Abstract
Fluctuating asymmetry are random deviations of an otherwise symmetrical body plan and arises from instability in development. Earlier studies suggest that levels of cranial fluctuating asymmetry may be influenced by lifestyle and quality of life in a population. It may, therefore, be useful as a stress indicator. We investigated whether cranial fluctuating asymmetry has changed in archaeological Danish populations over time, and between grave sites from the same time period. Our sample consisted of 219 adult individuals from the Neolithic Age (approx. 3000BC) to the Early Modern Age (approx. 1850). We collected 27 3-dimensional landmarks from the face, calvarium, and base of the cranium. Levels of shape variation were analyzed using Procrustes analysis of variance and principal component analysis. Cemeteries, time periods, and sex were compared using linear mixed models, one-way analysis of variance, and Kruskal-Wallis test. We found no statistically significant differences in cranial FA between grave sites from the same time period, nor did we find any statistically significant difference between time periods. We found that sex did not have an influence on levels of cranial FA. We found no measurable difference in levels of cranial FA between Danish populations over time. Further knowledge on genetics and other stress indicators in our sample may give more insight into the relationship between cranial fluctuating asymmetry and developmental instability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Investigating health and lifestyle of archaeological populations can help to understand more about life in the past. General health can be estimated using, e.g., mortality rates, demographic structures, and signs of pathological conditions, while physiological stress may be more difficult to illuminate. In biological anthropology, physiological stress has been measured using different indicators such as enamel hypoplasia (Geber, 2014; Goodman & Rose, 1990; Suckling, 1989), Harris lines (Geber, 2014; Harris, 1931), and body height (Geber, 2014; Victora, 1992). Fluctuating asymmetry (FA) is another less broadly used approach to measuring physiological stress. Fluctuating asymmetry is defined as random deviations from a symmetrical body plan. It is a process originating in early development and influences a given individual’s phenotype (Van Valen, 1962). The genotype for the body plan of most organisms is symmetry. However, environmental influences can disrupt this symmetry and introduce small changes in the body plan, resulting in asymmetry (Palmer & Strobeck, 1986; Van Valen, 1962). Depending on the level of environmental instability, these asymmetries may be more or less pronounced (Beasley et al., 2013; Møller & Thornhill, 1997; van Dongen, 2006; van Dongen & Gangestad, 2011). Models regarding the underlying causation behind FA suggest that changes in FA may not be exclusively caused by changes in environment, but also the genetic resilience of the individual (Farrera, 2022). These models are consistent with the principle behind the Developmental Origin of Health and Disease hypothesis (DoHaD) stating that early life environment and resilience to the lived environment has an influence on adult health outcomes (Gluckman et al., 2010; Hanson & Gluckman, 2008). Therefore, the prevailing hypothesis concerning FA is that since it is a consequence of developmental instability, the level of FA found in a sample can work as a proxy for environmental and genetic stress in populations. Indeed, many biological studies have been conducted on the subject (for a brief overview see: Beasley et al., 2013).
FA and developmental instability in human skeletal material have previously been investigated in crania (Bigoni et al., 2013; DeLeon, 2007; DeLeon & Richtsmeier, 2009; Gawlikowska et al., 2007; Gawlikowska-Sroka et al., 2017; Moes et al., 2022; Weisensee, 2013; Weisensee & Spradley, 2018) and in the post-cranial skeleton (e.g., Kujanová et al., 2008; Livshits et al., 1998; Mopin et al., 2018). For cranial FA specifically, studies have compared FA to developmental stress in two ways: firstly, by comparing FA to other stress indicators, or secondly, by comparing distinct populations stratified by time or socioeconomic status. Comparing levels of FA to enamel hypoplasia in archaeological material have shown contradicting results (Gawlikowska-Sroka et al., 2013, 2017; Moes et al., 2022). Weisensee (2013) has found that individuals from nineteenth and twentieth century Portugal that died from degenerative diseases (e.g., heart disease, diabetes, and vascular lesions) had higher levels of FA than individuals who died from infectious diseases, suggesting that FA may be connected to decreased resilience. Cranial FA has been compared between populations from different time periods, spanning from the Medieval Age to the twentieth century: DeLeon (2007) compared early and late medieval populations and Gawlikowska et al. (2007) compared medieval and modern populations—both studies found statistically significant decreases in levels of FA in connection with expected increase in living standards. A study by Kimmerle and Jantz (2005) found constant levels of FA over time in American populations from the nineteenth and twentieth century. Studies between FA and different socioeconomic groups show differing results: Bigoni et al. (2013) found constant levels of cranial FA for men, while levels of FA for women were higher in the group with expected higher living standards in a medieval population. Weisensee (2018) found that levels of cranial FA decreased with the increase in socioeconomic status between U.S. citizens and unauthorized border crossers at the Mexican border—the effect was only statistically significant for males and the sexes combined.
Thus, previous research indicates that there may be a link between levels of cranial FA in humans and changes in lifestyle and physiological stress. The aim of this study is to investigate if and how cranial FA has changed through time in adult Danish populations from the Neolithic (approximately 3000BC) until the Early Modern Age (approximately 1850). Differences in developmental instability between populations will be tested by comparing grave sites within the same time period, and by comparing different time periods. Cranial FA is measured using 3D landmarks and Geometric Morphometric methods to make our results comparable to most other recent research in cranial FA. The results are discussed in relation to lifestyle and environmental stress.
Materials and methods
Sample
The sample contained 219 adult individuals from 12 sites in Denmark dated from the Stone Age (Neolithic period, approximately 3300–2800BC) to the Early Modern Period (1650–1850) (see Table 1).
All crania were selected from the Anthropological Collection at the Laboratory of Biological Anthropology, Department of Forensic Medicine, University of Copenhagen (DK). Grave sites were chosen primarily according to the number of intact crania. Additionally, grave sites were chosen to cover as many time periods as possible. These criteria limited the sample size significantly, in particular for the early time periods. Crania with visible warping, and taphonomic damage and pathology in areas containing landmarks (see Fig. 1) were excluded.
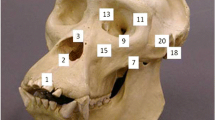
Landmark placement. Superior dataset (red dots): (1) Nasion, (2) Maxillonasofrontale, (3) Frontomalare orbitale, (4) Infraorbital foramen, (5) Nasal aperture, (6) Zygomaxillare inferior, (7) Jugale. Basal dataset (blue dots), (8) Asterion, (9) Lambda, (10) Opisthion, (11) Basion, (12) Foraminolaterale, (13) Caroticum mediale, (14) Spinale mediale, (15) Ovale mediale, and (16) Hormion
The sample consisted of 63 females, 152 males, and four individuals of undetermined sex (see Table 2). Age and sex of the skeletons were estimated by using standard methods on the cranium, pelvis, and ribs (J. L. Buckberry & Chamberlain, 2002; Buikstra, 1994; Dwight, 1905; Katz & Suchey, 1986; Meindl & Lovejoy, 1985; Phenice, 1969). Only adult individuals were included in the sample – cutoff was set as a minimum age of 18 and above.
Archaeological context of the grave sites
Stone Age (Neolithic)
The Neolithic Period in Denmark spanned around 3900BC–2800BC. This period is characterized by the shift from hunter-gatherer society to early agriculture and husbandry (Jensen, 2001).
Borreby and Rævehøj
Borreby and Rævehøj are both passage grave sites from the Neolithic Period and date to the late Tragtbæger culture from 3300 to 2800 BC. (Jensen, 2001).
Borreby passage grave is located near the village of Magleby, Slagelse on southwestern Zealand.
Rævehøj passage grave is located in a mount near the village of Dalby, near the Great Belt on Western Sealand (Jensen, 2001, p. 378).
Iron Age (Early Roman Age)
The Early Roman Age is a subcategory of the Iron Age in Denmark and spanned around 0-200AD. The period was characterized by agriculture and the founding of small villages and homesteads. (Jensen, 2003).
Simonsborg
Simonsborg is dated to around 0–200AD. It is situated in the central part of Zealand, near the town Sorø (Sellevold et al., 1984).
Viking Age
The Danish Viking Age spanned approximately 800–1050. In the Viking Age, most Danes lived as farmers. For a majority of the Danish Viking Age, society was generally divided into smaller family and village units.
Galgedil
The Galgedil grave site is located near Otterup on the northern part of Funen. It is dated to have been active between around 800 and 1060. It is a confirmed pagan grave site. Archaeological finds indicate that Galgedil was primarily a farmer community, which was socially stratified with masters and slaves. Grave goods indicate some contact with the larger world, and DNA and Strontium analyses show the presence of non-local individuals (Price et al., 2014).
Kaagården
Kaargården grave site is located on the southern part of the island of Langeland. Archaeological finds from the graves date the grave site to around 750–1066. (Grøn et al., 1994).
Medieval Age
The Danish Medieval Age dates from 1050 to 1536. The Medieval population lived generally in either rural farming communities or towns, where, e.g., tradesmen, craftsmen, and clergy lived and worked (Asmussen, 1997). Most villages had a local church and cemetery where inhabitants were buried, until the Reformation in 1536 and the years after, when several village cemeteries were discontinued.
Algade, Skælskør
The cemetery in Algade in Skælskør is located in the western part of Zealand, by the coast of the Great Belt. The cemetery was connected to a Carmelite monastery that was active from around 1400 until 1560 (Koch & Lynnerup, 2003).
Ahlgade, Holbæk
The cemetery beneath Ahlgade 15-17 is located in Holbæk in Northwestern Zealand at the bottom of the Issefjord. In Medieval times it was connected to the church St. Nicolai. It served a smaller town and was active from around 1200 until 1573 (Asmussen, 1997).
Tjærby
Tjærby cemetery is located close to Randers in eastern Jutland. It was active from around 1100 until the Reformation in 1536. The church served a rural community primarily comprised of farmers (Hyldgård, 2016).
Vor Frue, Aalborg
Vor Frue cemetery in central Aalborg was connected to a church and monastery complex that was active during most of the Medieval Age. Skeletons excavated on the site were dated to be from around 1100 to 1500. While it was expected that the cemetery had been reserved for the nuns in the monastery, excavations unveiled that the cemetery was used by the general population in the town (Springborg & Møller, 2016).
Early Modern Period
The samples from the Early Modern Period include individuals from Copenhagen from around 1650-1850. In this period, Copenhagen was a rapidly growing city with active commerce and military complexes. Life in the city was socially stratified with lower socioeconomic classes working in hard manual labor and higher-class citizens working as merchants, academics, or finer tradesmen such as goldsmiths. The military and naval complexes in the city also meant that Copenhagen had quite a large population of soldiers (Bech et al., 1980).
Østerbrogade
The Østerbrogade excavation covered an area connected to the general hospital “Almindeligt Hospital” and was active from 1770 to 1885. The burial site was not part of church grounds and was thus used to inter individuals that did not have the means to pay for a burial in a proper cemetery (Wendt, 1833).
Bremerholmen
Bremerholmen is located next to Holmens Church in Copenhagen. Bremerholmen was a large naval complex for ship building. It also contained a forced labor camp (1570–1741). The cemetery at Bremerholmen is thought to initially have been for men working at the naval complex and the convicts from the labor camp, but it also became a cemetery for inhabitants in Holmens Parish, and was used to inter plague victims during the epidemic in 1711–12 (Bobé, 1920). The cemetery has never been archaeologically excavated, but human remains have been recovered on several occasions during construction works in the twentieth century.
Holmens Church
Holmens Church in the center of Copenhagen was built on the naval docs in 1648–49 and had active burials until around 1800. Holmens church and cemetery was built for use by the Navy, and burials in the area are expected to be of middle-class individuals and individuals of higher middle-class status living in the Parish (Kjærgård, 2014).
Methods
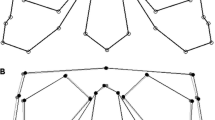
We collected 27 3-dimensional (3D) landmarks—11 bilateral and 5 midline, adapted from Olsen et al. (2022). Landmarks covered both face, calvarium, and base of the cranium (see Fig 1).
The 3D landmarks were collected as two separate datasets: one superior dataset covering the face and one basal dataset covering the base and calvarium (see Fig. 1). The two datasets were kept apart for statistical analysis. All landmarks were collected twice, as advised by Graham (2021) when studying small biological signals like FA.
3D landmarks were collected using a Revware Microscribe i 3D digitizer and Microscribe Utility Software (Revware, 2020). The 3D digitizer was clamped to a table to keep it stable and was calibrated daily. Crania were stabilized by placing them on a small bag filled with sand.
Missing landmarks
To accommodate the need for complete datasets when working with multivariate methods, any missing landmarks had to be estimated. Missing landmarks were estimated in RStudio using the geomorph package (Adams et al., 2022; Baken et al., 2021). All missing data were estimated using Thin Plate Spline interpolation as advised by Neeser (2009), which estimates missing landmarks by minimizing the bending energy between a complete reference cranium and the incomplete target crania (Mitteroecker & Gunz, 2009). All missing data were estimated by using a complete reference cranium from the same population as the incomplete target crania. The datasets containing estimated landmarks were compared to the complete datasets using unpaired Wilcoxon two-sample test to ensure there were no statistical differences between the two groups. Only Medieval and Early Modern samples were tested, due to sample size.
Statistical analyses
General procrustes analysis (GPA), principal component analysis (PCA), and individual FA values were calculated in MorphoJ (Klingenberg, 2011) and results were then exported to RStudio (RStudio Team, 2021) for further statistical analysis and visualization.
When analyzing fluctuating asymmetry and shape in general, certain preparations of the data are necessary. Namely that to analyze shape in isolation, one must remove any information on size, rotation, and orientation from the data. This is done by using GPA: all datasets are scaled to a centroid size of 1.0, moved to the origin of a common coordinate system, and rotated to minimize the sums of squared distances between all corresponding landmarks (Dryden & Mardia, 1998; Rohlf & Slice, 1990; Slice, 2006; Small, 2012). After the GPA, all information contained in the individual data sets is information on shape, which can then be analyzed.
Levels of shape variation were analyzed using Procrustes ANOVA, which is a nested ANOVA design adapted to analyze shape data. The Procrustes ANOVA compares levels of variation between the individuals (random effect), directional asymmetry (fixed effect), fluctuating asymmetry (interaction between random and fixed effects), and measuring error (residuals) (Klingenberg et al., 2002; Klingenberg & McIntyre, 1998). To illustrate the influence of the individual levels of variation, we calculated the percentage of variation for all levels by dividing a given levels sum of squares with the total sum of squares of all levels (Fruciano et al., 2017).
The asymmetric component of shape variation was analyzed using PCA to visualize the shape variation in the sample, as well as checking for outliers. The shape changes of the asymmetric component were visualized for the first two Principal Components. Individual levels of FA were calculated automatically in MorphoJ as the total individual asymmetry minus the mean asymmetry (directional asymmetry). Individual FA levels were expressed in Mahalanobis distance. Levels of FA between grave sites were compared using ANOVA or Kruskal-Wallis test, depending on whether the data sets met the assumptions of equal variance and normality. Due to small sample sizes in the Neolithic and Viking age, we did not test differences in levels of FA between grave sites in these time periods. Differences in levels of FA between time periods were tested using linear mixed models with FA as the dependent variable and sex and period as independent variables.
Results
Missing landmarks
In total, we collected 94% of all landmarks. We estimated 4.7% (n = 554) of the landmarks from the superior data set, and 6.7% (n = 792) from the basal dataset. We compared FA values in the datasets with and without estimated landmarks and found no statistically significant differences: Medieval Age superior: p = 0.0715, Medieval Age basal: = 0.1039, Early Modern Age superior: p = 0.1522, and Early Modern Age basal: p = 0.118.
Procrustes ANOVA
The Procrustes ANOVAs of the superior and basal data sets showed that all levels of shape variation (between individuals, directional asymmetry, fluctuating asymmetry, and measuring error) were statistically significant (Table 3). FA accounted for 14–16% of all shape variation. Measurement error in both data sets accounted for around 2% of shape variation.
PCA—asymmetrical component
Principal component analysis of the asymmetrical component of shape showed two different patterns between the superior and basal data sets.
The superior data set showed clustering around the mean shape for all time periods, and the Early Modern Period encompassed almost all other groups (Fig. 2). For the Medieval sample, PC1 represented a movement of the superior part of the nasal aperture to the left and a movement to the right for the maxilla and zygomatic (Fig. 3). For the Early Modern sample, PC1 represented a movement to the right of the superior part of the nasal aperture, and a movement to the left for the maxilla and zygomatic. For the Medieval Age sample, PC2 represented a movement of both maxilla and zygomatic bones to the right. PC2 in the Early Modern sample represented movement of the superior part of the nasal aperture to the right, a shortening of the left maxilla, and a broadening of the right maxilla (Fig. 3).
The basal data set showed more variation between groups. The Iron Age, Viking Age, and Early Modern Period groups still clustered around the mean shape, while the Stone Age and Medieval Age groups had individuals that showed higher levels of asymmetry (Fig. 4). In the medieval sample, both PC1 and PC2 represent some movement of all basal structures. In the Early Modern Age sample, PC1 and PC2 represent movement of posterior (lambda, asterion) and anterior structures (foramen caroticum, foramen spinale, and foramen ovale), while the foramen magnum shape stayed constant (Fig. 5).
Fluctuating asymmetry over time
Looking at FA over time, our data showed generally steady levels between time periods, with some larger variation present in especially the Neolitic and Viking Age samples. Individual FA values were between 1.997–8.45 for the superior dataset (Fig. 6) and 1.46–12.8 for the basal data set (Fig. 7).
Within cemeteries, we found the biggest differences between the two Viking Age cemeteries in the superior data set (Fig. 6), and the Stone Age grave site “Rævehøj” and all other cemeteries in the basal data set (Fig. 7).
Cemeteries
Both the Medieval and the Early Modern Period cemeteries were tested either using one-way ANOVA or Kruskal Wallis test (Table 4). None of the cemeteries in the four data sets were statistically different (Table 4).
Time periods
We tested differences in levels of FA between time periods using linear mixed models with time period and sex as independent factors. We found that sex was not a factor (superior: p = 0.225, basal: p = 0.198), and that there was no statistical difference between time periods (superior: p = 0.885, basal: p = 0.06).
Discussion
In this study we investigated cranial FA through time in adult Danish populations. Based on ANOVAs and Kruskal-Wallis test, we found no differences between cemeteries from neither the Medieval nor the Early Modern time periods. Based on mixed linear models, we found no statistical differences in cranial FA between time periods, and we found that sex did not influence levels of cranial FA.
Fluctuating asymmetry between grave sites from the same time period
We found no differences between cemeteries within the Medieval Age and Early Modern Age samples, which does not align with our hypothesis that FA is dependent on environmental stress and lifestyle. For both time periods, we expected to see some difference between cemeteries. The Medieval Age sample included cemeteries from both rural and urban areas, which may have meant some differences in levels of environmental stress. Village communities in the Danish Medieval Age were small compared to urban communities (Primeau et al., 2018), and we expected that this difference along with the higher levels of contact to other communities due to trade in bigger towns would lead to differences in genetic heterozygosity and lifestyle. However, as our results indicate, the differences between urban and rural communities in the Medieval Age might not have been big enough to cause differences in levels of cranial FA. The Early Modern Period sample included cemeteries only from Copenhagen, but different socioeconomic groups. We expected the lower socioeconomic groups to live with higher levels of environmental stress due to e.g., harder manual labour from an early age, less access to consistent nutrition, and higher disease loads caused by more cramped living spaces. We found that levels of cranial FA between the socioeconomic groups were not statistically different. This might be because the more advantaged groups lived with higher levels of environmental stress than we expected, or because the stratification of socioeconomic groups were not as static as we thought. Some differences were seen in the Stone Age and Viking Age samples. We saw a high level of FA in the basal data set from the Rævehøj grave site, which is interesting, though it is difficult to conclude anything about due to the small sample size (n = 4) and the lack of archaeological context. For the Viking Age, we noticed that the superior data sets showed different levels of FA between the two cemeteries included. Again, we were limited by the small samples size (n = 4 and n = 4), which makes conclusions vague. Additionally, levels of FA in these samples were not consistent between the superior and basal parts of the crania. In the Rævehøj sample, FA was very high in the basal data set, but it was comparable to the other grave sites in the superior data set. The two Viking Age sample FA levels only seemed to differ in the superior data sets. At present, we only have limited genetic and chemical context on the samples in our study, except for the Galgedil grave site. The results from Galgedil showed that the population included a number of non-local individuals, indicating that some level of admixing may have been present in the population (Melchior et al., 2008; Price et al., 2014). This may have had an effect on the levels of FA in the population compared to the other Viking Age grave site (Kaagården). However, the isotopic context of the Kaagården grave site is not known, so any proper comparisons would necessitate a similar analysis to be made on the remains there.
Fluctuating asymmetry between time periods
We found no differences in levels of cranial FA between the time periods. Our findings stand in opposition with previous studies, which found statistically significant differences between populations, both with regards to time (DeLeon, 2007; Gawlikowska et al., 2007) and socioeconomic status (Bigoni et al., 2013; Weisensee & Spradley, 2018). Our results are consistent with results found by Kimmerle and Jantz (2005) who found steady levels of FA over time. As with the comparison between cemeteries, we expected to see some change in FA through time periods. Since the Early Modern Period material is all from Copenhagen, the capital city of Denmark, and the other samples are from either smaller towns or rural communities, we expected to see a difference in FA between the time periods due to admixing, with the urban environment making inbreeding less likely. Moreover, we hypothesized the populations would differ in lifestyle based on changes in, e.g., jobs, working conditions, diet, and living quarters. Since the cemeteries within each period are not statistically different, we cannot attribute the lack of variation between the time periods to the mixing of different socioeconomic groups.
Fluctuating asymmetry, sex, and age
We chose to include sex as factor in our analysis both because of the lack of consensus on the subject in previous literature (Bigoni et al., 2013; Chovalopoulou et al., 2017; Gawlikowska-Sroka et al., 2013, 2017; Hershkovitz et al., 1992; Jung et al., 2016; Weisensee, 2013) and because we wanted to ensure that no bias was introduced by the skewed proportion of males to females that was present in our sample. We found that sex did not influence levels of cranial FA.
We chose not to test whether there was a connection between FA and age. This decision was primarily based on the inherent uncertainties related to age estimation in archaeological samples, such as lack of standards in combining age estimation methods and reference sample age distribution bias (Buckberry, 2015). This is especially true for our samples, as age estimations in the Anthropological Collection at the Laboratory of Biological Anthropology in Copenhagen have been performed by multiple researchers, and the bones present for age estimation vary greatly between samples, making age estimation inconsistent and age ranges very broad. Moreover, previous studies indicate that FA and age are not correlated (Chovalopoulou et al., 2017; Hershkovitz et al., 1992).
Limitations when measuring fluctuating asymmetry
There are multiple possible explanations as to why we found no differences in FA between populations. First, it may be that the placement of the landmarks used in our study did not detect FA. It has been suggested that FA stressors may be trait specific (DeLeon, 2007; DeLeon & Richtsmeier, 2009), which our choice of landmarks may not have covered. This hypothesis is supported by the fact that other studies have found differences in FA between groups. In this context, there may be some consequence of landmark choice: while there is some overlap between which landmarks different studies use, they are not consistent (Rupić et al., 2020), which could have some influence on the results. Other studies have chosen to include a higher number of landmarks to cover more of the cranial shape (Chovalopoulou et al., 2017; DeLeon, 2007; Jung & von Cramon-Taubadel, 2018), thus increasing the chances of registering traits exhibiting FA. In our instance, rather than to include a large number of traits, we chose to select our landmarks based on precision of the points and to avoid landmarks that were often missing due to taphonomic damage. Our list of landmarks was taken from Olsen et al. (2022), which is specifically tested for use in studies of cranial FA. We did this to ensure that any FA would not be obscured by measuring error, and because there is no definite answer to which traits are most appropriate to measure (Chovalopoulou et al., 2017; DeLeon, 2007; Jung & von Cramon-Taubadel, 2018; Olsen et al., 2022). Moreover, we collected only fixed landmarks. A possible alternative approach to cover more of the cranial shape could have been to include semi-landmarks in the study—however, semi-landmarks have not been previously used in studies of cranial FA in humans, and some research indicates that using semi-landmarks in studies of FA can introduce bias (Schlager, 2016).
Working with archaeological samples comes with its own set of unique complications. Not only are conclusions always dependent on the archaeological context—the material is often damaged, and it is almost certain that the skeletal samples used are not representative of the living population. Even though we excluded warped, pathological, and damaged crania from our sample, it is not guaranteed that all crania in our sample was then without bias. Crania may have been warped to such a small degree that it was not noticeable to the naked eye but was still registered by the 3D digitizer. Moreover, since it is hypothesized that FA is established in childhood, any illness in that time may have had an influence that is no longer visible. It could very well be the case that changes in populations have had an influence on FA, however heterogeneous frailty and selective mortality have obscured it (Wood et al., 1992). These mechanisms may have resulted in the weakest individuals in a population being selected out of said population early, leaving individuals with higher genetic resilience and therefore possibly lower FA. We only investigated adults in our study, to ensure that the crania had all finished development, which may mean that we have missed information of FA in frail populations. Having more knowledge of the demography of the populations and having more archaeological context could help clarify how levels of FA have developed.
Knowledge of the archaeological context becomes harder to obtain as the samples become older. Additionally, the amount of material we have available is also dependent on the age of the samples, making conclusions about our oldest samples vague. To put our results into clearer context, we would need to obtain more physiological information on the populations. This could be done by collecting data on stress indicators in the population, such as enamel hypoplasia or cribra orbitalia. Moreover, genetic information on heterozygosity of the population could be very useful to put into context whether admixing has had an influence on FA.
Division of datasets
We chose to analyze the crania in two parts: a superior part covering the face, and a basal part covering the calvarium and base of the crania. The division of the dataset was required due to the cranium needing to be flipped over to collect all landmarks. While it is possible to align the two datasets into one whole using alignment landmarks, we chose to forego this step because the alignment landmarks need to fulfil certain requirements (isotropic variation, very high precision) to not introduce bias (von Cramon-Taubadel et al., 2007). Furthermore, the divided datasets were an opportunity to analyze the neurocranium and viscerocranium separately, which may be reasonable as they have different embryonic origins (Schaefer et al., 2009). Other research touch upon the connection between FA and fetal development, arguing that some FA is established already in utero (Rossi et al., 2003; Russak et al., 2016), which could indicate that embryologic development could influence levels of FA in different structures. For cranial development specifically, there is firstly a difference in origin—the neurocranium originates in the crista neuralis and the paraxial mesoderm, while the viscerocranium originates in the first pharyngeal arch (Schaefer et al., 2009). Secondly, the two parts of the cranium have different growth trajectories, with the neurocranium growing faster than the viscerocranium to accommodate the rapid early growth of the brain (Zollikofer & Ponce de León, 2002). Thus, the neurocranium reaches adult morphology faster than the viscerocranium, possibly making the two parts of the cranium susceptible to environmental influence at different times during development. Indeed, others have found that levels of FA are lower in the face than in the base and calvarium (Chovalopoulou et al., 2017; DeLeon, 2007; DeLeon & Richtsmeier, 2009), indicating that this may be a factor, though other explanations such as sexual selection are proposed in these papers. We did not compare the levels of FA in the two cranial regions, due to the study design with different numbers of landmarks in the two datasets. However, it would be interesting to investigate this topic further.
Missing data estimation
For this study, we had to estimate around 6% of the data. This is almost unavoidable when working with archaeological material, which is often damaged. We followed guidelines set up by Neeser et al. (2009) to ensure as little bias as possible, though it is possible that some still exists. We tried to keep bias to a minimum by estimating missing data using a reference cranium from the same population as the target cranium. This way, any possible asymmetry from one population was not transferred to all populations.
Conclusion
The results of this study indicate that environmental stress and lifestyle have not had a measurable impact on cranial FA in the Danish population; we found no differences in FA over time in the Danish population, nor did we find any differences between cemeteries from the same time period. Additionally, we found no connection between FA and sex. Further knowledge of genetic variation and other stress indicators of the populations may help our understanding of the underlying mechanisms driving cranial FA.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Adams, D. C., Collyer, M. L., Kaliontzopoulou, A., & Baken, E. K. (2022). Geomorph: software for geometric morphometric analyses. R Package Version 4.0.4. https://cran.r-project.org/package=geomorph
Asmussen E (1997) Ahlgade 15-17, Holbæk - archaelogy and history from 1200 AD until the present. Det Kgl, Nordiske Oldskrifterselskab
Baken EK, Collyer ML, Kaliontzopoulou A, Adams DC (2021) geomorph v4. 0 and gmShiny: enhanced analytics and a new graphical interface for a comprehensive morphometric experience. Methods Ecol Evol 12(12):2355–2363
Beasley DAE, Bonisoli-Alquati A, Mousseau TA (2013) The use of fluctuating asymmetry as a measure of environmentally induced developmental instability: a meta-analysis. Ecol Indic 30:218–226. https://doi.org/10.1016/j.ecolind.2013.02.024
Bech SC, Kjersgaard E, Danielsen J (1980) Københavns historie, vol 2, Gyldendal
Bigoni L, Krajíček V, Sládek V, Velemínský P, Velemínská J (2013) Skull shape asymmetry and the socioeconomic structure of an early medieval Central European Society. Am J Phys Anthropol 150:349–364. https://doi.org/10.1002/ajpa.22210
Bobé L (1920) Bremerholms Kirke og Holmens Menighed gennem tre Aarhundreder. H. Hagerups Forlag
Buckberry J (2015) The (mis) use of adult age estimates in osteology. Ann Hum Biol 42(4):323–331
Buckberry JL, Chamberlain AT (2002) Age estimation from the auricular surface of the ilium: a revised method. AmePhysical Anthropology: Official Publ Ame Assoc Phys Anthropologists 119(3):231–239
Buikstra JE (1994) Standards for data collection from human skeletal remains. Arkansas Archaeol Survey Res Series 44
Chovalopoulou M-E, Bertsatos A, Papageorgopoulou C (2017) Age-related changes in the craniofacial region in a modern Greek population sample of known age and sex. Int J Legal Med 131(4):1103–1111
Chovalopoulou M-E, Papageorgopoulou C, Bertsatos A (2017) Cranium asymmetry in a modern Greek population sample of known age and sex. Int J Legal Med 131:803–812. https://doi.org/10.1007/s00414-016-1430-4
DeLeon VB (2007) Fluctuating asymmetry and stress in a medieval Nubian population. Am J Phys Anthropol 132(4):520–534
DeLeon VB, Richtsmeier JT (2009) Fluctuating asymmetry and developmental instability in sagittal craniosynostosis. Cleft Palate-Craniofacial J 46(2):187–196
Dryden IL, Mardia K, v. (1998) Statistical shape analysis. Wiley
Dwight T (1905) The size of the articular surfaces of the long bones as characteristic of sex; an anthropological study. Am J Anat 4(1):19–31
Farrera A (2022) Formal models for the study of the relationship between fluctuating asymmetry and fitness in humans. Ame J Biolog Anthropol 179(1):73–84. https://doi.org/10.1002/ajpa.24588
Fruciano C, Celik MA, Butler K, Dooley T, Weisbecker V, Phillips MJ (2017) Sharing is caring? Measurement error and the issues arising from combining 3D morphometric datasets. Eco Evol 7(17):7034–7046
Gawlikowska A, Szczurowski J, Czerwiński F, Miklaszewska D, Adamiec E, Dzieciołowska E (2007) The fluctuating asymmetry of medieval and modern human skulls. HOMO- J Comp Human Bio 58:159–172. https://doi.org/10.1016/j.jchb.2006.10.001
Gawlikowska-Sroka A, Dabrowski P, Szczurowski J, Dzieciolowska-Baran E, Staniowski T (2017) Influence of physiological stress on the presence of hypoplasia and fluctuating asymmetry in a medieval population from the village of Sypniewo. Int J Paleopathol 19:43–52. https://doi.org/10.1016/j.ijpp.2017.10.002
Gawlikowska-Sroka A, Dąbrowski P, Szczurowski J, Staniowski T (2013) Analysis of interaction between nutritional and developmental instability in mediaeval population in Wrocław. Anthropol Rev 76(1)
Geber J (2014) Skeletal manifestations of stress in child victims of the G reat I rish F amine (1845–1852): Prevalence of enamel hypoplasia, Harris lines, and growth retardation. Am J Phys Anthropol 155(1):149–161
Gluckman PD, Hanson MA, Buklijas T (2010) A conceptual framework for the developmental origins of health and disease. J Dev Orig Health Dis 1(1):6–18
Goodman AH, Rose JC (1990) Assessment of systemic physiological perturbations from dental enamel hypoplasias and associated histological structures. Am J Phys Anthropol 33(S11):59–110
Graham JH (2021) Fluctuating asymmetry and developmental instability, a guide to best practice. Symmetry 13(1):9
Grøn O, Hedeager Krag A, Bennike P (1994) Vikingetidsgravpladser på Langeland. Langelands Museum
Hanson MA, Gluckman PD (2008) Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol 102(2):90–93. https://doi.org/10.1111/j.1742-7843.2007.00186.x
Harris HA (1931) Lines of arrested growth in the long bones in childhood: the correlation of histological and radiographic appearances in clinical and experimental conditions. Br J Radiol 4(47):561–588
Hershkovitz I, Ring B, Kobyliansky E (1992) Craniofacial asymmetry in Bedouin adults. Am J Hum Biol 4:83–92. https://doi.org/10.1002/ajhb.1310040111
Hyldgård IM (2016) Tjærby Ødekirke og Kirkegård (1st ed., Vol. 3). Aarhus Universitetsforlag
Jensen J (2001) Danmarks oldtid – Stenalder. (1st ed., Vol. 1), Gyldendal
Jensen J (2003) Danmarks oldtid - Ældre Jernalder (1st ed., Vol. 3), Gyldendal
Jung H, von Cramon-Taubadel N (2018) Comparison of cranial fluctuating asymmetry between normal and pathological specimens from a modern Thai skeletal group. Homo - J Compar Human Bio 69:188–197. https://doi.org/10.1016/j.jchb.2018.07.004
Jung H, Woo EJ, Pak S (2016) A comparison of cranial fluctuating asymmetry between the two sexes in a Joseon Dynasty population of Korea. Anthropol Anz 73(3):215–223
Katz D, Suchey JM (1986) Age determination of the male os pubis. Am J Phys Anthropol 69(4):427–435
Kimmerle EH, Jantz RL (2005). Secular trends in craniofacial asymmetry studied by geometric morphometry and generalized procrustes methods. In: Modern morphometrics in physical anthropology (pp. 247–263). https://doi.org/10.1007/0-387-27614-9_11
Kjærgård, A. (2014). Beretning for arkæologisk undersøgelse ven Holmens Kirke 2013-2014.
Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11:353–357. https://doi.org/10.1111/j.1755-0998.2010.02924.x
Klingenberg CP, Barluenga M, Meyer A (2002) Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 56(10):1909–1920 http://apps.isiknowledge.com/full_record.do?product=WOS&search_mode=GeneralSearch&qid=61&SID=N1AMjKKKDN487ejm1B4&page=1&doc=1
Klingenberg CP, McIntyre GS (1998) Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with procrustes methods. Evolution 52(5):1363–1375. https://doi.org/10.2307/2411306
Koch HD, & Lynnerup N (2003). Skælskør Karmeliterkloster og dets kirkegård: senmiddelalderlig gravskik samt et limogeskrucifiks. Kongelige Nordiske Oldskriftselskab. https://books.google.dk/books?id=ECxoPAAACAAJ
Kujanová M, Bigoni L, Velemínská J, Velemínský P (2008) Limb bones asymmetry and stress in medieval and recent populations of Central Europe. Int J Osteoarchaeol 18(5):476–491
Livshits G, Yakovenko K, Kletselman L, Karasik D, Kobyliansky E (1998) Fluctuating asymmetry and morphometric variation of hand bones. Am J Phys Anthropol 107:125–136. https://doi.org/10.1002/(SICI)1096-8644(199809)107:1<125::AID-AJPA10>3.0.CO;2-2
Meindl RS, Lovejoy CO (1985) Ectocranial suture closure: a revised method for the determination of skeletal age at death based on the lateral-anterior sutures. Am J Phys Anthropol 68(1):57–66
Melchior L, Kivisild T, Lynnerup N, Dissing J (2008) Evidence of authentic DNA from Danish Viking Age skeletons untouched by humans for 1,000 years. PLoS One 3(5):e2214
Mitteroecker P, Gunz P (2009) Advances in geometric morphometrics. Evol Biol 36(2):235–247
Moes E, Willermet C, Hunley K, Ragsdale C, Edgar HJH (2022) Childhood stress and developmental instability: comparing microscopic enamel defects and cranial fluctuating asymmetry in a colonial Mexican sample. Ame J Biolog Anthropol 179(1):134–147. https://doi.org/10.1002/ajpa.24585
Møller AP, Thornhill R (1997) A meta-analysis of the heritability of developmental stability. J Evol Biol 10:1–16. https://doi.org/10.1007/s000360050001
Mopin C, Chaumoître K, Signoli M, Adalian P (2018) Developmental stability and environmental stress: a geometric morphometrics analysis of asymmetry in the human femur. Am J Phys Anthropol 167(1):144–160
Neeser R, Ackermann RR, Gain J (2009) Comparing the accuracy and precision of three techniques used for estimating missing landmarks when reconstructing fossil hominin crania. AmePhysical Anthropology: Official Publ Ame Assoc Phys Anthropologists 140(1):1–18
Olsen TB, García-Martínez D, Villa C (2022) Testing different 3D techniques using geometric morphometrics: Implications for cranial fluctuating asymmetry in humans. Ame J Biolog Anthropol
Palmer AR, Strobeck C (1986) Fluctuating asymmetry: measurement, analysis, patterns. Annu Rev Ecol Syst 17(1):391–421
Phenice TW (1969) A newly developed visual method of sexing the os pubis. Am J Phys Anthropol 30(2):297–301
Price TD, Prangsgaard K, Kanstrup M, Bennike P, Frei KM (2014) Galgedil: isotopic studies of a Viking cemetery on the Danish island of Funen, AD 800–1050. Danish J Archaeol 3(2):129–144
Primeau C, Homøe P, Lynnerup N (2018) Childhood health as reflected in adult urban and rural samples from medieval Denmark. Homo 69(1–2):6–16
Revware. (2020). Microscribe Utility Software (7.0). Revware. https://revware.net/revware-products/software/mus7/
Rohlf FJ, Slice D (1990) Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Biol 39(1):40–59
Rossi M, Ribeiro E, Smith R (2003) Craniofacial asymmetry in development: an anatomical study. Angle Orthodontist 73(4):381–385
RStudio Team. (2021). RStudio: integrated development environment for R. http://www.rstudio.com/
Rupić I, Čuković-Bagić I, Ivković V, Lauc T (2020) Assessment of facial landmarks for bone asymmetry in geometric morphometric studies: a review. South Eur Orthodont Dentofacial Res 7(specijalno izdanje):6–16
Russak ODF, Ives L, Mittal VA, Dean DJ (2016) Fluctuating dermatoglyphic asymmetries in youth at ultrahigh-risk for psychotic disorders. Schizophr Res 170:301–303. https://doi.org/10.1016/j.schres.2015.12.013
Schaefer M, Black SM, Schaefer MC, Scheuer L (2009) Juvenile osteology. Elsevier
Schlager S (2016) Sliding semi-landmarks on symmetric structures in three dimensions. Poster. https://doi.org/10.1002/ajpa.21502
Sellevold BJ, Hansen UL, Jørgensen JB (1984) Iron age man in Denmark: prehistoric man in Denmark, vol III. Kongelige Nordiske Oldskrift-Selskab
Slice DE (2006) Modern morphometrics in physical anthropology. Springer Science & Business Media
Small CG (2012) The statistical theory of shape. Springer Science & Business Media
Springborg, B., & Møller, S. B. (2016). Beretning for arkæologisk undersøgelse i kvarteret omkring Vor Frue kirke.
Suckling GW (1989) Developmental defects of enamel-historical and present-day perspectives of their pathogenesis. Adv Dent Res 3(2):87–94
van Dongen S (2006) Fluctuating asymmetry and developmental instability in evolutionary biology: Past, present and future. J Evol Biol 19:1727–1743. https://doi.org/10.1111/j.1420-9101.2006.01175.x
van Dongen S, Gangestad SW (2011) Human fluctuating asymmetry in relation to health and quality: A meta-analysis. Evol Hum Behav 32(6):380–398. https://doi.org/10.1016/j.evolhumbehav.2011.03.002
Van Valen L (1962) A study of fluctuating asymmetry. Evolution 16:125–142. https://doi.org/10.1038/157619d0
Victora CG (1992) The association between wasting and stunting: an international perspective. J Nutr 122(5):1105–1110
von Cramon-Taubadel N, Frazier BC, Lahr MM (2007) The problem of assessing landmark error in geometric morphometrics: theory, methods, and modifications. Am J Phys Anthropol 134(1):24–35
Weisensee KE (2013) Assessing the relationship between fluctuating asymmetry and cause of death in skeletal remains: a test of the developmental origins of health and disease hypothesis. Am J Hum Biol 25(3):411–417. https://doi.org/10.1002/ajhb.22390
Weisensee KE, Spradley MK (2018) Craniofacial asymmetry as a marker of socioeconomic status among undocumented Mexican immigrants in the United States. Econ Hum Biol 29:122–127. https://doi.org/10.1016/j.ehb.2018.02.007
Wendt JC, Wilhelm. (1833) Almindeligt Hospital i Kjøbenhavn : dets Indretning og Forfatning, Pengevæsen, Legater. Historie etc, Jens Hostrup-Schultz
Wood JW, Milner GR, Harpending HC, Weiss KM, Cohen MN, Eisenberg LE, Hutchinson DL, Jankauskas R, Cesnys G, Česnys G (1992) The osteological paradox: problems of inferring prehistoric health from skeletal samples [and comments and reply]. Curr Anthropol 33(4):343–370
Zollikofer CPE, Ponce de León MS (2002) Visualizing patterns of craniofacial shape variation in Homo sapiens. Proceedings of the Royal Society of London. Series B: Biolog Sci 269(1493):801–807
Acknowledgements
The authors would like to thank Eske Willerslev and the Lundbeck foundation (grant number R302-2018-2155) for the financial support. We would also like to thank the reviewers for their helpful comments.
Funding
Open access funding provided by Royal Library, Copenhagen University Library. This project was funded by Eske Willerslev and the Lundbeck foundation (grant number R302-2018-2155).
Author information
Authors and Affiliations
Contributions
Conceptualization: CV and NL; data curation: TBO; formal analysis: TBO, CV, and DG-M; investigation: TBO; methodology: TBO, CV, and DG-M; project administration: TBO and CV; Resources: MLSJ and NL; software: TBO, DG-M; supervision: CV, MLSJ; visualization: TBO; writing—original draft: TBO; and writing—review and editing: TBO, CV, MLSJ, NL, and DG-M.
Corresponding author
Ethics declarations
Disclaimer
The Lundbeck Foundation had no influence of study design, data collection and analysis, or preparation of the manuscripts.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olsen, T.B., García-Martínez, D., Lynnerup, N. et al. Cranial fluctuating asymmetry in Danish populations from the Neolithic to the Early Modern Age. Archaeol Anthropol Sci 15, 189 (2023). https://doi.org/10.1007/s12520-023-01888-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-023-01888-0