Abstract
Introduction
When electrical storm (ES) is amenable to neither antiarrhythmic drugs, nor deep sedation or catheter ablation, autonomic modulation may be considered. We report our experience with percutaneous left stellate ganglion block (PSGB) to temporarily suppress refractory ventricular arrhythmia (VA) in patients with structural heart disease.
Methods
A retrospective analysis was performed at our institution of patients with structural heart disease and an implantable cardioverter defibrillator (ICD) who had undergone PSGB for refractory VA between January 2018 and October 2021. The number of times antitachycardia pacing (ATP) was delivered and the number of ICD shocks/external cardioversions performed in the week before and after PSGB were evaluated. Charts were checked for potential complications.
Results
Twelve patients were identified who underwent a combined total of 15 PSGB and 5 surgical left cardiac sympathetic denervation procedures. Mean age was 73 ± 5.8 years and all patients were male. Nine of 12 (75%) had ischaemic cardiomyopathy, with the remainder having non-ischaemic dilated cardiomyopathy. Mean left ventricular ejection fraction was 35% (± 12.2%). Eight of 12 (66.7%) patients were already being treated with both amiodarone and beta-blockers. The reduction in ATP did not reach statistical significance (p = 0.066); however, ICD shocks (p = 0.028) and ATP/shocks combined were significantly reduced (p = 0.04). At our follow-up electrophysiology meetings PSGB was deemed ineffective in 4 of 12 patients (33%). Temporary anisocoria was seen in 2 of 12 (17%) patients, and temporary hypotension and hoarseness were reported in a single patient.
Discussion
In this limited series, PSGB showed promise as a method for temporarily stabilising refractory VA and ES in a cohort of male patients with structural heart disease. The side effects observed were mild and temporary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
-
A percutaneous block of the left stellate ganglion appeared effective in reducing the ventricular arrhythmia burden in patients with refractory ventricular arrhythmia in the presence of advanced structural heart disease in whom conventional treatments are either ineffective or technically infeasible.
-
A percutaneous block can be performed at the bedside using ultrasound. Though its effects are temporary, it can be used as a bridge to a more definitive treatment such as surgical left cardiac sympathetic denervation.
-
Only mild temporary side effects were seen.
Introduction
Ventricular arrhythmia (VA) can develop in apparently stable patients with structural heart disease due to subclinical neurohumoral hyperactivation and ventricular remodelling [1]. The most severe form of VA, electrical storm (ES), constitutes an important cause of morbidity and mortality, carrying a relative mortality risk of 3.15 [2]. Guideline-recommended first-line treatment aims to eliminate VA triggers and modulators by suppressing the cardiac sympathetic tone using a combination of beta-blockers, amiodarone and/or sedation [3]. Urgent catheter ablation (CA) and/or deep sedation are recommended when the former fail to control VA. However, CA might not be feasible for a variety of reasons, such as haemodynamic instability, substrate not amenable to ablation, ventricular thrombus or left-sided mechanical valvular prosthesis. Logistical considerations may also be a limiting factor in some centres. The temporary nature of deep sedation implies that it is primarily a bridge to more definitive treatment. The 2022 ESC guideline now classifies modulation of the autonomic nervous system (ANS) as a class IIB recommendation in selected patients with refractory VA ([3]; Fig. 1).
Coumel’s triangle of arrhythmogenesis provides a model for summarising the chief factors underlying ventricular arrhythmia in structural heart disease. Autonomic modulation by means of percutaneous stellate ganglion block and left cardiac sympathetic denervation aim to suppress the extrinsic sympathetic nervous system. This is suspected to be a prime modulator of arrhythmia by influencing both the trigger through altering myocardial calcium handling, which predisposes to early and later afterdepolarisations, as well as the substrate by altering conduction in the scar border zone
The pro-arrhythmogenic effects of the sympathetic branch of the ANS in structurally abnormal hearts have been described extensively [4]. Neural remodelling results in areas of sympathetic hyperinnervation and relative denervation predisposing to autonomically mediated heterogeneity of refractory periods. Cellular electrical remodelling predisposes to neurotransmitter-mediated intracellular Ca2+ overload and delayed afterdepolarisations. The sympathetic fibres innervating the ventricular myocardium derive from the ganglia of the cervicothoracic paravertebral chain, primarily from the left and right stellate ganglia (C7‑8 to Th1-2) [5, 6]. Surgical excision of the left stellate ganglion by means of video-assisted thoracoscopic left cardiac sympathetic denervation (LCSD) has been shown to reduce the VA burden in catecholaminergic polymorphic ventricular tachycardia and congenital long QT syndromes [7, 8]. More recently, a reduction in VA burden has also been described following percutaneous stellate ganglion block (PSGB) in patients with structural heart disease [9,10,11,12,13,14,15]. This percutaneous approach has the advantage of being a bedside intervention using ultrasound guidance, while major complications are rare [16]. Although its effects are temporary, expected to last no more than 2 weeks, PGSB provides a time window for scheduling definitive therapy. Since 2018, PSGB and LCSD procedures have been performed at our centre in selected patients with refractory VA.
Methods
Study population
This study was performed at the Medisch Spectrum Twente, Enschede, the Netherlands. Patients were identified by screening the weekly electrophysiology meetings between 1 January 2018 and 1 October 2021. Fourteen patients > 18 years of age with structural heart disease who had PSGB performed for refractory VA were identified. In two patients follow-up was incomplete, as they were transported to a transplantation centre following PSGB, so they were excluded.
Definitions
Sustained VT was defined as having a cycle length < 600 ms with a duration of ≥ 30 s or resulting in either antitachycardia pacing (ATP) or (intracardiac) defibrillation. ES was defined as a cluster of three or more episodes of either sustained VT, ventricular fibrillation or delivery of ATP or an electrical shock by a defibrillator in ≤ 24 h [17, 18]. Structural heart disease was considered to be either a left ventricular ejection fraction of ≤ 50% or the presence of late gadolinium enhancement on cardiac MRI.
Patient selection
Ventricular arrhythmia was treated according to the European Society of Cardiology Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death guideline and the judgement of the treating physician [3]. This generally consisted of antiarrhythmic drugs, sedation and CA when possible. PSGB was considered when VA was refractory to, or not amenable to, the aforementioned treatments. Cases in which PSGB was considered were discussed at our multidisciplinary electrophysiology meeting beforehand. In the case of ES an emergency meeting was scheduled. Only when other options such as CA were not feasible, for example a known inability to approach the substrate or severe comorbidities, was PSGB considered.
Ultrasound-guided PSGB
Percutaneous stellate ganglion block was performed by one of three anaesthesiologists with extensive experience with PSGB for chronic pain syndromes. We decided to perform a left-sided block in all cases. This decision was based on previous studies on LCSD in channelopathies as well as animal research, which suggests that the left stellate ganglion generally innervates a larger portion of the left ventricular myocardium [11, 19,20,21,22,23].
The procedure was performed at the bedside at the Cardiac Care Unit using an ultrasound-guided paratracheal approach. Ultrasound was used to visualise critical structures, including the carotid and vertebral arteries, the left jugular vein, the 6th cervical vertebra, the longus colli muscle and the stellate ganglion, in order to reduce the risk of major vascular complications [24]. Direct oral anticoagulants were discontinued 24 h in advance when possible. An INR of < 2.0 was desired if a vitamin‑K antagonist was administered.
While supine the patient’s head was rotated to the right and a sterile field was created around the neck. After local anaesthesia of the skin a 5-cm echogenic needle (Pajunk, Geisingen, Germany) was inserted in plane from lateral towards medial using the paratracheal approach with a route dorsal to the carotid artery and jugular vein to the stellate ganglion area. An aspiration test was performed and, if negative, the injection was performed. Initially, the type of anaesthetic agent used was determined by the operator. Subsequently, a protocol was implemented whereby 5 ml levobupivacaine 0.25% and 10 mg dexamethasone were used. Dexamethasone is co-administered to shorten the time to onset of the block and to increase the duration for up to 2 weeks [25].
Study endpoints
The primary outcome was the number of times ATP was delivered and the number of ICD shocks or external cardioversions performed for VA in the week before and the week following PSGB. External cardioversions were counted as shocks. This period was chosen to both account for the daily variation in VA episodes as well as PSGB being used as a bridge to a more definitive treatment which would influence the arrhythmia burden. Due to limitations of the patient monitoring system and ICD therapy being disabled in several patients with ES, we were unable to accurately reconstruct the exact onset of VA on the day of PSGB in several cases, and this was therefore not reported. Charts were checked for potential complications, such as local haematoma, paravertebral haematoma, vascular damage, or neurological symptoms.
Statistical analysis
Baseline characteristics (Tab. 1) were evaluated with a mean ± standard deviation being reported. Categorical data are presented as number/total cases (percentage). Event rates before and after PSGB were compared using the Wilcoxon signed rank test. A two-tailed probability value of < 0.05 was considered statistically significant. All statistical analyses were performed with IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp., Armonk, NY, USA).
Results
Twelve patients were identified who underwent PSGB for refractory VA and underlying structural heart disease during the study period. Median follow-up was 601 days (± 529). All patients were men, aged between 51 and 85 years of age, with monomorphic VT (Tab. 1). Gender did not play a role in patient selection for PSGB, and autonomic modulation has been performed for various indications in both genders at our centre. Time between either initial diagnosis of a cardiomyopathy or first myocardial infarction and PSGB was a mean of 19 years (± 8.5 years). The majority of patients (75%) had ischaemic cardiomyopathy with the remainder having a non-ischaemic dilated cardiomyopathy. Median left ventricular ejection fraction was 32% (± 12%). Endocardial VT ablation had previously been performed in 9 of 12 patients (75%). In the remaining 3 patients prior VT ablation had been deferred either due to comorbidity or because important technical limitations were expected due to patient-related factors. At baseline, 8 of 12 (67%) patients were already being treated with the combination of both beta-blocker and amiodarone. Three were on sotalol, and one patient was treated with bisoprolol (Tab. 2).
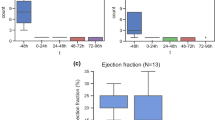
After PSGB a reduction in the absolute number of ATP episodes and ICD shocks was seen in 10 of 12 patients (83%) (Fig. 2), which was significant (p = 0.04) In the entire cohort the decrease in ATP, although showing a clear trend, did not reach statistical significance (p = 0.066), whereas ICD shocks did decrease significantly (p = 0.028). One patient ultimately developed refractory incessant VT after an initial reduction in VA (patient 12). In the presence of advanced heart failure and severe comorbidities treatment was discontinued. In total, 4 of 12 (33%) patients died during long-term follow-up at a median of 555 days (54–1327). The causes of death were refractory VA (one case), worsening heart failure/malignancy (one case) and complicated pneumonia (two cases) (Tab. 3).
Antitachycardia pacing (ATP) and implantable cardioverter defibrillator (ICD) shocks/external cardioversions delivered in the week before and following percutaneous left stellate ganglion block (PSGB). aSymptomatic recurrent slow ventricular tachycardia (VT) was not always registered by the ICD and thus the VT burden could not be reconstructed. bEstimated VT burden. The exact burden could not be retrospectively reconstructed due to limitations of the inpatient recording system
In three patients a second or even third PSGB was performed as a bridge to LCSD after VA recurred more than a week after the initial block. Two PSGBs resulted in a VT-free interval of 12 and over 14 days, allowing LCSD to be scheduled. In the third patient VT recurred at day 3 in the context of a COVID-19 infection.
Following PSGB one patient complained of temporary hoarseness. Anisocoria was reported in 2 of 12 (17%) blocks. In one patient temporary hypotension during pre-existing therapy-refractory incessant VT was seen immediately following PSGB but resolved after ‘spontaneous’ conversion to sinus rhythm 2 h later. No vascular complications were observed.
LCSD was performed in five patients following PSGB. Antiarrhythmic drugs had been unchanged in the weeks before and following surgery. One patient with cessation of VA following PSGB had a recurrence of ES 8 days after LCSD. We are unable to explain this difference in response, as his treatment had otherwise been unchanged before and following both PSGB and LCSD. In one patient with a mid-septal VT circuit not amenable to ablation LCSD did not reduce the VA burden, but higher amiodarone levels resulted in adequate VA suppression. The other three patients had substantial reduction of their VA burden with one remaining completely free of VA for 15 months, but he passed away due to a combination of disseminated bladder cancer and worsening heart failure. A second patient with 2281 instances of ATP in the 6 weeks preceding LCSD had only 1 h of recurrent VA terminated by ATP 13 days after LSCD. Only non-sustained VT was seen 6 months after LCSD. After these 6 months VT occurred only infrequently, but could be terminated by ATP. The third patient had only two episodes of VT terminated by ATP in the year following LCSD and a recurrence of ES a year later.
Discussion
We found PSGB to be efficacious in stabilising recurrent drug-refractory VA in the majority of patients with severe structural heart disease in whom urgent CA was deemed to be either technically infeasible or to carry an unacceptably high risk. We consider the comparative simplicity of the procedure and low risk of major complications to be advantages of PGSB. Another potential benefit of PGSB is identifying patients who could benefit from LCSD for long-term suppression of VA.
There are several limitations to the data presented here. With antiarrhythmic drugs and CA being the preferred treatment for VA, there is an implicit selection bias in opting for autonomic modulation. It was not feasible to create a matched historical cohort. Thus, in the absence of a control population the reduction in VA burden might in part be attributed to a late effect of the antiarrhythmic drugs administered. However, with two-thirds of patients already receiving long-term treatment with amiodarone/beta-blocker, an antiarrhythmic effect of additional amiodarone would not be expected. The absence of females in this series is also notable. Gender was not a factor when considering patients for autonomic modulation.
We speculate that some non-responders might have benefitted from a subsequent right-sided PSGB, as animal research has demonstrated that in canines the left and right stellate ganglion innervate different segments of the myocardium. It is plausible that the sympathetic innervation pattern is similar in humans [6, 21,22,23]. Secondly, in non-responders, e.g. those with scar-related arrhythmias, the ANS might be only a minor modulator. Finally, ineffective blockade of the stellate ganglion might be a factor in non-responders. However, this is unlikely, as extensive spread of agents injected into the paravertebral space has been previously demonstrated [26,27,28,29]. Methods to quantify cardiac autonomic tone would certainly be helpful in differentiating between the above hypotheses. We found traditional ANS markers, such as QTc dispersion and heart rate variability, to be impracticable in our patients due to the presence of frequent ventricular ectopia and/or permanent (bi-)ventricular pacing or antiarrhythmic drugs. MIBG (metaiodobenzylguanidine) scans were also logistically infeasible in unstable patients. Further research into novel methods, such as sensory skin nerve activity, are of particular interest for evaluating the response to PSGB and potentially selecting patients who could benefit from autonomic modulation.
Limitations
Only a limited number of patients were studied. PSGB/LCSD was performed only on male patients during the study period. No reliable data are available on the exact change in VA in the first 24 h following PSGB. Moreover, a reliable method is lacking for measuring the cardiac autonomic tone before and following PSGB in patients with refractory VA.
Conclusion
In this retrospective series, left-sided PSGB showed significant promise as a treatment to temporarily suppress VA and ES in patients with extensive structural heart disease considered not amenable to urgent CA. Cessation of VA was seen in several patients for whom no other evidence-based acute treatment options were available, allowing definitive treatment to be scheduled. Further randomised studies are required to determine the role PSGB might play in the treatment of VA. Moreover, methods for quantifying the change in cardiac sympathetic tone, which has been achieved following a percutaneous block, are of particular interest.
References
Borovac JA, D’Amario D, Bozic J, et al. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J Cardiol. 2020;12:373–408.
Guerra F, Shkoza M, Scappini L, et al. Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: A meta-analysis. Europace. 2014;16:347–53.
Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997–4126.
Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–15.
Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–21.
Irie T, Yamakawa K, Hamon D, et al. Cardiac sympathetic innervation via middle cervical and stellate ganglia and antiarrhythmic mechanism of bilateral stellectomy. Am J Physiol Heart Circ Physiol. 2017;312:H392–405.
Schwartz PJ, Priori SG, Cerrone M, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–33.
de Ferrari GM, Dusi V, Spazzolini C, et al. Clinical management of catecholaminergic polymorphic ventricular tachycardia: The role of left cardiac sympathetic denervation. Circulation. 2015;131:2185–93.
Fudim M, Qadri YJ, Waldron NH, et al. Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias. JACC Clin Electrophysiol. 2020;6:562–71.
Reinertsen E, Sabayon M, Riso M, et al. Stellate ganglion blockade for treating refractory electrical storm: A historical cohort study. Can J Anaesth. 2021;68:1683–9.
Meng L, Tseng C‑H, Shivkumar K, et al. Efficacy of stellate ganglion blockade in managing electrical storm: A systematic review. JACC Clin Electrophysiol. 2017;3:942–9.
Tian Y, Wittwer ED, Kapa S, et al. Effective use of percutaneous stellate ganglion blockade in patients with electrical storm. Circ Arrhythm Electrophysiol. 2019;12:e7118.
Fudim M, Boortz-Marx R, Patel CB, et al. Autonomic modulation for the treatment of ventricular arrhythmias: therapeutic use of percutaneous stellate ganglion blocks. J Cardiovasc Electrophysiol. 2017;28:446–9.
Fudim M, Boortz-Marx R, Ganesh A, et al. Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias: A systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2017;28:1460–7.
Savastano S, Dusi V, Baldi E, et al. Anatomical-based percutaneous left stellate ganglion block in patients with drug-refractory electrical storm and structural heart disease: A single-centre case series. Europace. 2021;23:581–6.
Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: A systematic review. Reg Anesth Pain Med. 2019;44:669–78.
Exner D v, Pinski SL, Wyse DG, et al. Electrical storm presages nonsudden death: The antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation. 2001;103:2066–71.
Greene M, Newman D, Geist M, et al. Is electrical storm in ICD patients the sign of a dying heart? Outcome of patients with clusters of ventricular tachyarrhythmias. Europace. 2000;2:263–9.
Schwartz PJ, Snebold NG, Brown AM. Effects of unilateral cardiac sympathetic denervation on the ventricular fibrillation threshold. Am J Cardiol. 1976;37:1034–40.
García-Calvo R, Chorro FJ, Sendra M, et al. The effects of selective stellate ganglion manipulation on ventricular refractoriness and excitability. Pacing Clin Electrophysiol. 1992;15:1492–503.
Vaseghi M, Zhou W, Shi J, Ajijola OA, et al. Sympathetic innervation of the anterior left ventricular wall by the right and left stellate ganglia. Heart Rhythm. 2012;9:1303–9.
Opthof T, Misier AR, Coronel R, et al. Dispersion of refractoriness in canine ventricular myocardium. Effects of sympathetic stimulation. Circ Res. 1991;68:1204–15.
Yanowitz F, Preston JB, Abildskov JA. Functional distribution of right and left stellate innervation to the ventricles. Production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ Res. 1966;18:416–28.
Aleanakian R, Chung B‑Y, Feldmann RE, et al. Effectiveness, safety, and predictive potential in ultrasound-guided stellate ganglion blockades for the treatment of sympathetically maintained pain. Pain Pract. 2020;20:626–38.
Huynh TM, Marret E, Bonnet F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: A meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2015;32:751–8.
Christie JM, Martinez CR. Computerized axial tomography to define the distribution of solution after stellate ganglion nerve block. J Clin Anesth. 1995;7:306–11.
Feigl GC, Rosmarin W, Stelzl A, et al. Comparison of different injectate volumes for stellate ganglion block: an anatomic and radiologic study. Reg Anesth Pain Med. 2007;32:203–8.
Honma M, Murakami G, Sato TJ, et al. Spread of injectate during C6 stellate ganglion block and fascial arrangement in the prevertebral region: An experimental study using donated cadavers. Reg Anesth Pain Med. 2000;25:573–83.
Guntamukkala M, Hardy PA. Spread of injectate after stellate ganglion block in man: An anatomical study. Br J Anaesth. 1991;66:643–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N.P. Monteiro de Oliveira reports research grants from Pioneers in Health Care, Averitas Pharma Inc. and Stayble Therapeutics. V.R. van der Pas, J.M. van Opstal, M.F. Scholten, R.G.H. Speekenbrink and P.F.H.M. van Dessel declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Pas, V.R., van Opstal, J.M., Scholten, M.F. et al. Percutaneous left stellate ganglion block for refractory ventricular tachycardia in structural heart disease: our single-centre experience. Neth Heart J (2024). https://doi.org/10.1007/s12471-024-01880-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s12471-024-01880-w