the exact duration of their life had not yet been stablished (Martin et al. 2002).
Haplosyllis chamaeleon is chiefly found on the apical portions of the gorgonian branches, where the high density of polyps probably maximises feeding opportunities (Laubier 1960; Martin et al. 2002). As previously reported in Martin et al. (2002), the syllids were observed to directly enter the coelenteron through the polyp pharynx (Fig. 5), likely to steal food, without inducing modifications of the host morphology; moreover, juveniles of our population from Portofino, were also observed completely to disappear inside the polyp, suggesting that they may enter also the stem canals (Fig. 5). This appeared to be a typical kleptoparasitic behaviour (i.e. stealing food captured by the host polyp) (Martin et al. 2002). However, the coincidence in colour morphs between host and symbiont observed in their different populations, including those collected in this study in Italian and Croatian waters, does not allow to discard the possibility of the symbiont feeding on host tissues, a behaviour that has also been reported in this species and in other symbiotic syllids (Pawlik 1983; Magnino and Gaino 1998; Martin et al. 2002; Lattig and Martin 2011). Kleptoparasitism has also been reported for different cnidarian associates. Amongst them, the caprellids Pseudoprotella phasma (Montagu, 1804), living on the hydroid Eudendrium glomeratum Picard, 1952 (Bavestrello et al. 1996), and Paracaprella pusilla Mayer, 1890, living on Eudendrium racemosum (Cavolini, 1785) (Ros and Guerra-García 2012) In turn, the nudibranch Cratena peregrina (Gmelin, 1791), associated to E. racemosum, feeds preferentially on hydroids that have recently captured preys, thus combining kleptoparasitism with predation (Willis et al. 2017). In light of our data, we cannot discard such a combination for H. chamaeleon. It has also been suggested that the presence of the symbiont could generate some benefit for the host, particularly by cleaning its surface (Martin et al. 2002), a possibility that would move the association towards mutualism and that we cannot discard in light of our data. This possibility certainly merits further behavioural observations.
Even though H. chamaeleon probably lives its whole adult benthic stage on the gorgonian host, there was no evidence of branch damage or injury either when being sheltered on the host surface or whilst using the gorgonians to stole planktonic preys from inside their polyps. Also, they did not induce the hosts to generate any malformation (Martin et al. 2002, this work). Conversely, the congeneric H. anthogorgicola excavates galleries in the coenenchyme, inducing the formation of protuberances on the branches, whereas H. villogorgicola forms gall-like structures by bending several branches, which suggested they were in different steps of the evolution of their respective associations (Martin et al. 2002). Nevertheless, the behaviour of these two species was also considered as being kleptoparasitic (Martin et al. 2002). Kleptoparasitisms differ from parasitisms in that the symbionts did not cause direct damage to the host (Dales 1957; Margulis et al. 1982), being thus more closely related to commensalisms (Martin and Britayev 2018). The real status of the association between H. chamaeleon and its gorgonian hosts is still an open question that could possibly be answered by analysing the biochemical composition of both partners or by studying the composition of the faecal pellets produced by the symbionts, but these two interesting approaches were far from the objectives of the present paper, and certainly merit further studies.
References
Abdelnaby FA (2014) On some new recorded Syllidae (Polychaeta: Phyllodocida) for Mediterranean waters. Ann Res Rev Biol 4(24):4314–4335. https://doi.org/10.9734/ARRB/2014/11738
Aguado MT, San Martín G, Siddall ME (2012) Systematics and evolution of syllids (Annelida, Syllidae). Cladistics 28:234–250. https://doi.org/10.1111/j.1096-0031.2011.00377.x
Aguado MT, Murray A, Hutchings P (2015) Syllidae (Annelida: Phyllodocida) from Lizard Island, Great Barrier Reef, Australia. Zootaxa 4019(1). https://doi.org/10.11646/zootaxa.4019.1.5
Arizmendi-Mejía R, Linares C, Garrabou J, Antunes A, Ballesteros E, Cebrian E, Diaz D, Ledoux JB (2015) Combining genetic and demographic data for the conservation of a Mediterranean marine habitat-forming species. PLoS One 10(3):e0119585. https://doi.org/10.1371/journal.pone.0119585
Augener H (1913) Polychaeta I. Errantia. In: Michaelsen W and Hartmeyer R (Eds) Die Fauna Südwest-Australiens. Ergebnisse der Hamburger südwest-australischen Forschungsreise 1905, Gustav Fischer, Jena, 4(5):65–304
Aurivillius M (1931) The Gorgonarians from Dr. Sixten Bock’s Expedition to Japan and Bonin Islands 1914. Kungliga Svenska Vetenskaps Akademiens Handlingar, Tredje Serien, Band 9. Almqvist and Wiksell: Stockholm
Balss H (1921) Über eine neue Pontoniide aus dem Golf von Neapel. Pubbl Staz Zool Napoli 22:523–526
Baratech L, San Martín G (1987) Contribución al conocimiento de lo Anélidos Poliquetos (Annelida: Polychaeta) de las costas andaluzas. Bol Inst Esp Oceanogr 4(2):37–48
Barnich R, Beuck L, Freiwald A (2013) Scale worms (Polychaeta: Aphroditiformia) associated with cold-water corals in the eastern Gulf of Mexico. J Mar Biol Assoc UK 93:2129–2143. https://doi.org/10.1017/S002531541300088X
Bavestrello G, Cattaneo-Vietti R, Cerrano C, Sarà M (1996) Relations between Eudendrium glomeratum (Cnidaria, Hydromedusae) and its associated vagile fauna. Sci Mar 60:157–163
Bo M, Di Camillo CG, Puce S, Canese S, Giusti M, Angiolillo M, Bavestrello G (2011) A tubulariid hydroid associated with anthozoan corals in the Mediterranean Sea. Ital J Zool 78(4):487–496. https://doi.org/10.1080/11250003.2011.568015
Britayev T, Gil J, Altuna A, Calvo M, Martin D (2014) New symbiotic associations involving polynoids (Polychaeta, Polynoidae) from Atlantic waters, with redescriptions of Parahololepidella greeffi (Augener, 1918) and Gorgoniapolynoe caeciliae (Fauvel, 1913). Mem Mus Vic 71:27–43
Calcinai B, Bavestrello G, Bertolino M, Pica D, Wagner D, Cerrano C (2013) Sponges associated with octocorals in the Indo-Pacific, with the description of four new species. Zootaxa 3617(1):001–061. https://doi.org/10.11646/zootaxa.3617.1.1
Carvalho S, Cúrdia J, Pereira F, Guerra-García JM, Santos MN, Cunha MR (2014) Biodiversity patterns of epifaunal assemblages associated with the gorgonians Eunicella gazella and Leptogorgia lusitanica in response to host, space and time. J Sea Res 85:37–47. https://doi.org/10.1016/j.seares.2013.10.001
Castelli A, Bianchi CN, Cantone G, Çinar ME, Gambi MC, Giangrande A, Iraci Sareri D, Lanera P, Licciano M, Musco L, Sanfilippo R (2008) Annelida Polychaeta. In Relini G (Ed) Checklist della flora e della fauna dei mari italiani (Parte I). Biol Mar Med 15 (Supplement 1):327-377
Cavolini, F (1785) Memorie per servire alla storia de’ polipi marini di Filippo Cavolini. pp 160
Cerrano C, Bavestrello G, Bianchi CN, Cattaneo-Vietti R, Bava S, Morganti C, Morri C, Picco P, Sara G, Schiaparelli S, Siccardi A, Sponga F (2000) A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (NW Mediterranean), summer 1999. Ecol Lett 3:284–293
Cerrano C, Arillo A, Azzini F, Calcinai B, Castellano L, Muti C, Valisano L, Zeg G, Bavestrello G (2005) Gorgonian population recovery after a mass mortality event. Aquat Conserv 15:147–157
Cerrano C, Danovaro R, Gambi MC, Pusceddu A, Riva A, Schiaparelli S (2010) Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers Conserv 19:153–167. https://doi.org/10.1007/s10531-009-9712-5
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial (Plymouth routines in multivariate ecological research). Primer-E Ltd., Plymouth
Clarke KR, Warwick RM (2001) A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Mar Ecol Prog Ser 216:265–278
Coma R, Zabala M, Gili JM (1995) Sexual reproduction effort in the Mediterranean gorgonian Paramuricea clavata. Mar Ecol Prog Ser 117:185–192
Cúrdia J, Carvalho S, Pereira F, Guerra-García JM, Santos MN, Cunha MR (2015) Diversity and abundance of invertebrate epifaunal assemblages associated with gorgonians are driven by colony attributes. Coral Reefs 34:611–624. https://doi.org/10.1007/s00338-015-1283-1
Dales RP (1957) Interrelations of organisms. A Commensalisms In treatise on marine ecology and paleoecology Hedgpet JW (ed) Memories of the Geological Society of America 67:391–412
Di Camillo CG, Ponti M, Bavetrello G, Krzelj M, Cerrano C (2018a) Building a baseline for habitat-forming corals by a multi-source approach, including web ecological knowledge. Biodivers Conserv 27:1257–1276. https://doi.org/10.1007/s10531-017-1492-8
Di Camillo CG, Gravili C, De Vito D, Pica D, Piraino S, Puce S, Cerrano C (2018b) The importance of applying standardised integrative taxonomy when describing marine benthic organisms and collecting ecological data. Inv Syst 32(4):794–802. https://doi.org/10.1071/IS17067
Dias IM, Cúrdia J, Cunha MR, Santos MN, Carvalho S (2015) Temporal variability in epifaunal assemblages associated with temperate gorgonian gardens. Mar Environ Res 112:140–151
Dohrn A (1881) Die Pantopoden des Golfes von Neapel und der angrenzenden Meeresabschnitte. Mon Fauna Flora Golfes Neapel 3:1–252
Franke HD (1986) The role of light and endogenous factors in the timing of the reproductive cycle of Typosyllis prolifera and some other polychaetes. Am Zool 26:433–445
Franke HD (1999) Reproduction of Syllidae (Annelida: Polychaeta). Hydrobiologia 402:39–55
Garrabou J, Coma R, Bensoussan N, Bally M, Chevaldonné P, Cigliano M, Diaz D, Harmelin JG, Gambi MC, Kersting DK, Ledoux JB, Lejeusne C, Linares C, Marschal C, Pérez T, Ribes M, Romano JC, Serrano E, Teixido V, Torrents O, Zabala M, Zuberer F, Cerrano C (2009) Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob Chang Biol 15:1090–1103. https://doi.org/10.1111/j.1365-2486.2008.01823.x
Glasby CJ, Watson C (2001) A new genus and species of Syllidae (Annelida: Polychaeta) commensal with octocorals. Beagle Records of the Northern Territory Museum of Arts and Sciences 17:43–51
Gmelin JF (1791). Vermes. In Gmelin JF (Ed) Caroli a Linnaei Systema Naturae per Regna Tria Naturae, Ed. 13. Tome 1(6) G.E. Beer Lipsiae pp 3021-3910
Goh NKC, Ng PKL, Chou LM (1999) Notes on the shallow water gorgonian-associated fauna on coral reefs in Singapore. Bull Mar Sci 65(1):259–282
Hammer ØD, Harper AT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Pal Electr 4:1–9
Huete-Stauffer C, Vielmini I, Palma M, Navone A, Panzalis P, Vezzulli L, Misic C, Cerrano C (2011) Paramuricea clavata (Anthozoa, Octocorallia) loss in the Marine Protected Area of Tavolara (Sardinia, Italy) due to mass mortality event. Mar Ecol 32(1):107–116. https://doi.org/10.1111/j.1439-0485.2011.00429.x
Johnson JY (1861) Description of a second species of Acanthogorgia (JE Gray) from Madeira. Proc Zool Soc London 1861:296–298
Koch A (1887) Echiniden der obertertiären Ablagerungen Siebenbürgens. Értes Kolozvári Orvos-Termés Tars 12:225–271
Kowalevsky A (1880) Bau und die Lebeuserscheinungen von Neomenia gorgonophilus n. sp. Zool Anz 3:190–191
Krohn A (1852) Über die Erscheinungen bei der Fortpflanzung von Syllis prolifera und Autolytus prolifer. Arch Naturgesch 18(1):66–76
Lattig P, Martin D (2009) A taxonomic revision of the genus Haplosyllis Langherans, 1887. Zootaxa 2220:1–40
Lattig P, Martin D (2011) Two new endosymbiotic species of Haplosyllis (Polychaeta: Syllidae) from the Indian Ocean and Red Sea, with new data on H. djiboutiensis from the Persian Gulf. Italian Journal of Zoology 78:112–123. https://doi.org/10.1080/11250003.2011.569373
Laubier L (1960) Une nouvelle sous-espèce de Syllidien: Haplosyllis depressa Augener ssp. nov. chamaeleon, ectoparassite sur l’octocorallaire Muricea chamaeleon von Koch. Vie et milieu 11:75–87
Linares C, Coma R, Mariani S, Díaz D, Hereu B, Zabala M (2008a) Early life history of the Mediterranean gorgonian Paramuricea clavata: implications for population dynamics. Invertebr Biol 127(1):1–11. https://doi.org/10.1111/j.1744-7410.2007.00109.x
Linares C, Coma R, Garrabou J, Díaz D, Zabala M (2008b) Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis. J Appl Ecol 45:688–699
López E, San Martin G, Jiménez M (1996) Syllinae (Syllidae, Annelida, Polychaeta) from Chafarinas Islands (Alboran Sea, W. Mediterranean). Misc Zool 19:105–118
Magnino G, Gaino E (1998) Haplosyllis spongicola (Grübe) (Polychaeta, Syllidae) associated with two species of sponges from East Africa (Tanzania, Indian Ocean). PSZN I: Marine Ecology 19:77–87
Margulis L, Esch GW, Holmes JC, Schad GA KAM (1982) The use of ecological terms in parasitology (report of a committee of the American Society of Parasitologists). J Parasitol 68:131–133
Martin D, Britayev TA (1998) Symbiotic polychaetes: review of known species. Oceanogr Mar Biol 36:217–340
Martin D, Britayev TA (2018) Symbiotic polychaetes revisited: an update of the known species and relationships (1998–2017). Oceanogr Mar Biol 56:371–448
Martin D, Núñez J, Reira R, Gil J (2002) On the associations between Haplosyllis (Polychaeta, Syllidae) and gorgonians (Cnidaria, Octocorallia), with the description of a new species. Biol J Linn Soc Lond 77:455–477
Martin D, Meca MA, Gil J, Drake P, Nygren A (2017) Another brick in the wall: population dynamics of a symbiotic Oxydromus (Annelida, Hesionidae), described as a new species based on morphometry. Contrib Zool 86:181–211. https://doi.org/10.1163/18759866-08603001
Mayer P (1890) Die Caprelliden des Golfes von Neapel und der angrenzenden meeres-abschnitte. Nachtrag zur monographie derselben Fauna und Flora des Golfes von Neapel und der angrenzenden Meeres-Abschnitte 17:1–157
Meca MA, Drake P, Martin D (2019) Does polyxenous symbiosis promote sympatric divergence? A morphometric and phylogeographic approach based on Oxydromus okupa (Annelida, Polychaeta, Hesionidae). Contrib Zool 88:173–200. https://doi.org/10.1163/18759866-20191403
Micac B (2015) A sea of worms: polychaete checklist of the Adriatic Sea. Zootaxa 3943(1). https://doi.org/10.11646/zootaxa.3943.1.1
Micac B, Musco L (2010) Faunal and biogeography analysis of Syllidae (Polychaeta) from Rovinj (Croatia, northern Adriatic Sea). Sci Mar 74(2):353–370
Mifsud C, Mastrototaro F, Taviani M (2008) On the occurrence of Anamenia gorgonophila (Kowalevsky, 1880) (Solenogastres, Strophomaniidae) and its host Paramuricea macrospina (Koch, 1882) in the Maltese waters (Mediterranean Sea). Boll Malacol 44:5–8
Molodtsova TN, Britayev TA, Martin D (2016) Cnidarians and their polychaete symbionts. The Cnidaria, past, present and future, pp:387–413. https://doi.org/10.1007/978-3-319-31305-4_25
Montagu G (1804) Description of several marine animals found on the south coast of Devonshire. Trans Linn Soc London 7:61–85
Mori M, Morri C, Bianchi CN (1995) Notes on the life history of the pontonine shrimp Balssia gasti (Balss, 1921). Oebalia XX:129–137
Musco L, Giangrande A (2005) Mediterranean Syllidae (Annelida: Polychaeta) revisited: biogeography, diversity and species fidelity to environmental features. Mar Ecol Prog Ser 304:143–153
Nygren A, Pleijel F (2010) Redescription of Imajimaea draculai a rare syllid polychaete associated with the sea pen Funiculina quadrangularis. J Mar Biol Assoc UK 90:1441–1448. https://doi.org/10.1017/S0025315409991536
Pallas PS (1766) Elenchus zoophytorum sistens generum adumbrationes generaliores et specierum cognitarum succinctas descriptiones, cum selectis auctorum synonymis. Hagae-Comitum XVI:17–28
Patton WK (1972) Studies on the animal symbionts of the gorgonian coral, Leptogorgia virgulata (Lamarck). Bull Mar Sci 22:419–431
Pawlik JR (1983) A sponge-eating worm from Bermuda: Branchiosyllis oculata (Polychaeta, Syllidae). PSZN I: Mar Ecol 4:65–79
Pica D, Bastari A, Vaga CF, Di Camillo CG, Montano S, Puce S (2017) Hydroid diversity of Eilat Bay with the description of a new Zanclea species. Mar Biol Res 13(5):469–479. https://doi.org/10.1080/17451000.2016.1236202
Pica D, Calcinai B, Poliseno A, Trainito E, Cerrano C (2018) Distribution and phenotypic variability of the Mediterranean gorgonian Paramuricea macrospina (Cnidaria: Octocorallia). Eur Zool J 85(1):392–408
Picard J (1952) Les Hydrozoaires des herbiers de Zostéracées des côtes françaises de la Méditerranée. Vie Milieu suppl 2:217–233
Ponti M, Perlini RA, Ventra V, Grech D, Abbiati M, Cerrano C (2014) Ecological shifts in Mediterranean coralligenous assemblages related to gorgonian forest loss. PLoS One 9(7):e102782
Ponti M, Grech D, Mori M, Perlini RA, Ventra V, Panzalis PA, Cerrano C (2016) The role of gorgonians on the diversity of vagile benthic fauna in Mediterranean rocky habitats. Mar Biol 163:120. https://doi.org/10.1007/s00227-016-2897-8
Ponti M, Turicchia E, Ferro F, Cerrano C, Abbiati M (2018) The understorey of gorgonian forests in mesophotic temperate reefs. Aquat Conserv Mar Freshw Ecosyst 28:1153–1166. https://doi.org/10.1002/aqc.2928
Ponti M, Turicchia E, Costantini F, Gori A, Bramanti L, Di Camillo CG, Linares C, Rossi S, Abbiati M, Garrabou J, Cerrano C (2019) Mediterranean gorgonian forests: distribution patterns and ecological roles. 3ème Symposium Méditerranéen sur la conservation du Coralligène at autres Bio-Concrétions (Antalya, Turquie, 15-16 janvier 2019):7–14
Puce S, Di Camillo CG, Bavestrello G (2008) Hydroids symbiotic with octocorals from the Sulawesi Sea, Indonesia. J Mar Biol Assoc UK 88(8):1643–1654. https://doi.org/10.1017/S0025315408001094
Risso A (1818) Mémoire sur quelques Gastropodes nouveaux, Nudibranches et Tectibranches observés dans la mer de Nice. J Phys Chim Hist Nat Arts 87:368–377
Risso A (1826) Histoire naturelle des principales productions de l’Europe Méridionale et particulièrement de celles des environs de Nice et des Alpes Maritimes. Paris: Levrault 5:400
Ros M, Guerra-García JM (2012) On the occurrence of the tropical caprellid Paracaprella pusilla Mayer, 1890 (Crustacea: Amphipoda) in Europe. Mediterr Mar Sci 13:134–139
San Martín G, López E (2002) New species of Autolytus Grube, 1850, Paraprocerastea San Martín & Alós, 1989, and Sphaerosyllis Claparède, 1863 (Syllidae, Polychaeta) from the Iberian Peninsula. Sarsia 87(2):135–143. https://doi.org/10.1080/003648202320205210
Seveso D, Montano S, Pica D, Maggioni D, Galli P, Allevi V, Bastari A, Puce S (2016) Pteroclava krempfi-octocoral symbiosis: new information from the Indian Ocean and the Red Sea. Mar Biodivers 46:483–487. https://doi.org/10.1007/s12526-015-0368-y
Sokal RR, Rohlf FJ (1981) Biometry: the principle and practice of statistics in biological research. Freeman WH and Company, New York
Utinomi H (1956) On the so-called "Umi-utiwa", a peculiar flabellate gorgonacean, with notes on a syllidean polychaete commensal. Publ Seto Mar Biol Lab 5(2):243–250
Valisano L, Notari F, Mori M, Cerrano C (2016) Temporal variability of sedimentation rates and mobile fauna inside and outside a gorgonian garden. Mar Ecol ISSN:0173–9565. https://doi.org/10.1111/maec.12328
Willis TJ, Berglöf KT, McGill RAR, Musco L, Piraino S, Rumsey CM, Fernández TV, Badalamenti F (2017) Kleptopredation: a mechanism to facilitate planktivory in a benthic mollusc. Biol Lett 13(11):20170447. https://doi.org/10.1098/rsbl.2017.0447
Wilson GDF (2006) Taxonomic Results. Arafura Sea Biological Survey, Report on Benthic Fauna collected during R/V Southern Surveyor Voyage, 05-2005
Wissocq JC (1966) La sexualisation du stolon chez Syllis spongicola Grube. Cah Biol Mar 7:337–342
Acknowledgements
We are grateful to the Portofino marine Protected Area authorities for support and authorization and to Fabrizio Torsani and Ubaldo Pantaleo for their field assistance.
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement. This research has been performed within the frame of the European Union’s Horizon 2020 research and innovation programme, as part of the project MERCES: Marine Ecosystem Restoration in Changing European Seas (grant agreement No. 689518) and is a contribution of Daniel Martin to the Consolidated Research Group on Marine Benthic Ecology of the Generalitat de Catalunya (2017SGR378) and to the Research Project PopCOmics (CTM2017-88080), funded by the ‘Ministerio de Ciencia, Innovación y Universidades’ of Spain (MICINU), the ‘Agencia Española de Investigación’ (AEI) and the European Funds for Regional Development (FEDER).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements. The study is compliant with CBD and Nagoya protocols..
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Author contribution
CC and DM conceived and designed research. LP, BC, CGDC conducted experiments. CC, DP and DM performed the underwater sampling. LP, BC, CGCD and DM analyzed data. LP, BC and CGDC wrote the original draft. All authors read and approved the manuscript. CC covered the costs of field activities and lab analyses.
Additional information
Communicated by P. Lana
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 222 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pola, L., Calcinai, B., Pica, D. et al. Updating the current knowledge on the relationships between Haplosyllis chamaeleon Laubier, 1960 (Annelida, Syllidae) and Paramuricea clavata (Risso, 1826) (Cnidaria, Plexauridae) in the Mediterranean Sea. Mar. Biodivers. 50, 105 (2020). https://doi.org/10.1007/s12526-020-01127-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-020-01127-y