Abstract
Stable isotope-based dietary information on the tenth century CE Hungarian population serves as a valuable comparative tool to other dietary stable isotope studies in the region. This study presents a multiproxy approach involving skeletal samples with the least diagenetic alteration and the best-preserved primary signals. Dental and bone samples collected from three cemeteries in northeastern Hungary were investigated using a combination of Fourier-transform infrared spectroscopy (FTIR), stable isotope geochemistry, and strontium isotope analysis. FTIR measurements were used to detect the addition of secondary carbonate to the skeletal apatite carbonate component and to differentiate diagenetically altered samples. Our results suggest that all but the bone apatite carbonate stable isotope values and 87Sr/86Sr ratios are trustworthy and may be used to interpret the diet and migration of the researched individuals. We also determined that the inhabitants of the three tenth century CE Hungarian settlements relied primarily on C3 plants, but that C4 plants, most likely broomcorn millet (P. miliaceum), comprised a moderate portion of their plant-based diet and that animal products were generally consumed in moderate-high amounts. Coupled analyses of carbonate oxygen and strontium isotope compositions revealed that most of the bone apatite carbonate was heavily altered, whereas enamel apatite carbonate samples largely preserved their primary compositions. The bones showing the least amount of diagenetic alteration may partially preserve their primary apatite carbonate compositions, as well as show a combination of primary and secondary signals, which should be taken into consideration when bone apatite carbonate data are interpreted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the pioneering research of the 1980s (see Ambrose 1990), hundreds of studies have used stable carbon, nitrogen, and oxygen isotope measurements, as well as 87Sr/86Sr ratio analyses of human bone and dental samples to decipher the migration histories and dietary habits of ancient societies. The Carpathian Basin has been the crossroad for peoples’ migrations from the east and south to the west for thousands of years, making it an excellent topic of several stable oxygen isotope and 87Sr/86Sr composition studies (Giblin 2009; Gerling et al. 2012; Giblin et al. 2013; Alt et al. 2014; Noche-Dowdy 2015; Giblin and Yerkes 2016; Hakenbeck et al. 2017; Gamarra et al. 2018; Gugora et al. 2018; Knipper et al. 2020; Depaermentier et al. 2020; 2021). Ancient Hungarian tribes settled the Carpathian Basin in 895/896 CE, bringing with them the genetic and cultural characteristics from several boundary regions between Europe and Asia, while also adopting habits of the local inhabitants (e.g., Avars and Slavs) (Fóthi et al. 2020, and references therein). As such, the cemeteries of the tenth century CE Hungarian populations, ranging from commoners (at Tiszanána in Eastern Hungary) to the military upper class and their families (at Kenézlő-Fazekaszug and Karos-Eperjesszög in northeastern Hungary), may provide insight into the migrations and dietary habits of the people in the region. The studied populations appeared to have possessed the same culture, with differences, such as diet, likely due to differing food procurement practices as a result of site-specific environmental constraints (e.g., marshy Kenézlő and Karos vs. dryer Tiszanána). A complex study involving stable C-N–O isotope (13C/12C, 15 N/14 N, and 18O/16O ratios) analyses and Sr isotope (87Sr/86Sr ratio) measurements (Gugora et al. 2021; 2022) was begun to determine diet and migration at several tenth century CE Hungarian Conquest period sites. The study includes stable carbon and nitrogen isotope analyses of dentin and bone collagen, as well as stable carbon and oxygen isotope analyses of enamel and bone apatite carbonate. If well preserved, the carbon isotope composition of apatite can provide information about carbohydrate consumption, whereas the carbon and nitrogen isotope compositions of collagen reflect the protein portion of diet (Krueger and Sullivan 1984). The stable oxygen isotope composition of apatite carbonate may be used to infer diet and environmental conditions, provided that diagenesis has not altered the carbonate component (Sponheimer and Lee-Thorp 1999; Koch et al. 1997; Salesse et al. 2014; Wang and Cerling 1994). Previous studies have recommended chemical treatment procedures to remove the contaminating secondary carbonate or methods to detect diagenetic alteration by crystallinity analyses (Crowley and Wheatley 2014; Garvie-Lok et al. 2004; Koch et al. 1997; Person et al. 1995; Stuart-Williams et al. 1996; Weiner and Bar-Yosef 1990). Additionally, strontium isotope ratios may yield information about migration and diet due to local differences in environmental strontium isotope compositions where bioavailable Sr is incorporated into the human body (Bentley 2006). Stable isotope compositions of teeth reflect childhood, whereas those of bones reflect the final 3–10 years before death (depending on the bone type), allowing for a complex evaluation of diet and migration that potentially spans the entire lifetime of the studied individuals (Arneborg et al. 1999; Hedges et al. 2007). However, to determine if the apatite and collagen analyses provide reliable data, the effects of potential post-depositional diagenetic alteration in the soil-hosted graves must be determined. Fourier-transform infrared spectroscopy (FTIR) is a powerful tool to detect diagenetic alteration in skeletal material (Roche et al. 2010). This study presents a combination of FTIR and isotope analyses to achieve a reliable selection of unaltered skeletal samples and pristine compositions.
Materials and methods
Materials
Skeletal samples were collected from three tenth century CE cemeteries: Karos-Eperjesszög (i.e., Karos), Kenézlő-Fazekaszug (i.e., Kenézlő), and Tiszanána, in eastern and northeastern Hungary (Fig. 1). Kenézlő is located in northeastern Hungary, along the Tisza River’s northern bank (Upper Tisza region), and contains opulent burials of high-status individuals, likely military leaders and their family, as well as other warriors and household servants (Révész 2014, 2020) (Fig. 1), indicating it was likely part of the princely retinue of the Upper Tisza region. It was discovered in 1913 and excavated in the 1920s (Révész 2014).
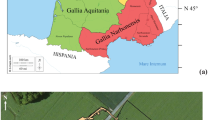
Locations of the tenth century CE cemeteries studied in this paper. The sites investigated by Depaermentier et al. (2021) (Mezőzombor-Községi temető [MEKÖ], Tiszadob-Okenéz [TISO], Kompolt-Kígyós-ér [KOKI], Tiszabura Bonishat [TIBO], and Pusztataksony Ledence [PULE]) are also shown
Karos is situated near Kenézlő in northeastern Hungary and also contains several upper class individuals, including military leaders, their family, and upper class warriors, as well as warriors and other members of the lower classes, such as household and personal servants (Nagy and Révész 2003) (Fig. 1). Because the site is composed of three adjacent and contemporaneous cemeteries (I-III), they were each discovered at different times by different individuals. Karos I is the smallest of the three and was found in 1899 by fieldhands (Nagy and Révész 2003; Révész 1996). Karos II is the largest and was initially discovered in 1936 and rediscovered in 1985 by a resident of Karos village (Nagy and Révész 2003; Révész 1996). Karos III was the final cemetery to be processed, between 1988 and 1990 (Wolf and Révész 1996). Karos is presently the most opulent tenth century CE Hungarian cemetery excavated (Nagy and Révész 2003). Both Karos and Kenézlő are located in a marshy floodplain called the Bodrogköz, which made the cultivation of many common C3 plants, such as wheat (T. aestivum), difficult and impractical. Instead, the Hungarians living here relied more heavily on broomcorn millet (P. miliaceum) (a C4 plant), as it is much quicker to cultivate (40–90 days) than C3 cereal grains, thus allowing it to be harvested before the area flooded (Balassa 1994; Motuzaite-Matuzeviciute et al. 2013).
In contrast, Tiszanána is in northern Hungary (Fig. 1) and likely was discovered at a sand mine in 1958 by a member of the mining company (Révész 2008). The cemetery was extensively damaged by plowing and looted by mine workers, as well as area residents. Tiszanána appears to have had a fairly egalitarian society, with few, if any, upper class individuals, as the graves contained relatively modest material artifacts. Additionally, warriors likely did not inhabit the site, because weapons were not found (Révész 2008).
The areas of studied cemeteries are characterized by Quaternary fluvial deposits, clays, and mudstones (interactive geological map of Hungary, Mining and Geological Survey of Hungary, https://map.mbfsz.gov.hu/fdt100/).
These three tenth century CE Hungarian sites were chosen because the archaeological and anthropological evidence suggest that they contained a variety of social statuses and potential indications of non-native individuals. They are therefore considered to be representative of the Hungarians living in that period, as well as anthropologically interesting.
The number of samples (n) collected is as follows: 71 teeth and 83 bones from Karos, 19 teeth and 11 bones from Kenézlő, and 8 teeth and 37 bones from Tiszanána (Supplementary Table S1).
Methods
Biological sex and age at death were determined for the collected samples by Tóth (1958) and Kustár (1993; 1996) (Supplementary Table S1). For apatite analyses, tooth enamel and bone samples were ground in a ceramic mortar and pestle, after which the skeletal powder was repeatedly sieved using a 180-μm sieve, so that the grain size was approximately 180 μm (Garvie-Lok et al. 2004).
Attenuated total reflection (ATR) FTIR analyses were performed using a Bruker Vertex 70 Fourier Transform Infrared spectroscope controlled by OPUS 8.1 software. For each sample, 64 scans were recorded in the 4000–400 cm−1 spectral range, with a resolution of 4 cm−1. FTIR spectra were baseline-corrected using the Bruker Opus software. Peak area OH/PO4 and CO3/PO4 ratios were calculated on the basis of the following wave number intervals: 890–1190 cm−1 (PO4), 1320–1530 cm−1 (CO3), 3000–3600 cm−1 (OH). Instead of peak intensities, which are based on single values for OH, CO3, and PO4, integrated peak area values were used (Demény et al. 2019). Keeping the sample powder grain size just below 180 μm allowed for the exclusion of large variations in grain size, which can significantly affect the measured CO3/PO4 ratios (Kontopoulos et al. 2018).
Apatite samples were prepared following the procedures adapted from Garvie-Lok et al. (2004) and described by Gugora et al. (2018). Distilled water was used to clean the teeth, which were then dried overnight in a drying oven at 60 °C. Bone was similarly prepared. Using a ceramic mortar and pestle, 20–30 mg of skeletal sample (likewise for both enamel and bone) was ground to a fine powder (< 180 μm) and placed into 2 mL plastic tubes. Then, 0.04 mL of a 2% sodium hypochlorite (bleach) solution per mg of sample was added to the sample for 24 h and 72 h, respectively, for enamel and bone. The samples were rinsed 5 times with distilled water, after which 0.04 mL of 0.1 M acetic acid was added to each sample. The samples were soaked in the acetic acid solution at room temperature for 4 h and were rinsed 5 times with distilled water. The sample containers were then placed in the freezer at − 40 °C for 2 days and were freeze-dried at − 60 °C and at 200 millitorr for 2 days to remove all moisture, to prevent possible isotopic exchange during stable isotope analysis. Finally, the samples were weighed and the apatite percent yields were calculated.
Dentin and bone collagen were extracted following the adapted Longin (1971) procedure, which was previously also used in Gugora et al. (2018). When the teeth and bone were ultrasonically cleaned with distilled water, the dentin was isolated from the enamel and placed into 10 mL glass culture test tubes, while 1–2 g of bone was fragmented into smaller pieces and placed into 50 mL plastic centrifuge tubes. Bone was immersed in 10 mL of 2:1 chloroform:methanol for 20-min intervals, until the liquid was clear, to ensure the removal of lipids that may potentially skew collagen isotope results. Afterwards, the bone underwent 3 rinses in distilled water and was dried overnight. Dentin was demineralized in ~ 5 mL of a 0.5 M hydrochloric (HCl) acid solution, while bone was demineralized in ~ 10 mL of a 0.25 M HCl solution, with daily HCl changes. Complete demineralization occurred in about 2.5 months for dentin and in about 1 month for bone. Three rinses with distilled water followed for both dentin and bone. Then, 5 mL (for dentin) and 10 mL (for bone) of a 0.5 M sodium hydroxide (NaOH) solution were added to the samples in 20-min intervals to remove humic acids, until the solution was clear. Samples were rinsed in distilled water to achieve a pH of 7 ± 1. Next, the samples were rinsed with 0.25 M HCl acid, decanted, and then rinsed in approximately 5 mL of distilled water, until the pH was between 2.5 and 3. The sample tubes were baked (with caps) in a drying oven at 90 °C for 24 h (dentin) and 16 h (bone) to solubilize the collagen. Afterwards, the dentin and bone collagen solutions were pipetted into dram vials and baked uncovered in the oven at 60 °C, until the water evaporated and the collagen solidified. Finally, the samples were weighed and the collagen percent yields were calculated.
Stable isotope compositions are expressed in ‰ as δ13C, δ18O, and δ15N values relative to VPDB (δ13C), VSMOW (δ18O), and AIR (δ15N), respectively. Stable carbon and oxygen isotope compositions of the carbonate component of skeletal apatite were measured by applying an orthophosphoric acid reaction at 72° C (Spötl and Vennemann 2003) and an automated GASBENCH II sample preparation device attached to a Thermo Finnigan Delta Plus XP Isotope Ratio Mass Spectrometer at the Institute for Geological and Geochemical Research (IGGR). The raw data were calibrated using three in-house standards (calibrated using the NBS-18, NBS-19, and LSVEC standards from the International Atomic Energy Agency). Three replicates were run, when sample weight allowed. A δ13C value of − 6.34 ± 0.05‰ (n = 24) was obtained for the NBS-120c phosphate rock standard (carbonated fluorapatite, whose δ13C value is frequently reported; e.g., Passey et al., 2007). The general reproducibility of the sample measurements is about 0.1‰ (see Demény et al. 2019).
C and N concentrations (wt%) as well as stable carbon and nitrogen isotope compositions of collagen samples were determined using an automated Flash 2000 Elemental Analyzer attached to a Thermo Finnigan Delta V Advantage Isotope Ratio Mass Spectrometer at the IGGR. Two-point linear normalization was used to recalculate raw isotope values to VPDB (δ13C) or AIR (δ15N) scales based on analyses of IAEA-CH-6 and IAEA-CH-7 standards for carbon and IAEA-N1 and IAEA-N2 standards for nitrogen. Standard deviations of δ13C and δ15N values of the reference standards were about 0.05‰ and 0.15‰, respectively. δ13Cap and δ13Ccoll are used to represent carbon isotope compositions of the carbonate component of apatite and of the collagen samples, respectively, while δ18O refers to the oxygen isotope composition of skeletal carbonate, as other components of apatite (e.g., phosphate) were not analyzed.
For strontium isotope analyses, bone and dental enamel samples were physically pre-cleaned using a Dremel 300 drill equipped with a diamond tipped tool to remove any surface contaminants. Samples were then cut with a diamond saw. Any remaining dentin on the teeth or cancellous bone on the cortical bone was removed using a diamond tipped Dremel tool attachment. The samples were examined under a microscope to ensure that all contaminants had been eliminated. Bones were repeatedly cleaned of dust by ultrasonication in milliQ water, after which they were rinsed in a 1 M acetic acid solution to remove diagenetic carbonate. Then, the samples were rinsed 3 times in milliQ water and dried overnight at 60 °C in a drying oven. Twenty milligrams of enamel and 50 mg of bone samples, respectively, were weighed and placed into cleaned, labelled PFA beakers. Additional blank and standard solutions were included to verify the blank and accuracy of the chemical preparation. Enamel samples were digested twice in 1 mL of 14 M HNO3 and placed on a hotplate at 120 °C to evaporate to dryness. Bone samples were digested twice in 1 mL of 14 M HNO3 and 100 µL of HClO4. When the sample was fully demineralized, it was taken up in in 3% HNO3 and loaded into the pre-cleaned Sr spec column to separate Sr from the other matrix elements. All sample preparation occurred in a Class 1000 cleanroom. Ultrapure water (UPW) (Merck, 18 MΩ cm) and doubly distilled 14 mol L−1 nitric acid were used for sample digestion, resin cleaning, and elutions. Crown ether-based Sr-Spec Resin (100–150 µm particle size) from Triskem International, France, was pre-cleaned with 8 M HNO3 and UPW prior to use. Sr was isolated from the matrix components and from Rb to avoid an isobaric overlap of 87Sr+ and 87Rb+. Following this separation, the solution was evaporated to dryness, and 1 mL of 14 mol L−1 nitric acid was added, and the sample was evaporated again. The residue was taken up in 4 mL 3% HNO3 solution. Standard solutions were prepared from the NBS987 SrCO3. Strontium isotope ratios were measured using a Neptune Plus MC-ICPMS (Thermo Scientific), equipped with an Aridus-3 (CETAC) desolvating system at the Isotope Climatology and Environmental Research Centre (ICER) in Debrecen, Hungary. The 87Sr/86Sr ratio was corrected for instrumental mass discrimination using 88Sr/86Sr = 8.375209, as well as by applying an interference correction for 87Rb+ and 86Kr+ with 85Rb+ and 83Kr+, respectively. All values were normalized to the accepted value of 0.710240 for NBS987 (Wall et al. 2013).
Results and discussion
This study is based on 226 δ13C-δ18O analyses of apatite carbonate and 220 δ13C-δ15N analyses of collagen samples extracted from teeth and bones, supplemented with 82 FTIR measurements and 78 87Sr/86Sr ratio determinations of enamel and bone samples. The data are listed in Supplementary Tables 2–5 (see Supporting Information Material).
Detection of diagenetic alteration
The δ13C and δ18O values of the carbonate component of enamel and bone apatite, as well as their average values, 1σ standard deviation, and minimum and maximum values are listed in Supplementary Table S2. The enamel apatite δ13C and δ18O values from Karos, Kenézlő, and Tiszanána overlap (Fig. 2A), with the Karos data showing the largest scatter and the Tiszanána data the smallest. This difference may derive from the greater magnitude of data at Karos (68 samples) than at Kenézlő (10 samples) or Tiszanána (6 samples). In contrast, the bone apatite samples show significant δ18O shifts from the enamel fields (with − 2.4‰ at Karos, − 0.8‰ at Kenézlő, and − 2.7‰ at Tiszanána), while the δ13C ranges are either similar in enamel and bones or the bone fields are slightly shifted towards lower δ13C values (Fig. 2, Supplementary Table S2). It is interesting to note that the Kenézlő samples are characterized by the least amount of difference between the enamel and bone δ18O values, whereas the Karos and particularly the Tiszanána data indicate larger shifts.
Stable carbon and oxygen isotope compositions of A the carbonate component of bone and enamel apatite, and B contaminating carbonate from soil debris collected along with skeletal samples. Limestone compositions that the green arrow points to are from Haas and Demény (2002)
The enamel-bone difference may originate from migration and dietary changes during the lifetimes of the individuals, with the enamel preserving information during childhood and the bones reflecting environmental and dietary conditions over the final ~ 3–10 years of the individual’s life. Thus, it is essential to determine if the observed δ18O differences reflect primary compositions or if the bone data are related to secondary, post-depositional diagenetic alteration due to the porous structure of the bones, which makes them vulnerable to contamination, compared to the dense, compact material of teeth, particularly of the enamel portion. The isotopic compositions of skeletal apatite are determined from the carbonate component, so the suspected alteration is likely due to infiltration of groundwater and incorporation of a secondary carbonate component to the apatite structure, leading to elevated carbonate concentration (Demény et al. 2019). This effect may be present if the bone carbonate data resemble data of soil carbonate compositions. As such, soil samples collected alongside or scraped from the surface of the skeletal elements were also analyzed. The isotopic compositions of the potential contaminating material are plotted in Fig. 2B.
The distribution of δ13C and δ18O values indicates the presence of two main contaminating components: marine sedimentary carbonate (limestone and dolomite debris), with elevated δ13C and δ18O values (Haas and Demény 2002), and authigenic soil carbonate, with low δ13C and δ18O values (Fórizs et al. 2017). The negative δ13C shift in bone compared to enamel data suggests that the limestone debris is not truly a contaminating material, as it is easily removed by pre-treatment. However, groundwater infiltration and incorporation of a secondary carbonate appear to have caused the alteration observed in bone carbonate δ18O (Demény et al. 2019). Secondary, soil-related carbonate is precipitated from infiltrating groundwater, covering the outer, cortical surface of bones, and may infiltrate the bone itself, as well. Figure 2B shows that most of the contaminating carbonate scraped from the bone surfaces has low δ18O values compared to the bone compositions, while limestone debris has elevated δ13C values. As the limestone grains are removed by washing the bones, the negative δ18O shift of the bone samples compared to the enamel samples can be attributed to the presence of secondary carbonate precipitated from infiltrating groundwater or incorporated into the apatite structure (Demény et al. 2019).
To investigate the carbonate content of the enamel and bone samples, FTIR analyses were conducted. Peak areas were computed, and OH/PO4 and CO3/PO4 peak area ratios were plotted in Fig. 3A. The enamel samples have low OH/PO4 and CO3/PO4 ratios, with a slight positive correlation, which continues in the Kenézlő bone data. In contrast, the Karos and Tiszanána bones show elevated CO3 contents, in accordance with their suspected diagenetic alteration. The shift from the Kenézlő enamel and bone trend is expressed by the ΔCO3/PO4 value, which is the difference between the measured ratio and the value corresponding to the measured OH/PO4 ratio on the Kenézlő trend (see Fig. 3A). The isotopic data were next plotted as a function of ΔCO3/PO4 values to investigate the effect of secondary carbonate formation on the δ13C and δ18O values. The δ13C values did not indicate a relationship with the CO3 contents (Fig. S1 shown in the Supplementary Tables excel file). The δ18O data, however, display systematic changes with the ΔCO3/PO4 values (Fig. 3B). The plot clearly shows that the enamel data are unaffected, the Kenézlő bones are slightly altered, and increasing alteration appears in the Karos and Tiszanána bone samples (with the bone carbonate δ18O values at Karos and Tiszanána shifted by − 2.4 and − 2.7‰, respectively, and the CO3/PO4 ratios elevated from 0.08 ± 0.01 to 0.14 ± 0.04 at Karos and from 0.07 ± 0.03 to 0.22 ± 0.03 at Tiszanána; Supplementary Tables S2 and S3). The unaltered nature (i.e., free of diagenetic alteration) of the enamel data suggests that, although the carbonate component of skeletal apatite is generally more prone to late-stage alteration than is the phosphate component, enamel data may still provide primary information.
Collagen resistance to alteration
Diagenetic collagen alteration may result in differential removal of C and N, which appears if the atomic C:N ratio is plotted as a function of C% (following Ambrose 1990). Unaltered collagen has an atomic C:N ratio between 2.9 and 3.6 (DeNiro 1985; Ambrose 1990); thus, samples outside this range, with low C and N concentrations, may be regarded as altered, which may affect the C and N isotope compositions, as well (Ambrose 1990). Following this rationale, collagen C and N concentrations and δ13C and δ15N values were determined (Supplementary Table S4) and plotted in Fig. 4.
Figure 4A shows the atomic C:N ratio as a function of C wt% data. Apart from two outliers, all the data fall within the 2.9 to 3.6 atomic C:N range, regardless of whether the sample was dentin or bone collagen. However, some of the Karos and Tiszanána bone collagen data are shifted towards lower C wt% values, with slightly elevated C:N ratios, whereas the dentin and bone collagen from Kenézlő plot in narrow ranges. This corresponds with the general degree of alteration inferred from the FTIR analyses, with the Karos and Tiszanána samples showing signs of enhanced diagenetic alteration and with the Kenézlő samples displaying the least. The next task is to determine how, if at all, this alteration affects the C and N isotopic compositions, as it is important to select unaltered compositions when interpreting dietary differences with collagen δ13C and δ15N values. Figure 4 B and C show that there are no relationships between collagen biochemistry and isotopic composition. Consequently, we can confidently conclude that the δ13Ccoll and δ15Ncoll values can be used to infer dietary variations, which will be the subject of a follow-up study (Gugora et al. 2022).
Unaltered δ13C data can also be used to examine plant protein consumption. If the δ13Cap values are plotted as a function of δ13Ccoll data, the consumption of C3 and C4 plants can be graphically determined (Kellner and Schoeninger 2007), as shown in Fig. 5. Dental enamel and dentin data from Karos, Kenézlő, and Tiszanána plot between the C3 and C4 plant protein lines, indicating that a significant portion of the diet was comprised of C4 plant foods. The most likely candidate is millet, as it was detected in earlier (sixth century CE, Alt et al. 2014) and later (thirteenth century CE, Gugora et al. 2018) medieval Hungary. This observation is somewhat different to what is seen among the Avars (sixth–ninth centuries CE), who, like the tenth century CE Hungarians, had a history of the pastoral nomadic traditions typical of the Eurasian Steppe peoples (Herold 2008; Noche-Dowdy 2015; Vidal-Ronchas et al. 2019). Despite this, the Hungarians consumed more C4 plants (i.e., millet) than did the Avars (Noche-Dowdy 2015). This may be due to environmental conditions, such as at Karos and Kenézlő, where the terrain was marshy and prone to flooding (Nagy and Révész 2003). This type of environment likely necessitated the cultivation of quick-growing plants, like millet (Gugora et al. 2021; Nagy and Révész 2003). Tiszanána, however, was much dryer, so C3 cereal crops were viable, but even they consumed slightly more millet than did the Avars. Interestingly, the C4 plant percentages at tenth century CE Karos and Kenézlő more closely resemble those of the Scythians from north of the Black Sea (Ventresca-Miller et al. 2021) than the Avars (Herold 2008; Noche-Dowdy 2015; Vidal-Ronchas et al. 2019) or medieval Europeans (Gugora et al. 2018; Leggett et al. 2021). Although the tenth century CE Hungarians had a history of nomadism, like the Black Sea Scythians did, they practised sedentary agriculture, rather than traditional nomadism by the time they reached the Carpathian Basin in 895/896 CE (Nagy and Révész 2003). This suggests that the increased C4 plant consumption is likely explained primarily by environmental constraints (i.e., marshy Karos and Kenézlő), with potentially a moderate cultural component (i.e., preserving dietary customs from ancestors’ nomadic past), as well. Most of the bone δ13C data overlap with the dental values, indicating that no significant δ13C alteration occurred, but the low and high δ13C ends indicate changes in the bone apatite carbonate content and slight collagen alteration. The red arrows in Fig. 5 mark the effect of soil carbonate invasion into the bone apatite carbonate. The low δ13Cap values around − 14‰ may indicate direct addition of low-δ13C soil-related carbonate, whereas the elevated δ13Ccoll values up to − 12‰ may suggest collagen alteration due to groundwater infiltration. This observation supports the selection procedure based on the combined FTIR and stable isotope analyses.
Stable carbon isotope compositions of apatite as a function of collagen data obtained from teeth. C3, C4, and marine protein lines are from Kellner and Schoeninger (2007). Bone data are shown as shaded field for comparison. Red arrows indicate the effect of soil carbonate addition
Strontium isotopic compositions: alteration, migration, and diet
Strontium isotope compositions of dental and bone samples were analyzed to investigate the preservation of the original isotopic compositions. The samples were pre-treated both mechanically and chemically to remove superficial secondary carbonate.
The skeletal strontium isotope data (along with averages, 1σ standard deviations, and minimum and maximum values) are listed in Supplementary Tables S5 and are plotted together with the apatite carbonate δ18O values (Fig. 6). As expected, the bone data differ significantly from the enamel compositions (Fig. 6). The Kenézlő samples are an exception, as both the bone and enamel samples have similar oxygen and strontium isotope ranges, excepting only two bone samples, KF1023 and KF1030, with low δ18O values (of about − 23.3‰) and low 87Sr/86Sr ratios (of 0.7091). In contrast, the Karos enamel and bone data are clearly different, particularly due to the differences in the δ18O values (mean enamel δ18O = 25.5 ± 0.6‰, mean bone δ18O = 23.1 ± 0.6‰). The Tiszanána enamel and bone samples show even larger δ18O and 87Sr/86Sr differences, although it should be noted that only 3 enamel and 4 bone samples were measured for both isotope compositions. The Tiszanána bones plot close to the Karos bones, although with slightly higher Sr isotope ratios and lower δ18O values. The bone-enamel differences raise the possibility of secondary carbonate contamination; however, the powdered and oxidized samples were soaked in 0.1 M acetic acid for 4 h, which should remove secondary carbonate from the bioapatite. As Demény et al. (2019) showed, calcite is not detected after the acid treatment, so the carbonate can be attributed entirely to structural carbonate within the apatite. Thus, the elevated CO3/PO4 ratios and low δ18O values are related to the incorporation of carbonate into the apatite structure due to groundwater infiltration in the grave soil. This carbonate may be considered to be analogous to soil carbonate. Depaermentier et al. (2021) used human bone strontium isotope compositions as representative values for burial soils, assuming thorough alteration. The nearest locations studied by Depaermentier et al. (2021) with sedimentary environments (fluvial sediments) similar to those of Karos and Kenézlő are Mezőzombor-Községi temető (MEKÖ) and Tiszadob-Okenéz (TISO), whereas the Tiszanána cemetery is close to the Kompolt-Kígyós-ér (KOKI), Tiszabura Bonishat (TIBO), and Pusztataksony Ledence (PULE) sites investigated by Depaermentier et al. (2021). The comparison of these sites is also supported by the similarities of local geological characteristics, as Fig. 6 shows the 87Sr/86Sr range from these sites, as well as the δ18O range of the estimated secondary carbonate. Secondary carbonate is precipitated as a product of alteration induced by groundwater infiltration, primarily in the warmer seasons (Újvári et al. 2019). Summer temperatures range from 19 to 22 °C in the area (Demény et al. 2012; Újvári et al. 2019), which serve as an upper limit of soil-related carbonate formation. The lower limit is the annual average of 10–11 °C (Czuppon et al. 2018; Újvári et al. 2019). However, because carbonate formation predominantly occurs during the warmer seasons, the bone carbonate formation temperatures are closer to the upper limit. The groundwater compositions can be estimated from the δ18O data of precipitation (Czuppon et al. 2018) to be around − 9‰ (annual average) to − 7‰ (summer average). The expected bone carbonate composition can be calculated from the bone carbonate–water oxygen isotope fractionation determined by Lécuyer et al. (2010). The observed δ18O values range from 20.0 to 25.8 ‰, with most compositions fitting the lower δ18O limit that corresponds to δ18Owater = − 9‰ and t = 22 °C. This is presented on the δ18O axis of Fig. 6. As shown in Fig. 6 by the grey arrows, the bone compositions are shifted from the enamel data towards the estimated soil carbonate δ18O and 87Sr/86Sr ranges. However, because bone alteration and, consequently, the stable isotopic shifts are not uniform, the bone data may represent mixed compositions between enamel data and local soil values. This should be taken into account when bone isotope values are used as representative of soil compositions (e.g., Depaermentier et al. 2021).
Stable oxygen isotope compositions as a function of strontium isotope ratios. The blue arrow marks the coupled δ18O-87Sr/86Sr variation due to migration. Grey arrows indicate shifts from enamel data towards bone compositions. Estimated soil carbonate 87Sr/86Sr range and δ18O values are from Depaermentier et al. (2021) and from carbonate-water oxygen isotope fractionation calculations (see text)
Unlike the bone data, the enamel data may represent primary compositions that can be used in dietary and migration studies. Most of the Karos and Kenézlő enamel apatite samples overlap in Fig. 6, which is in agreement with the proximity and similar environmental conditions of the sites. However, one of the Kenézlő enamel apatite samples (KF 10.940) plots far away from the other samples and displays the largest 87Sr/86Sr ratio (0.71164) of the present dataset, but a relatively low δ18O value (24.0‰), which is the lowest value among the Kenézlő enamel samples. This difference may be explained by the migration of this individual from a mountainous area that has old crustal rocks with elevated strontium isotope ratios (Mason et al. 1996) and low δ18O values of the precipitation water (Kern et al. 2019). Another Kenézlő enamel sample (KF 1027-t) plots within the Tiszanána enamel group, suggesting that this individual may have been originated from a similar place to Tiszanána. The Tiszanána enamel data have higher 87Sr/86Sr ratios than do the Karos and Kenézlő enamel data, which may mirror the local sedimentary environmental characteristics or may be evidence of migration. The aforementioned locations studied by Depaermentier et al. (2021) are geographically close to the sites researched in this paper and do not show systematic Sr isotope differences along the Tisza river. The Sr isotope difference between the Tiszanána and the Karos-Kenézlő data may derive from inhomogeneities in the fluvial sediments. Alternatively, the Tiszanána enamel data may indicate that the analyzed individuals migrated from a place with higher 87Sr/86Sr ratios. To support the latter possibility, detailed investigations with more samples and additional archaeological information are required, which are beyond the scope of this paper.
Conclusions
Skeletal remains from three tenth century CE cemeteries in northeastern Hungary (Karos, Kenézlő, and Tiszanána) were investigated using a combination of Fourier-transform infrared (FTIR) spectroscopy and C-N–O-Sr isotope geochemistry. The primary aim of this study was to determine the degree of alteration among teeth and bone samples, as well as to select isotope data that may be used to infer primary migration and dietary information. Stable carbon and oxygen isotope compositions of enamel and bone apatite carbonate suggest significant diagenetic alteration, and additional, independent data were supplied by CO3, OH, and PO4 contents by means of FTIR analyses. The FTIR analyses detected surplus carbonate in the bones that was formed by the infiltration of groundwater and secondary carbonate incorporation, whereas the enamel remained unaltered. In contrast, collagen samples extracted from dentin and bone showed only slight alteration on the basis of typical bioarchaeological markers of preservation, such as atomic C:N ratios and C and N contents. This indicates that the collagen C and N isotopic compositions did not undergo diagenetic alteration and are therefore appropriate for dietary analysis. Stable carbon isotope values of enamel apatite carbonate and dentin collagen suggest that C3 plants were the primary plant type consumed but that a significant portion of their vegetal diet also included C4 plants, most likely from millet. Additionally, these observations indicate a dietary similarity with some Eurasian Steppe peoples (e.g., Scythians living north of the Black Sea, Ventresca-Miller et al. 2021) instead of with Avars (sixth–eighth centuries CE, Herold 2008; Noche-Dowdy 2015; Vidal-Ronchas et al. 2019) or medieval Europeans (Gugora et al. 2018; Leggett et al. 2021), although environmental conditions favouring C4 plant cultivation at Karos and Kenézlő can also induce such dietary differences.
Another way that filtering out diagenetically altered samples may be useful is in the interpretation of strontium isotope ratios, specifically relating to migration and diet. Some of the bone apatite 87Sr/86Sr ratios indicate secondary carbonate formation, but the enamel samples do not and thus provide primary Sr information. The stable isotope data from Karos, Kenézlő, and Tiszanána predominantly reflect local compositions, with only limited migrations, as just one individual is assumed to have originated from outside the research area. This study indicates that careful investigation of the effects of diagenetic alteration can lead to the selection of primary compositions that may be used to detect subtle differences in diet and/or migration.
Data availability
The authors confirm that the data supporting the findings of this study are available in the article Supporting Information Material.
Code availability
Not relevant.
References
Alt KW, Knipper C, Peters D, Müller W, Maurer A-F, Kollig I, Nicklisch N, Müller C, Karimnia S, Brandt G, Roth C, Rosner M, Mende B, Schöne BR, Vida T, von Freeden U (2014) Lombards on the move - an integrative study of the migration period cemetery at Szólád Hungary. PLoS ONE 9:e110793. https://doi.org/10.1371/journalpone0110793
Ambrose SH (1990) Preparation and characterization of bone and tooth collagen for isotopic analysis. J Archaeol Sci 17:431–451. https://doi.org/10.1016/0305-4403(90)90007-R
Arneborg J, Heinemeier J, Lynnerup N, Nielsen HL, Rud N, Sveinbjörnsdóttir ÁE (1999) Change of diet in Greenland Vikings determined from stable carbon isotope analysis and 14C dating of their bones. Radiocarbon 41(2):157–168. https://doi.org/10.1017/S0033822200019512
Balassa I (1994) A magyar földművelés emlékei. In: Kovács L (ed) A honfoglalásról sok szemmel: Honfoglalás és régészet; Balassi Kiadó: Budapest, Hungary, p 235–246
Bentley AR (2006) Strontium isotopes from the Earth to the archaeological skeleton: a review. J Archaeol Method Theory 13:135–187. https://doi.org/10.1007/s10816-006-9009-x
Christoph, N, Rossmann, A, Schlicht, C, & Voerkelius, S (2007) Wine authentication using stable isotope ratio analysis: significance of geographic origin, climate, and viticultural parameters. In: Ebeler, SR, Takeoka, GR, Winterhalter, P (Eds), Authentication of Food and Wine ACS Symposium Series; American Chemical Society: Washington, DC, 2006, p 166–179.
Crowley BE, Wheatley PV (2014) To bleach or not to bleach? Comparing treatment methods for isolating biogenic carbonate. Chem Geol 381:234–242. https://doi.org/10.1016/j.chemgeo.2014.05.006
Czuppon G, Demény A, Leél-Őssy S, Óvári M, Molnár M, Stieber J, Kiss K, Kármán K, Surányi G, Haszpra L (2018) Cave monitoring in the Béke and Baradla caves (Northeastern Hungary): implications for the conditions for the formation cave carbonates. Int J Speleol 47:13–28. https://doi.org/10.5038/1827-806X.47.1.2110
Demény A, Schöll-Barna G, Fórizs I, Osán J, Sümegi P, Bajnóczi B (2012) Stable isotope compositions and trace element concentrations in freshwater bivalve shells (Unio sp.) as indicators of environmental changes at Tiszapüspöki, eastern Hungary. Central European Geology 55:441–460. https://doi.org/10.1556/CEuGeol.55.2012.4.5
Demény A, Gugora AD, Kesjár D, Lécuyer C, Fourel F (2019) Stable isotope analyses of the carbonate component of bones and teeth: the need for method standardization. J Archaeol Sci 109:104979. https://doi.org/10.1016/j.jas.2019.104979
DeNiro MJ (1985) Post-mortem preservation and alteration of in-vivo bone collagen isotope ratios in relation to paleodietary reconstruction. Nature 317:806–809. https://doi.org/10.1038/317806a0
Depaermentier MLC, Kempf M, Bánffy E, Alt KW (2020) Tracing mobility patterns through the 6th-5th millennia BC in the Carpathian Basin with strontium and oxygen stable isotope analyses. PLoS ONE 15:e0242745. https://doi.org/10.1371/journal.pone.0242745
Depaermentier MLC, Kempf M, Bánffy E, Alt KW (2021) Modelling a scale-based strontium isotope baseline for Hungary. J Archaeol Sci 135:105489. https://doi.org/10.1016/j.jas.2021.105489
Fórizs, I, Bajnóczi, B, Mozgai, V, Szabó, M, Hegyi, I, & Tóth, M (2017) Isotope geochemistry of carbonates of loess-related soils in Western Hungary. In: Ionete, RE (szerk) The XIVth Workshop of the European Society for Isotope Research - ESIR 2017 : Book of Abstracts Băile Govora, Románia : National Institute of Research and Development for Cryogenic and Isotopic Technologies ICSI (2017) pp 89–90 , 2 p ISBN: 978–973–0–24503–5
Fóthi E, Gonzalez A, Fehér T, Gugora A, Fóthi Á, Bíró O, Keyser C (2020) Genetic analysis of male Hungarian Conquerors: European and Asian paternal lineages of the conquering Hungarian tribes. Archaeol Anthropol Sci 12:31. https://doi.org/10.1007/s12520-019-00996-0
Gamarra B, Howcroft RM, McCall A, Dani J, Hajdú Z, Nagy E, Szabó L, Domboróczki L, Pap I, Raczky P, Marcsik A, Zoffmann Z, Hajdu T, Feeney R, Pinhasi R (2018) 5000 years of dietary variations of prehistoric farmers in the Great Hungarian Plain. PLoS ONE 13:e0197214. https://doi.org/10.1371/journalpone0197214
Garvie-Lok SJ, Varney TL, Katzenberg MA (2004) Preparation of bone carbonate for stable isotope analysis: the effects of treatment time and acid concentration. J Archaeol Sci 31:763–776. https://doi.org/10.1016/j.jas.2003.10.014
Gerling, C, Heyd, V, Pike, A, Bánffy, E, Dani, J, Köhler, K, Kulcsár, G, Kaiser, E, & Schier, W (2012) Identifying kurgan graves in Eastern Hungary: a burial mound in the light of strontium and oxygen isotope analysis. de Gruyter, Berlin, p 12. https://doi.org/10.1515/9783110266306165
Giblin JI (2009) Strontium isotope analysis of Neolithic and Copper Age populations on the Great Hungarian Plain. J Archaeol Sci 36:491–497. https://doi.org/10.1016/jjas200809034
Giblin JI, Yerkes RW (2016) Diet, dispersal and social differentiation during the Copper Age in eastern Hungary. Antiquity 90:81–94. https://doi.org/10.15184/aqy20163
Giblin JI, Knudson KJ, Bereczki Zs, Pálfi Gy, Pap I (2013) Strontium isotope analysis and human mobility during the Neolithic and Copper Age: a case study from the Great Hungarian Plain. J Archaeol Sci 40:227–239. https://doi.org/10.1016/jjas201208024
Gugora A, Dupras TL, Fóthi E (2018) Pre-dating paprika: reconstructing childhood and adulthood diet at medieval (13th century CE) Solt-Tételhegy, Hungary from stable carbon and nitrogen isotope analyses. J Archaeol Sci Reports 18:151–160. https://doi.org/10.1016/jjasrep201712036
Gugora, A, Dupras, TL, Fóthi, E, & Demény, A (2021) New home, new diet? Reconstruction of diet at the 10th century CE Hungarian Conquest period site of Kenézlő-Fazekaszug from stable carbon and nitrogen isotope analyses. Journal of Archaeological Science: Reports, 38 Paper: 103033. https://doi.org/10.1016/j.jasrep.2021.103033
Gugora, A, Demény, A, & Fóthi, E (2022) The impact of social status and biological sex on diet at 10th century CE Hungarian sites from stable carbon and nitrogen isotope analyses. (manuscript in preparation)
Haas J, Demény A (2002) Early dolomitization of Late Triassic platform carbonates in the Transdanubian Range (Hungary). Sediment Geol 151:225–242. https://doi.org/10.1016/S0037-0738(01)00259-7
Hakenbeck SE, Evans J, Chapman H, Fóthi E (2017) Practising pastoralism in an agricultural environment: an isotopic analysis of the impact of the Hunnic incursions on Pannonian populations. PLoS ONE 12:e0173079. https://doi.org/10.1371/journalpone0173079
Hedges REM, Reynard LM (2007) Nitrogen isotopes and the trophic level of humans in archaeology. J Archaeol Sci 34(8):1240–1251. https://doi.org/10.1016/j.jas.2006.10.015
Herold, M (2008) Sex differences in mortality in Lower Austria and Vienna in the Early Medieval period: an investigation and evaluation of possible contributing factors. PhD Dissertation, University of Vienna.
Kellner CM, Schoeninger MJ (2007) A simple carbon isotope model for reconstructing prehistoric human diet. Am J Phys Anthropol 133:1112–1127. https://doi.org/10.1002/ajpa20618
Kern, Z, Demény, A, Persoiu, A, & Hatvani, IG (2019) Speleothem records from the eastern part of Europe and Turkey—discussion on stable oxygen and carbon isotopes. Quaternary, 2, paper 31. https://doi.org/10.3390/quat2030031
Knipper C, Koncz I, Ódor JG, Mende BG, Rácz Z, Kraus S, van Gyseghem R, Friedrich R, Vida T (2020) Coalescing traditions —coalescing people: community formation in Pannonia after the decline of the Roman Empire. PLoS ONE 15:e0231760. https://doi.org/10.1371/journalpone0231760
Koch PL, Tuross N, Fogel ML (1997) The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J Archaeol Sci 24:417–429. https://doi.org/10.1006/jasc.1996.0126
Kontopoulos I, Presslee S, Penkman K, Collins MJ (2018) Preparation of bone powder for FTIR-ATR analysis: the particle size effect. Vib Spectrosc 99:167–177. https://doi.org/10.1016/j.vibspec.2018.09.004
Krueger HW, Sullivan CH (1984) Models for carbon isotope fractionation between diet and bone. In: Turnlund JR, Johnson PE (eds) Stable isotopes in nutrition Washington. American Chemical Society Symposium Series, DC, pp 205–220
Kustár, Á (1993) Adatok a honfoglaló magyarság antropológiájához [Data for the anthropology of the conquering Hungarians]. Diploma thesis Budapest 50 pp. (in Hungarian)
Kustár, Á (1996) A karos-eperjesszögi I-II-III honfoglalás kori temetők embertani vizsgálata [Anthropological examination of the Hungarian Conquering period cemeteries of Karos-Eperjesszög]. In: Révész L A karosi honfoglalás kori temetők [Hungarian Conquering period cemeteries of Karos-Eperjesszög], Miskolc, pp 395–456. (in Hungarian)
Lécuyer C, Balter V, Martineau F, Fourel F, Bernard A, Amiot R, Gardien V, Otero O, Legendre S, Panczer G, Simon L, Martini R (2010) Oxygen isotope fractionation between apatite-bound carbonate and water determined from controlled experiments with synthetic apatites precipitated at 10–37 °C. Geochim Cosmochim Acta 74:2072–2081. https://doi.org/10.1016/j.gca.2009.12.024
Leggett S, Rose A, Praet E, Le Roux P (2021) Multi-tissue and multi-isotope (δ13C, δ15N, δ18O and 87/86Sr) data for early medieval human and animal palaeoecology. Ecology 102(6):e03349. https://doi.org/10.1002/ecy.3349
Longin R (1971) New method of collagen extraction for radiocarbon dating. Nature 230:241–242. https://doi.org/10.1038/230241a0
Mason P, Downes H, Thirlwall MF, Seghedi I, Szakács A, Lowry D, Mattey D (1996) Crustal assimilation as a major petrogenetic process in the East Carpathian Neogene and Quaternary continental margin arc, Romania. J Petrol 37:927–959. https://doi.org/10.1093/petrology/374927
Motuzaite-Matuzeviciute G, Staff RA, Hunt HV, Liu X, Jones MK (2013) The early chronology of broomcorn millet (Panicum miliaceum) in Europe. Antiquity 87(338):1073–1085. https://doi.org/10.1017/S0003598X00049875
Nagy, G, & Révész, L (2003) Karos. Száz Magyar Falu Könyvesháza Kht, Budapest pp 7–66 (in Hungarian).
Noche-Dowdy, L (2015) Multi-isotope analysis to reconstruct dietary and migration patterns of an Avar population from Sajópetri, Hungary, AD 568–895. PhD Thesis, University of South Florida, p 137.
Palmer MR, Edmond JM (1989) The strontium isotope budget of the modern ocean. Earth and Planetary Sci Lett 92:11–26. https://doi.org/10.1016/0012-8
Passey BH, Cerling TE, Levin NE (2007) Temperature dependence of oxygen isotope acid fractionation for modern and fossil tooth enamels. Rapid Commun Mass Spectrom 21:2853–2859. https://doi.org/10.1002/rcm.3149
Person A, Bocherens H, Saliege J, Paris F, Zeitoun V, Gerard M (1995) Early diagenetic evolution of bone phosphate: an X-ray diffractometry analysis. J Archaeol Sci 22:211–221. https://doi.org/10.1006/jasc.1995.0023
Révész, L (1996) A Karos honfoglalás kori temetők: Régészeti adatok a Felső-Tisza-vidék X. századi történetéhez, Miskolc, Hungary: Herman Ottó Múzeum-Magyar Nemzeti Múzeum (in Hungarian).
Révész, L (2008) Heves megye 10–11. századi temetői. Budapest: Magyar Nemzeti Múzeum (in Hungarian).
Révész, L (2014) A magyar honfoglalás kora. Budapest, Hungarian National Museum (in Hungarian).
Révész, L (2020) A 10–11. századi temetők regionális jellemzői a Keleti-Kárpátoktól a Dunáig. Budapest, Hungary: Martin Opitz Kiadó (in Hungarian).
Roche D, Ségalen L, Balan E, Delattre S (2010) Preservation assessment of Miocene-Pliocene tooth enamel from Tugen Hills (Kenyan Rift Valley) through FTIR, chemical and stable-isotope analyses. J Archaeol Sci 37:1690–1699. https://doi.org/10.1016/jjas20100102921X(89)90017-4
Salesse K, Dufour E, Lebon M, Wurster C, Castex D, Bruzek J, Zazzo A (2014) Variability of bone preservation in a confined environment: the case of the catacomb of Sts Peter and Marcellinus (Rome, Italy). Palaeogeogr Palaeoclimatol Palaeoecol 416:43–54. https://doi.org/10.1016/j.palaeo.2014.07.021
Seghedi I, Downes H, Pécskay Z, Thirlwall MF, Szakács A, Prychodko M, Mattey D (2001) Magmagenesis in a subduction-related post-collisional volcanic arc segment: the Ukrainian Carpathians. Lithos 57:237–262. https://doi.org/10.1016/S0024-4937(01)00042-1
Sponheimer M, Lee-Thorp J (1999) Oxygen isotopes in enamel carbonate and their ecological significance. Journal of Archaeological Science 26:723–728. https://doi.org/10.1006/jasc19980388
Spötl C, Vennemann TW (2003) Continuous-flow IRMS analysis of carbonate minerals. Rapid Commun Mass Spectrom 17:1004–1006. https://doi.org/10.1002/rcm1010
Stuart-Williams HLQ, Schwarcz HP, White CD, Spence MW (1996) The isotopic composition and diagenesis of human bone from Teotihuacan and Oaxaca, Mexico. Palaeogeogr Palaeoclimatol Palaeoecol 126:1–14. https://doi.org/10.1016/S0031-0182(96)00066-1
Thomas F, Jamin E, Hammond D (2013) 18O internal referencing method to detect water addition in wines and fruit juices: interlaboratory study. J AOAC Int 96:615–624. https://doi.org/10.5740/jaoacint12-339
Tóth T (1958) Profilation horizontale du crane facial de la population et contemporaine de la Hongrie. Crania Hungarica 3:1–126
Újvári G, Kele S, Bernasconi SM, Haszpra L, Novothny Á, Bradák B (2019) Clumped isotope paleotemperatures from MIS 5 soil carbonates in southern Hungary. Palaeogeogr Palaeoclimatol Palaeoecol 518:72–81. https://doi.org/10.1016/j.palaeo.2019.01.002
Ventresca Miller AR, Johnson J, Makhortykh S, Gerling C, Litvinova L, Andrukh S et al (2021) Re-evaluating Scythian lifeways: isotopic analysis of diet and mobility in Iron Age Ukraine. PLoS ONE 16(3):e0245996. https://doi.org/10.1371/journal.pone.0245996
Vidal-Ronchas R, RajićŠikanjić P, Premužić Z, RapanPapeša A, Lightfoot E (2019) Diet, sex, and social status in the Late Avar period: stable isotope investigations at Nuštar cemetery, Croatia. Archaeol Anthropol Sci 11:1727–1737. https://doi.org/10.1007/s12520-018-0628-4
Wall AJ, Capo RC, Stewart BW, Phan TT, Jain JC, Hakala JA, Guthrie GD (2013) High throughput method for Sr extraction from variable matrix waters and 87Sr/86Sr isotope analysis by MC-ICP-MS. J Anal at Spectrom 28:1338. https://doi.org/10.1039/C3JA30350K
Wang Y, Cerling TE (1994) A model of fossil tooth and bone diagenesis: implications for paleodiet reconstruction from stable isotopes. Palaeogeogr Palaeoclimatol Palaeoecol 107:281–289. https://doi.org/10.1016/0031-0182(94)90100-7
Weiner S, Bar-Yosef O (1990) States of preservation of bones from prehistoric sites in the Near East: a survey. J Archaeol Sci 17:187–196. https://doi.org/10.1016/0305-4403(90)90058-D
Wolf, M, & Révész, L (1996) A magyar honfoglalás korának régészeti emlékei. Miskolc, Hungary: Otto Herman Museum (in Hungarian).
Acknowledgements
The MC-ICP-MS facility of the Institute for Nuclear Research was supported by the European Union and the State of Hungary, co-financed by the European Regional Development Fund in the GINOP-2.3.2-15-2016-00009 ‘ICER’ project. The work was conducted in the framework of a Thematic Excellence Program, financed by the Ministry of Innovation and Technology (TKP2020-NKA-20).
Funding
Open access funding provided by ELKH Research Centre for Astronomy and Earth Sciences. European Union, the State of Hungary, co-financed by the European Regional Development Fund in the GINOP-2.3.2–15-2016–00009; Ministry of Innovation and Technology (TKP2020-NKA-20).
Author information
Authors and Affiliations
Contributions
AG and AD: data interpretation, paper writing; EF: anthropological and archaeological studies; AH and LP: Sr isotope analyses; MK: FTIR analyses and evaluation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gugora, A., Demény, A., Fóthi, E. et al. Detection of diagenetic alteration in bones and teeth for migration and dietary studies — a combined FTIR and C-N–O-Sr isotope study on tenth century CE cemeteries in northern and northeastern Hungary. Archaeol Anthropol Sci 14, 58 (2022). https://doi.org/10.1007/s12520-022-01532-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-022-01532-3