Abstract
Background
Stellate ganglion blockade (SGB) has been used to treat electrical storm (ES) refractory to antiarrhythmic therapy or to stabilize patients before more definitive intervention. Nevertheless, its efficacy is not well understood, with only a few case reports and retrospective case series in the literature.
Methods
We conducted a historical cohort study on patients with drug-refractory ES who underwent ultrasound-guided unilateral SGB from 1 January 2010 until 19 July 2019 at two hospital sites. Stellate ganglion blockade was performed with variable combinations of bupivacaine, lidocaine, ropivacaine, and dexamethasone. We collected data on demographic and procedural characteristics, the number of arrhythmias and defibrillation episodes, antiarrhythmic and anticoagulant medication, left ventricular ejection fraction (EF), and respiratory support requirement.
Results
We identified N = 13 patients; their mean (standard deviation [SD]) age was 64 (13) yr, and 10 (77%) were male. The baseline mean (SD) number of overall arrhythmia and defibrillation episodes per day were 9 (6) and 4 (3), respectively; the mean (SD) pre-SGB EF was 23 (7)%. Seven patients (54%) received dexamethasone in addition to local anesthetic for SGB. One patient experienced hypotension after SGB. Arrhythmias and defibrillation episodes significantly decreased at 24, 48, 72, and 96 hr after SGB; at 96 hr, 62% and 92% of patients had no VA and defibrillation episodes, respectively (P < 0.001 for all time points). Ejection fraction and the number of patients receiving antiarrhythmic medications or requiring respiratory support were unchanged.
Conclusions
Unilateral SGB was associated with a reduction in arrhythmias and defibrillation episodes, but did not affect antiarrhythmic medication, respiratory support, or EF. Randomized controlled trials on larger cohorts are needed to confirm these findings.
Résumé
Contexte
Le bloc du ganglion stellaire (BGS) a été employé pour traiter les tempêtes électriques réfractaires à la thérapie antiarythmique ou pour stabiliser les patients avant une intervention plus définitive. Néanmoins, son efficacité n’est pas bien comprise, et il n’existe que quelques présentations de cas et séries de cas rétrospectives dans la littérature.
Méthode
Nous avons mené une étude de cohorte historique auprès de patients souffrant de tempêtes électriques réfractaires aux médicaments qui ont subi un BGS unilatéral échoguidé entre le 1er janvier 2010 et le 19 juillet 2019 dans deux sites hospitaliers. Le bloc du ganglion stellaire a été réalisé à l’aide de combinaisons variables de bupivacaïne, de lidocaïne, de ropivacaïne et de dexaméthasone. Nous avons colligé les données touchant aux caractéristiques démographiques et procédurales, au nombre d’arythmies et d’épisodes de défibrillation, aux traitements antiarythmique et anticoagulant, à la fraction d’éjection (FE) ventriculaire gauche, et au besoin d’assistance respiratoire.
Résultats
Nous avons identifié N = 13 patients; leur âge moyen (écart type [ÉT]) était de 64 (13) ans, et 10 (77 %) patients étaient des hommes. Globalement, le nombre moyen (ÉT) d’épisodes d’arythmie et de défibrillation de base par jour était de 9 (6) et 4 (3), respectivement; la FE moyenne (ÉT) pré-BGS était de 23 (7) %. Sept patients (54 %) ont reçu de la dexaméthasone en plus de l’anesthésique local pour le BGS. Un patient a souffert d’hypotension après le BGS. Les arythmies et les épisodes de défibrillation ont diminué de manière significative à 24, 48, 72, et 96 heures après le BGS; à 96 heures, 62 % et 92 % des patients ne subissaient plus aucun épisode d’arythmie ventriculaire et de défibrillation, respectivement (P < 0,001 pour tous les temps). La fraction d’éjection et le nombre de patients recevant des médicaments antiarythmiques ou nécessitant une assistance respiratoire sont demeurés inchangés.
Conclusion
Un BGS unilatéral a été associé à une réduction des épisodes d’arythmies et de défibrillation, mais n’a pas eu d’impact sur le traitement antiarythmique, l’assistance respiratoire, ou la FE. Des études randomisées contrôlées réalisées avec des cohortes plus importantes sont nécessaires pour confirmer ces résultats.
Similar content being viewed by others
Electrical storm (ES) is defined as three or more episodes of ventricular arrhythmia (VA) over 24 hr or sustained VA for more than 12 hr.1 The primary arrhythmia is typically monomorphic ventricular tachycardia (VT), but can also be polymorphic VT or ventricular fibrillation (VF).2 Electrical storm occurs in patients with severe structural heart disease and/or channelopathies such as Brugada and long QT syndromes.2 The development of ES is associated with death, heart transplantation, and hospitalization.3,4
Electrical storm is acutely managed via sympathetic blockade with beta blockers, correcting triggers (electrolyte disturbances, ischemia, etc.), antiarrhythmic medications (amiodarone, lidocaine), and advanced cardiac life support. Intubation and sedation can blunt sympathetic drive and reduce the frequency of VAs. Intravenous amiodarone is the first line antiarrhythmic agent for treating VT with reduced risk of defibrillations and improved survival.1,2,5 In patients with ES refractory to medical management, catheter radiofrequency VT ablation, renal sympathetic denervation, or cardiac sympathetic denervation are more definitive therapeutic options, although unfeasible in unstable patients.2,5,6,7,8,9
Bedside interventions include thoracic epidural anesthesia and percutaneous stellate ganglion blockade (SGB).5 Cardiac sympathetic outflow emerges from the T1-5 spinal levels.10 High thoracic epidural sympatholysis showed therapeutic response in 54% of 11 patients with ES but is contraindicated in patients treated with anticoagulant and antiplatelet drugs because of possible epidural hematoma and negative hemodynamic consequences.5 Moreover, reductions in ventricular contractility may be poorly tolerated by patients with limited cardiac reserve.10
Sympathetic neurons from the upper thoracic spine transmit excitatory signals through the cervicothoracic (stellate) ganglion as well as cervical sympathetic ganglia.10,11,12 Stellate ganglion block is a minimally invasive procedure that transiently blocks sympathetic outflow, thus serving as a temporizing measure for patients with ES if medical management fails.13 Case reports,14,15,16,17,18 three retrospective series,19,20,21 and two systematic reviews13,19 of SGB for ES have reported benefit. We conducted a historical cohort study to evaluate VA episodes, defibrillations, patients requiring antiarrhythmic medications and respiratory support, and EF before vs after SGB. We also contextualize SGB with the broader literature and propose a framework for managing ES.
Methods
This study was approved by the Emory Institutional Review Board. We queried our database for patients above the age of 18 who underwent SGB from 1 January 2010 until 19 July 2019 at Emory University Hospital and Emory University Hospital Midtown (Atlanta, GA, USA). Patients with Current Procedural Terminology codes for SGB and with an International Classification of Diseases-10 code for heart failure were included. Of these, patients who were admitted to critical care units, who underwent SGB and who had three or more sustained episodes of VT, VF, or shocks from an implantable cardioverter defibrillator (ICD) within 24 hr, were identified by chart review to confirm the reason for SGB. Patients who underwent SGB for other reasons were excluded. Patients receiving SGB for recurrent drug-refractory VT and/or VF were included in the final analysis.
Unilateral SGBs were performed at the bedside by one of six anesthesiologists staffing the Regional Anesthesia and Acute Pain Medicine service via ultrasound guidance and the lateral approach described previously.22 For each patient, arrhythmia episodes, defibrillation episodes, administration of antiarrhythmic medications, and requirement of respiratory support were determined at five time-windows relative to SGB: − 48-0, 0-24, 24-48, 48-72, and 72-96 hr. Left ventricular ejection fraction (EF) was obtained via transthoracic echocardiography (TTE). The pre-SGB EF was obtained from the first TTE performed from admission up until SGB and the post-SGB EF was obtained from the first TTE performed after the SGB.
At each time point, we evaluated the number of arrhythmia and defibrillation episodes, number of patients receiving antiarrhythmic medications, number of patients requiring respiratory support, and EF. A paired two-sided Wilcoxon signed-rank test was performed with α = 0.05. Data were analyzed in Python (Python Software Foundation, Beaverton, OR, USA).
Results
Thirteen patients met the inclusion criteria. Four of these patients were excluded from intensive care unit (ICU) length of stay calculations because they were transferred to Emory University Hospital for advanced therapies including left ventricular assist devices and heart transplants, which could confound the analysis of length of stay due to ES. Baseline characteristics of the cohort, including arrhythmia type, subsequent surgical cardiac sympathectomy, antiarrhythmic drugs administered, and site and type of injectate, are shown in the Table. Ten (77%) were male, the mean (standard deviation [SD]) age was 64 (13) yr, ICU length of stay was 16 (9) days, overall arrhythmia episodes per day was 9 (6), and overall defibrillation episodes per day were 4 (3) (Electronic Supplementary Material [ESM] eTable). The number of patients in the cohort did not change over time. Injectate was chosen at the discretion of the anesthesiologist. For 11 blocks, bupivacaine was injected; of these, 9 (82%) were performed using the 0.25% concentration and two (18%) were with the 0.5% concentration. Lidocaine 2% was used for one injection and ropivacaine 0.2% for one injection. Dexamethasone was added in 54% of SGBs to prolong duration of nerve blockade; doses ranged from 4 to 12 mg with the 8-mg dose used in 57%. The volume of injectate varied from 8 to 20 mL; the average volume injected was 11 mL. Of the blocks, 85% were performed on the left, 15% on the right, and none bilaterally; 93% were performed at the C6 level and one block was performed at the C5 level because the carotid artery was close to the C6 Chassaignac’s tubercle, precluding a safe lateral approach. For this injection, 12 mL was utilized given the greater distance from the stellate ganglion. Ninety-two percent of SGBs were performed on patients receiving anticoagulant and antiplatelet medications, including warfarin, enoxaparin, clopidogrel, ticagrelor, heparin intravenous infusion, subcutaneous heparin, and aspirin; 38% of patients received two or more anticoagulant or antiplatelet drugs simultaneously. One patient experienced hypotension after SGB, but no other complications were noted. Horner’s syndrome and hoarseness were not assessed due to confounding with infused vasopressors and intubation limiting evaluation. One patient (7.7%) required repeated SGB.
After SGB, significant reductions were observed in the number of VA (Figure 1A) and defibrillation (Figure 1B) episodes at all time points. At the last window (96 hr), 62% and 92% of patients had no VA episodes and defibrillation episodes, respectively. The mean (SD) EF across the cohort was 23 (7)% pre-SGB and 22 (7)% post-SGB (P = 0.32; Figure 1C). The number of patients receiving antiarrhythmic medications or requiring respiratory support also did not change after SGB (ESM eTable).
Total number of A) arrhythmia episodes (P < 0.001 for all time points versus pre-SGB baseline) and B) defibrillation episodes (P < 0.001 for all time points versus pre-SGB baseline) from all patients in the series (y axis) vs time (hr) relative to stellate ganglion block (SGB; x axis). An asterisk above a column indicates a statistically significant difference (P < 0.05) at the time point of that column vs -48 to 0 hr relative to SGB, via two-sided Wilcoxon signed-rank test. C) Left ventricular ejection fraction (y axis; percent) before ES (pre-storm) vs after SGB (post-SGB) (P = 0.32). The box shows quartiles and the whiskers show the 1.5 interquartile ranges of the lower and upper quartiles. ES = electrical storm, SGB = stellate ganglion blockade.
Discussion
In our single-institution historical cohort study of a series of 13 patients with ES, SGB was associated with a rapid and significant reduction in VA and defibrillation episodes, which persisted for 96 hr. Nevertheless, EF and the number of patients receiving antiarrhythmic drugs or requiring respiratory support did not change after SGB in the time frame of this study. Electrical storm may be due to the interplay between structural, autonomic, and electrophysiologic vulnerability as well as triggers such as electrolyte disturbances or ischemia.2 Electrical storm is associated with a 3.4-fold increased combined risk of death, cardiac transplantation, or hospitalization for severe heart failure or cardiogenic shock.3 In unstable patients with ES for whom optimal medical management has failed, SGB can reduce VA episodes and bridge patients to more definitive therapy.
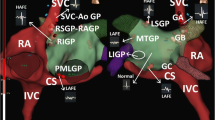
Autonomic sympathetic dysregulation initiates and maintains VAs.5 Preganglionic myocardial sympathetic neurons originate in the intermediolateral cell column of the thoracic spinal cord and synapse on noradrenergic cardiac nerves located in the paravertebral cervical and thoracic ganglia including the stellate (cervicothoracic), ventral/vertebral, middle, and superior cervical ganglia.10,12,23 Direct percutaneous blockade of the stellate ganglion, located at the C7-T1 vertebral level, is challenging because of its proximity to the lung apex, exposed vertebral artery, and costocervical trunk.11 The cervical sympathetic chain is located deep to the cervical prevertebral fascia but superficial to the longus colli muscle13,23 (Figs 2A-C). Injecting 5 mL of fluid with ultrasound guidance at the C6 middle cervical ganglion has shown reliable spread from C4 to T1, encompassing the stellate ganglion.12,23,24
A) Schematic depiction of anterior and axial views with selected anatomical structures involved in middle cervical and stellate ganglion sympathetic blockade. B) Ultrasound image at left C6 level depicting lateral needle block trajectory (green arrow) to the middle cervical sympathetic ganglion/stellate ganglion (red asterisk). AT = anterior tubercle, C = carotid artery, E = esophagus, IJ = internal jugular vein (compressed), LCa = longus capitis muscle, LCo = longus colli muscle, PVF = prevertebral fascia, SCM = sternocleidomastoid muscle, T = trachea, Th = thyroid gland. C) Cervical magnetic resonance imaging, transverse/axial T2 sequence at C6 vertebral body level, depicting lateral needle trajectory (green arrow) for stellate ganglion block and surrounding anatomical structures; red star = middle cervical sympathetic ganglion, AS = anterior scalene muscle, AT = anterior tubercle of C6 (Chassaignac’s tubercle), C = carotid artery, CPM = cervical paraspinal muscles, E = esophagus, IJ = internal jugular vein, LC = longus colli muscle, MS = middle sclene muscle, NR = nerve root, PVF = prevertebral fascia, SCM = sternocleidomastoid muscle, T = trachea, Th = thyroid, VA = vertebral artery, VB = vertebral body, SC = spinal cord
The literature on SGB for ES consists of case reports, three case series,20,21,25 and two systematic reviews.13,19 In 2017, Meng et al. evaluated 38 patients across 23 studies and reported age, sex, prevalence of ischemic cardiomyopathy, mean left ventricular EF, type of local anesthetic used for SGB, VA burden and ICD shocks before vs after SGB, and survival to discharge. The mean number of VA episodes per day decreased from 12.4 to 1.04, although the time windows pre- and post-SGB were not defined. That same year, Fudim et al. published their literature review and reported similar results from 35 patients across 22 unique case series.19 Mean VA episodes per day decreased from 16.5 to 1.4 in the 24 hr pre- vs post-SGB, which is a more rapid decrease in VA episodes than observed in our cohort. The distributions of demographic information, type and dose of anesthetic agent, and mean EF were similar between the two reviews, although many of the same studies were cited. Both reviews reported a similar majority of ischemic cardiomyopathy, order-of-magnitude decrease in number of VA episodes per day, and lack of significant complications.
In our cohort, 62% of patients did not have VA episodes at 96 hr, and 92% were free from defibrillation episodes. Although Fudim et al. note that publication bias can result in the exclusion of ineffective or neutral cases from being published as case series and thus not appear in systematic reviews, the prevalence of VA or defibrillation episodes before vs after SGB reported in systematic reviews are consistent with what is reported in retrospective case series, including ours.
We propose that SGB has a role in the management of refractory ES. When the VA is unresponsive to antiarrhythmic drugs, defibrillation, treatment of reversible contributors, and ICD reprogramming, we recommend concurrent evaluation for mechanical circulatory support, intubation and sedation, catheter ablation, and SGB. While SGB can provide temporary benefit, after the procedure we recommend concurrent evaluation for repeat left-sided SGB, bilateral SGB, a left stellate ganglion catheter, or surgical cardiac sympathetic denervation.19 While high thoracic epidural anesthesia has also provided sympatholysis, the absolute contraindication in anticoagulated patients renders it less useful in most refractory ES patients. Most published cases of SGB utilize a left-sided approach except when impractical because other hardware or aberrant anatomy is present, where a right-sided block could be performed instead. Caution is recommended regarding bilateral SGB due to potential risks including airway compromise from bilateral recurrent laryngeal nerve block as well as respiratory impairment with possible bilateral phrenic blockade, although Fudim’s series of 20 consecutive bilateral SGBs revealed no cases of dyspnea in eight spontaneously ventilating patients.25 While the ideal injected local anesthetic remains unclear, we recommend a longer duration agent such as ropivacaine or bupivacaine (with consideration of dexamethasone for additional potential prolongation) at a volume of 5-10 mL at the C6 middle cervical ganglion level, which has shown adequate spread to the C7-T1 stellate ganglion level.23
This report has several limitations. We did not assess long-term outcomes or other measures of illness severity, precluding direct comparison to case series from other institutions. Longer term outcomes such as hospital length of stay, resource utilization, survival, and post-discharge quality of life have not been assessed in the literature. Summaries are reported, but underlying source data are not accessible for pooled analyses. Lastly, given the relative rarity of ES, clinical urgency, and “last resort” nature of SGB as an intervention for patients who fail medical management, there are no randomized trials with sham control arms. These factors limit our ability to assess predictors of response, estimate treatment effect, and recommend the update of clinical management guidelines based on evidence.
Conclusion
Stellate ganglion blockade is a temporizing option for patients with refractory ES who are too unstable for definitive sympathectomy. We contribute to the sparse literature on this topic with a single-institution retrospective case series of administering ultrasound-guided unilateral SGB to 13 patients with ES. Stellate ganglion blockade was associated with reduced VA and defibrillation events. No serious complications occurred. Our results add to the findings of prior reports and support the safety and efficacy of SGB for ES. Moreover, we contextualize how SGB fits in the management of ES. Standardized reporting of larger cohorts and prospective randomized trials are required to better understand mechanisms of action, therapeutic efficacy, and the role of SGB in the management of ES.
References
Gao D, Sapp JL. Electrical storm: definitions, clinical importance, and treatment. Curr Opin Cardiol 2013; 28: 72-79.
Geraghty L, Santangeli P, Tedrow UB, Shivkumar K, Kumar S. Contemporary management of electrical storm. Heart Lung Circ 2019; 28: 123-133.
Guerra F, Shkoza M, Scappini L, Flor M, Capucci A. Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: a meta-analysis. Eurospace 2014; 16: 347-353.
Noda T, Kurita T, Nitta T, et al. Significant impact of electrical storm on mortality in patients with structural heart disease and an implantable cardiac defibrillator. Int J Cardiol 2018; 255: 85-91.
Bradfield JS, Ajijola OA, Vaseghi M, Shivkumar K. Mechanisms and management of refractory ventricular arrhythmias in the age of autonomic modulation. Heart Rhythm 2018; 15: 1252-1260.
Irie T, Yamakawa K, Hamon D, Nakamura K, Shivkumar K, Vaseghi M (2017) Cardiac sympathetic innervation via middle cervical and stellate ganglia and antiarrhythmic mechanism of bilateral stellectomy. Am J Physiol Heart Circ Physiol 312: H392-405.
Buckley U, Yamakawa K, Takamiya T, Armour JA, Shivkumar K, Ardell JL. Targeted stellate decentralization: Implications for sympathetic control of ventricular electrophysiology. Heart Rhythm 2016; 13: 282-288.
Ajijola OA, Howard-Quijano K, Scovotti J, et al. Augmentation of cardiac sympathetic tone by percutaneous low-level stellate ganglion stimulation in humans: a feasibility study. Physiol Rep 2015; DOI:https://doi.org/10.14814/phy2.12328.
Elliott IA, DeJesus M, Dobaria V, et al . Minimally invasive bilateral stellate ganglionectomy for refractory ventricular tachycardia. JACC Clin Electrophysiol 2021; 7: 533-535.
Wink J, Veering BT, Aarts LP, Wouters PF. Effects of thoracic epidural anesthesia on neuronal cardiac regulation and cardiac function. Anesthesiology 2019; 130: 472-491.
Ates Y, Asik I, Ozgencil E, Açar HI, Yagmurlu B, Tekdemir I . Evaluation of the longus colli muscle in relation to stellate ganglion block. Region Anesth Pain Med 2009; 34: 219-223.
Park C, Suh CH, Shin JE, Baek JH. Characteristics of the middle cervical sympathetic ganglion: a systematic review and meta-analysis. Pain Physician 2018; 21: 9-18.
Meng L, Tseng CH, Shivkumar K, Ajijola O. Efficacy of stellate ganglion blockade in managing electrical storm: a systematic review. JACC Clin Electrophysiol 2017; 3: 942-949.
Tan AY, Abdi S, Buxton AE, Anter E. Percutaneous stellate ganglia block for acute control of refractory ventricular tachycardia. Heart Rhythm 2012; 9: 2063-2067.
Hayase J, Patel J, Narayan SM, Krummen DE (2013) Percutaneous stellate ganglion block suppressing VT and VF in a patient refractory to VT ablation. J Cardiovasc Electrophysiol 24: 926-928.
Gadhinglajkar S, Sreedhar R, Unnikrishnan M, Namboodiri N. Electrical storm: role of stellate ganglion blockade and anesthetic implications of left cardiac sympathetic denervation. Indian J Anaesth 2013; 57: 397-400.
Yang SC, Wu CC, Hsieh YJ. Left stellate ganglion block, a rescue treatment for ventricular arrhythmia refractory to radiofrequency catheter ablation: a care-compliant case report. Medicine (Baltimore) 2019; DOI:https://doi.org/10.1097/MD.0000000000017790.
Patel RA, Priore DL, Szeto WY. Slevin KA. Left stellate ganglion blockade for the management of drug‐resistant electrical storm. Pain Med 2011; 12: 1196-8.
Fudim M, Boortz-Marx R, Ganesh A, et al. Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias: a systematic review and meta-analysis. J Cardiovasc Electrophysiol 2017; 28: 1460-1467.
Sanghai S, Abbott NJ, Dewland TA, et al. Stellate ganglion blockade with continuous infusion versus single injection for treatment of ventricular arrhythmia storm. JACC Clin Electrophysiol. 2020; DOI:https://doi.org/10.1016/j.jacep.2020.09.032.
Tian Y, Wittwer ED, Kapa S, et al. Effective use of percutaneous stellate ganglion blockade in patients with electrical storm. Circ Arrhythm Electrophysiol. 2019; DOI:https://doi.org/10.1161/CIRCEP.118.007118.
Bhatia A, Flamer D, Peng PW. Evaluation of sonoanatomy relevant to performing stellate ganglion blocks using anterior and lateral simulated approaches: an observational study. Can J Anesth 2012; 59: 1040-1047.
Gofeld M, Bhatia A, Abbas S, Ganapathy S, Johnson M. Development and validation of a new technique for ultrasound-guided stellate ganglion block. Reg Anesth Pain Med 2009; 34: 475-479.
Shin JE, Baek JH, Ha EJ, Choi YJ, Choi WJ, Lee JH. Ultrasound features of middle cervical sympathetic ganglion. Clin J Pain 2015; 31: 909-913.
Fudim M, Qadri YJ, Waldron NH, et al. Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias. JACC Clin Electrophysiol 2020; 6: 562-571.
Author contributions
Erik Reinertsen, Muhie Sabayon, Michael Lloyd, and Boris Spektor contributed to study conception and design. Erik Reinertsen, Muhie Sabayon, Margaret Riso, and Boris Spektor contributed to data analysis and interpretation and drafting the article. Erik Reinertsen, Boris Spektor, Muhie Sabayon, and Margaret Riso contributed to critical revision of the article. Michael Lloyd and Boris Spektor approved the article. Muhie Sabayon, Michael Lloyd, and Boris Spektor contributed to data collection.
Disclosures
None.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Sheila Riazi, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reinertsen, E., Sabayon, M., Riso, M. et al. Stellate ganglion blockade for treating refractory electrical storm: a historical cohort study. Can J Anesth/J Can Anesth 68, 1683–1689 (2021). https://doi.org/10.1007/s12630-021-02068-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-021-02068-1