Abstract
Purpose of Review
Myocardial fibrosis is a response to myocardial injury and plays a pivotal role in ventricular remodeling. Different patterns of fibrosis are associated with different disease states, but the presence and amount of fibrosis provide a different impact on prognosis.
Recent Findings
In the latest years, fibroblast activation protein inhibitor (FAPi) positron emission tomography (PET) gain interest for its potential in detecting myocardial fibrosis, in differentiating between active and chronic disease, and in the assessment of disease progression and response to treatment.
Summary
We aim to highlight the most relevant current applications of FAPi PET/CT in cardiovascular imaging, focusing on its applications, advantages, limitations, and to underline future clinical perspective.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
68Ga-labeled fibroblast activation protein (FAP) inhibitor (FAPi) is a positron emission tomography (PET) tracer, whose use has been consistently increasing in recent years, especially in the oncological field [1]. This tracer targets a membrane-anchored peptidase (FAP) with dipeptidyl peptidase, endopeptidase, and gelatinase activity related to fibroblast activation [1, 2].
FAP is expressed by myofibroblasts, a subpopulation of fibroblasts with contraction properties similar to smooth muscles, that are a part of the tumor microenvironment (TME) in the extracellular matrix (ECM), together with blood vessels, growth factors and cytokines [3•]. These favorable characteristics render 68Ga-FAPi imaging suitable to assess the presence and the metastatic involvement of various cancers [4,5,6].
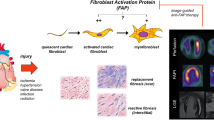
More recently, the use of 68Ga-FAPi has been investigated also in cardiovascular imaging [7], based on the demonstration of FAP overexpression in inflamed tissues [8]. In the heart, an initial inflammation with subsequent fibroblast activation [9] is followed by a reparative process, characterized by fibroblast proliferation and differentiation in myofibroblast, resulting in ECM deposition and apoptosis of the granulation tissue [10]. It is important to note that timing and physiologic balance between inflammatory and reparative processes are key elements to guarantee proper healing [11] and their dysregulation plays a pivotal role in almost all form of myocardial disease, by worsening tissue damage therefore increasing the probability of adverse outcome [12].
The aim of the present paper is to review the current applications of FAPi PET computer tomography (FAPi PET/CT) in cardiovascular imaging, focusing on its applications, advantages, limitations, and future perspectives in the context of clinical indication.
Materials and Methods
A comprehensive computer literature search strategy using PubMed databases was carried out looking for articles on the applications of FAPi in cardiovascular imaging. The string used for the search included a combination of the terms: “FAP” or “FAPi” or “fibroblast activation protein” or “myocardial fibrosis” or “myocardial infarction” and “cardiac PET” or “cardiac positron emission tomography.” The search was updated to May 2023, taking into consideration both clinical and preclinical studies published in English that used FAP-specific PET in cardiovascular field. The references of the retrieved articles were also checked as not to miss important clinical studies. Review articles, articles not in the field of interest, and commentaries were excluded. Papers on future perspectives in the field and experimental data were also considered eligible.
Three researchers (CEP, PF, and IG) independently reviewed the titles and the abstracts of the retrieved literature, selecting relevant articles according to the inclusion criteria mentioned above. Disagreements were resolved in a consensus meeting.
Results
Applying the search terms, 44 articles were retrieved. Titles and abstracts were carefully checked. Of all the articles, we identify 30 articles meeting the inclusion criteria. More specifically, 24 articles were related to clinical studies including 11 case report, 2 articles were translational studies, and 4 articles were preclinical studies. An overview of published articles and case reports on cardiac FAPi PET imaging is summarized in Table 1.
Correlation with Cardiovascular Risk Factors
In the largest retrospective analysis to date, Heckmann and colleagues [13••] investigated the correlation between myocardial FAPi-uptake in 229 patients undergoing 68Ga-FAPi PET/CT scan during oncologic follow-up. In their study, increased 68Ga-FAPi uptake correlated well with established cardiovascular risk factors (i.e., overweight and type II diabetes mellitus) but not with coronary artery disease (CAD) or prior myocardial infarction (MI). In addition, increased FAPi uptake was associated with reduced left ventricular ejection fraction (LVEF) in a sub-group of 44 patients. Similar findings were reported by Siebermair et al., wherein FAPi uptake correlated with age and LVEF [7]. Finally, also a retrospective study with a small cohort found a relationship between myocardial FAPi uptake, age, and high blood glucose levels [14•]. Given the retrospective nature of these studies, no firm conclusions can be drawn. Nevertheless, they support the concept of the interconnection between fibroblast activation and cardiac disease, thus suggesting a potential factor involved in their progression.
Coronary Artery Disease
Several reports also exist on FAPi imaging in patients with acute myocardial infarction (AMI). Back in 2015, Tillmanns et al. [40] investigated in a rat model the expression of FAP in activated fibroblasts after AMI. From this preclinical study, it seems that FAP expression follows a dynamic timecourse, with highest activity in the first week after AMI, especially in the peri-infarct area. These findings were confirmed by Varasteh et al. [15], wherein a peak in 68Ga-FAPi uptake was detected at day 6 post-AMI in rats, mainly in the ischemic borderzone, while FAP expression returned to near baseline by 2 weeks. Conversely, in another preclinical FAPi imaging study with 68Ga-MHLL1, persistent FAP expression was seen in the infarct relative to the non-infarcted remote myocardium between 7 and 21 days after AMI, suggesting a more stable expression over time compared to the previous 2 studies [16].
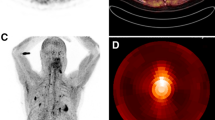
The possibility to reveal areas of inflamed myocardium after AMI has been confirmed in subsequent clinical studies. Both Xie et al. [17••] and Diekmann et al. [18••] showed in their work increased 68Ga-FAPi uptake after AMI. In both papers, patients exhibited intense 68Ga-FAPi uptake in the infarct zone, exceeding the perfusion defect identified either by nuclear perfusion imaging or cardiac magnetic resonance (CMR) late gadolinium enhancement (LGE) (Fig. 1), also consistent with previous case reports [18••, 19].
Coronary angiographic images before (first column) and after PCI (second column) with the treated culprit vessel (white arrows). Polar maps (third column) and the selected short-axis images of FAPI (fourth column), LGE (fifth column), and T2WI (sixth column). FAPI uptake was observed in the culprit territory, which was larger than the corresponding edematous and infarcted area (yellow arrows). PCI percutaneous coronary intervention, FAPI fibroblast activation protein inhibitor, SA short-axis, LGE late gadolinium enhancement, T2WI T2-weighted imaging. Reprinted with permission of Springer from [17••]. No changes were made
Following these observations, FAPi PET imaging has been directed toward the identification of early signs of ventricular remodeling post-AMI. In this regard, it was shown that FAPi uptake well correlates to signs of myocardial injury including reduced LVEF and peak creatinine kinase levels [20•]. Hence, it is conceivable that the amount of activated fibroblasts in the infarcted and non-infarcted myocardium directly affects subsequent remodeling.
Consistent with this concept, a recent study [21] suggested that FAPi PET can noninvasively monitor the activated fibroblasts early after AMI, which are ultimately responsible for the occurrence of reparative fibrosis. Also, Zhang et al. [41••] showed that the FAPi uptake of injured myocardium as detected early after AMI has a predictive value for late LV remodeling at 12-month follow-up. This prognostic value probably pertains to the observation that a long persistence of activated myocardial fibroblasts is related to the occurrence of adverse cardiac events. However, no correlation between either FAPi signal intensity or volume and the level of acute inflammation or myocardial damage-related markers was observed in the work by Zhang. This observation is consistent with other studies, wherein no correlation between FAPi uptake and the level of TGF-β1, TNF-α, IL-6, hsCRP, CKpeak, and LDHpeak could be demonstrated [17••, 20•]. It is conceivable that the uptake of 68Ga-FAPI within the myocardium reflects a different degree of ventricular remodelling, but, in order to fully understand its significance, further studies are needed to elucidate the interaction among other subtype of fibroblasts and macrophages.
Heart Failure in Non-ischemic Disease
Active fibroblasts and ventricular remodeling play an essential role in the onset and progression of heart failure (HF). In a rat model [23], activated fibroblasts in the heart and liver after pressure overload were linked in an early fibrotic environment, able to predict the occurrence of HF. Consistent results were reported in another translational study [24], wherein high FAPi uptake early after isoproterenol (ISO)-induced HF was reported, with a peak 7 days after HF induction. Over time, myocardial fibrosis invariably occurred and was associated to increased degree of myocardial injury. Of note, the degree of FAPi uptake decreased with the increase of myocardial fibrosis, thus suggesting that 68Ga-FAPi PET selectively identifies active myocardial fibrosis. Hence, the detection of early FAP expression may assist treatment decision-making in HF patients.
Right Ventricular Imaging in Pulmonary Arterial Hypertension
The value of FAPi-PET was also investigated to directly visualize fibrotic remodeling of the right ventricle in patients with pulmonary arterial hypertension (PAH). Specifically, two case reports [25, 26] and two prospective studies [27, 28] showed significant tracer uptake of the right heart including the right ventricular free wall which correlated positively with wall thickness and negatively with right ventricular function. On the contrary, no 68Ga-FAPi uptake of LV myocardium was seen in these studies. Additionally, a significant positive correlation was observed between cardiac FAPi uptake and total pulmonary resistance and the level of N-terminal pro b-type natriuretic peptide [28].
Chemotherapy-Induced Cardiotoxicity
Some studies support the use of FAPi-PET in patients undergoing chemotherapy to assess cardiotoxicity [29•, 30]. In these reports, the described high 68Ga-FAPi uptake within the LV myocardium in patients with previous systemic antineoplastic therapies may reveal FAP activation due to cardiotoxicity. Same considerations pertain to cardiac damage after radiotherapy. In another two studies, patients treated with anthracyclines or alkylating agents or patients with previous radiotherapy had high myocardial 68Ga-FAPi uptake [13••]. Of note, it seems that FAPi-PET imaging can detect radiation-induced myocardial damage before evidence of decreased LVEF, with evident implications for early monitoring of cardiac toxicity [31].
Hypertrophic, Dilatated, and Hypertensive Cardiomyopathy
Due to the similar fibroblast-mediated pathophysiological mechanism leading to myocardial fibrosis, FAPi imaging was investigated in the assessment of myocardial injury in hypertrophic cardiomyopathy (HCM). In a prospective observational study, high myocardial FAPi uptake was predictive of increased risk of sudden cardiac death within 5 years [32]. Two case reports also highlighted the possible role of FAPi imaging in dilated cardiomyopathy [42] and hypertensive cardiomyopathy [33], showing increased uptake within the LV. However, the precise role of FAPi imaging in these conditions needs to be fully elucidated.
Other Cardiomyopathies
A recent study using 68Ga-FAPi-46 PET/CT reported high sensitivity (87%) and specificity (90%) in the detection of fibroblasts activity in cardiac sarcoidosis (CS) [34]. Other case reports reported an intense FAP uptake in affected myocardial areas in patients with amyloidosis, related to ventricular remodeling [35, 36], which may provide complementary information on cardiac molecular characterization and staging of disease. Also, FAPi imaging was investigated in systemic sclerosis-related myocardial fibrosis [37], eosinophilic myocarditis (EM) [38] and in the assessment of myocarditis induced by checkpoint inhibitors (CI). In this latter setting, Finke et al. [39] report a high myocardial FAPi uptake in patients with typical clinical and serum hallmarks of myocarditis related to immune-checkpoint inhibitors therapy. Of note, 68Ga-FAPi PET imaging may also be used to predict HF due to CI-related myocarditis, as recently described by Zhang et al. [43].
General Remarks
To now, evidences on the role of 68Ga-FAPi PET/CT in cardiovascular imaging are scarce, but not poor. As a matter of fact, studies are mostly preliminary, but cumulating information indicates a clear potential in the diagnostic and prognostic assessment of patients with cardiovascular diseases.
To date, the most studied area of application of FAPi imaging is CAD, especially in the evaluation of post-AMI inflammatory alterations within the myocardium. The enhanced efforts in the latest years well fit the need for early markers of HF progression after AMI.
In this regard, increasing evidence suggests that inflammation plays a pivotal role in the progression to HF [44]. Among possible mechanisms of inflammation, the migration of macrophages after an insult plays conceivably a major role [22]. Early after an acute event, proinflammatory, M1-like macrophages are recruited, followed by the expression of reparative, M2-like macrophages [45]. While the former subtype is responsible for the production of proinflammatory molecules, including cytokines and proteases, the latter facilitates the secretion of factors stimulating angiogenesis and extracellular matrix reorganization, which are responsible for the promotion of fibrosis, as occurs in the post-infarction myocardial scar [46, 47].
Fibroblasts also possess a wide range of functions and phenotypes, which are likely to evolve over the healing process. But in contrast to the macrophage activation, which occurs immediately after the insult, the activation of myocardial fibroblasts is relatively delayed and sustained by TGF-ꞵ and other growth factors including platelet-derived growth factor [22, 48]. Consistent with this concept, there is evidence that distinct signatures of fibroblasts are expressed after AMI [49].
But if the expression of different fibroblasts follows a similar timecourse in the infarcted myocardium across individuals, but with conceivable, significant interindividual difference, how can we apply FAPi imaging in clinical practice? Is there a chance that we are not capturing the entire disease burden if we perform FAPi imaging at wrong time intervals after AMI? Furthermore, can we be absolutely sure that the interaction of different subtypes of fibroblasts does not play a more important role than FAP expression itself?
As a matter of fact, there is a contention on the correlation between FAPi uptake and the level of acute inflammation or myocardial damage-related markers. While some reports found no correlation [17••], another study demonstrated a correlation with markers of myocardial injury [20•]. This discrepancy represents a major issue for clinical applications, since the intensity of 68Ga-FAPi uptake can have a therapeutic importance only if it highlights a subtending, intensive inflammation, which may be treated with immune-modulators.
An inconsistency in the peak time between these serum markers in the blood and that of myocardial 68Ga-FAPI uptake can be an explanation but the influence of the interaction among other subtype of fibroblasts and/or macrophages requires further clarifications.
Besides its role in post-AMI setting, FAPi also brings the potential for the diagnostic and prognostic assessment of other cardiac diseases. However, the full role of FAPi imaging needs to be elucidated, as well as its performance in comparison to already established modalities such as fluorodeoxyglucose (FDG) PET and CMR.
Many trials have been registered in the latest months, and their results will clarify whether we now identified a workhorse in the prognostic assessment of patients with cardiac diseases or just a nice tool with academic interest but without any reasonable clinical application.
Data Availability
Data will be made available upon request.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin Appl. 2014;8(5–6):454–63. https://doi.org/10.1002/prca.201300095.
Brilla CG. Renin-angiotensin-aldosterone system and myocardial fibrosis. Cardiovasc Res. 2000;47(1):1–3. https://doi.org/10.1016/s0008-6363(00)00092-4.
Sidrak MMA, De Feo MS, Corica F, Gorica J, Conte M, Filippi L, et al. Fibroblast activation protein inhibitor (FAPI)-based theranostics-where we are at and where we are heading: a systematic review. Int J Mol Sci. 2023;24(4):3863. https://doi.org/10.3390/ijms24043863. This is a recent Review paper on the theranostic potential of FAPI imaging.
Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115. https://doi.org/10.1038/s41573-018-0004-1.
Cheng JD, Dunbrack RL, Valianou M, Rogatko A, Alpaugh RK, Weiner LM. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res. 2002;62(16):4767–72.
Santos AM, Jung J, Aziz N, Kissil JL, Puré E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119(12):3613–25. https://doi.org/10.1172/JCI38988.
Siebermair J, Köhler MI, Kupusovic J, Nekolla SG, Kessler L, Ferdinandus J, et al. Cardiac fibroblast activation detected by Ga-68 FAPI PET imaging as a potential novel biomarker of cardiac injury/remodeling. J Nucl Cardiol. 2021;28(3):812–21. https://doi.org/10.1007/s12350-020-02307-w.
Iking J, Staniszewska M, Kessler L, Klose JM, Lückerath K, Fendler WP, et al. Imaging inflammation with positron emission tomography. Biomedicines. 2021;9(2):212. https://doi.org/10.3390/biomedicines9020212.
Bengel FM, Ross TL. Emerging imaging targets for infiltrative cardiomyopathy: inflammation and fibrosis. J Nucl Cardiol. 2019;26(1):208–16. https://doi.org/10.1007/s12350-018-1356-y.
Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119(1):91–112. https://doi.org/10.1161/CIRCRESAHA.116.303577.
Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol. 2014;109(6):444. https://doi.org/10.1007/s00395-014-0444-7.
Kempf T, Zarbock A, Vestweber D, Wollert KC. Anti-inflammatory mechanisms and therapeutic opportunities in myocardial infarct healing. J Mol Med (Berl). 2012;90(4):361–9. https://doi.org/10.1007/s00109-011-0847-y.
Heckmann MB, Reinhardt F, Finke D, Katus HA, Haberkorn U, Leuschner F, et al. Relationship between cardiac fibroblast activation protein activity by positron emission tomography and cardiovascular disease. Circ Cardiovasc Imaging. 2020;13(9):e010628. https://doi.org/10.1161/CIRCIMAGING.120.010628. This is an important paper demonstrating the correlation between cardiovascular diseases and FAPI uptake.
Lyu Z, Han W, Zhao H, Jiao Y, Xu P, Wang Y, et al. A clinical study on relationship between visualization of cardiac fibroblast activation protein activity by Al18F-NOTA-FAPI-04 positron emission tomography and cardiovascular disease. Front Cardiovasc Med. 2022;9:921724. https://doi.org/10.3389/fcvm.2022.921724. In this paper, further evidence are reported for a correlation between FAPI uptake and cardiovascular diseases.
Varasteh Z, Mohanta S, Robu S, Braeuer M, Li Y, Omidvari N, et al. Molecular imaging of fibroblast activity after myocardial infarction using a 68Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J Nucl Med. 2019;60(12):1743–9. https://doi.org/10.2967/jnumed.119.226993.
Langer LBN, Hess A, Korkmaz Z, Tillmanns J, Reffert LM, Bankstahl JP, et al. Molecular imaging of fibroblast activation protein after myocardial infarction using the novel radiotracer [68Ga]MHLL1. Theranostics. 2021;11(16):7755–66. https://doi.org/10.7150/thno.51419.
Xie B, Wang J, Xi XY, Guo X, Chen BX, Li L, et al. Fibroblast activation protein imaging in reperfused ST-elevation myocardial infarction: comparison with cardiac magnetic resonance imaging. Eur J Nucl Med Mol Imaging. 2022;49(8):2786–97. https://doi.org/10.1007/s00259-021-05674-9. This is a very important paper, highlighting the correlation between post-infarction scar and FAPI uptake, which is related to reparative processes exceeding the extent of myocardial scar.
Diekmann J, Koenig T, Zwadlo C, Derlin T, Neuser J, Thackeray JT, et al. Molecular imaging identifies fibroblast activation beyond the infarct region after acute myocardial infarction. J Am Coll Cardiol. 2021;77(14):1835–7. https://doi.org/10.1016/j.jacc.2021.02.019. Also in this paper, there is evidence that the area affected by reparative processes after myocardial infarction exceeds that of myocardial scar.
Notohamiprodjo S, Nekolla SG, Robu S, Villagran Asiares A, Kupatt C, Ibrahim T, et al. Imaging of cardiac fibroblast activation in a patient after acute myocardial infarction using 68Ga-FAPI-04. J Nucl Cardiol. 2022;29(5):2254–61. https://doi.org/10.1007/s12350-021-02603-z.
Kessler L, Kupusovic J, Ferdinandus J, Hirmas N, Umutlu L, Zarrad F, et al. Visualization of fibroblast activation after myocardial infarction using 68Ga-FAPI PET. Clin Nucl Med. 2021;46(10):807–13. https://doi.org/10.1097/RLU.0000000000003745. In this paper, a correlation between markers of cardiac injury and FAPI uptake is shown.
Qiao P, Wang Y, Zhu K, Zheng D, Song Y, Jiang D, et al. Noninvasive monitoring of reparative fibrosis after myocardial infarction in rats using 68Ga-FAPI-04 PET/CT. Mol Pharm. 2022;19(11):4171–8. https://doi.org/10.1021/acs.molpharmaceut.2c00551.
Caobelli F, Nappi C. A spotlight on fibroblast-activated protein inhibitor (FAPi) cardiovascular imaging. Clin Transl Imaging. 2023. https://doi.org/10.1007/s40336-023-00548-6.
Wang G, Yang Q, Wu S, Xu X, Li X, Liang S, et al. Molecular imaging of fibroblast activity in pressure overload heart failure using [68 Ga]Ga-FAPI-04 PET/CT. Eur J Nucl Med Mol Imaging. 2023;50(2):465–74. https://doi.org/10.1007/s00259-022-05984-6.
Song W, Zhang X, He S, Gai Y, Qin C, Hu F, et al. 68Ga-FAPI PET visualize heart failure: from mechanism to clinic. Eur J Nucl Med Mol Imaging. 2023;50(2):475–85. https://doi.org/10.1007/s00259-022-05994-4.
Wang L, Zhang Z, Zhao Z, Yan C, Fang W. 68Ga-FAPI right heart uptake in a patient with idiopathic pulmonary arterial hypertension. J Nucl Cardiol. 2022;29(3):1475–7. https://doi.org/10.1007/s12350-020-02407-7.
Xing H-Q, Gong J-N, Chen B-X, Guo X-J, Yang Y-H, Huo L, et al. Comparison of 68Ga-FAPI imaging and cardiac magnetic resonance in detection of myocardial fibrosis in a patient with chronic thromboembolic pulmonary hypertension. J Nucl Cardiol. 2022;29(5):2728–30. https://doi.org/10.1007/s12350-020-02517-2.
Chen BX, Xing HQ, Gong JN, Guo XJ, Xi XY, Yang YH, et al. Imaging of cardiac fibroblast activation in patients with chronic thromboembolic pulmonary hypertension. Eur J Nucl Med Mol Imaging. 2022;49(4):1211–22. https://doi.org/10.1007/s00259-021-05577-9.
Gu Y, Han K, Zhang Z, Zhao Z, Yan C, Wang L, et al. 68Ga-FAPI PET/CT for molecular assessment of fibroblast activation in right heart in pulmonary arterial hypertension: a single-center, pilot study. J Nucl Cardiol. 2023;30(2):495–503. https://doi.org/10.1007/s12350-022-02952-3.
Niu N, Huo L, Zhang S, Liu Y, Li X. Immune checkpoint inhibitor-associated cardiotoxicity detected by 68Ga-DOTATATE PET/CT and 68Ga-FAPI PET/CT. Eur Heart J Cardiovasc Imaging. 2022;23(3):e123. https://doi.org/10.1093/ehjci/jeab189. This paper highlights the potential role of FAPI to predict inflammatory cardiac alterations in patients under therapy with immune checkpoint inhibitors.
Totzeck M, Siebermair J, Rassaf T, Rischpler C. Cardiac fibroblast activation detected by positron emission tomography/computed tomography as a possible sign of cardiotoxicity. Eur Heart J. 2020;41(9):1060. https://doi.org/10.1093/eurheartj/ehz736.
Wei Y, Sun Y, Liu J, Zhang G, Qin X, Xu S, et al. Early detection of radiation-induced myocardial damage by [18F]AlF-NOTA-FAPI-04 PET/CT imaging. Eur J Nucl Med Mol Imaging. 2023;50(2):453–64. https://doi.org/10.1007/s00259-022-05962-y.
Wang L, Wang Y, Wang J, Xiao M, Xi XY, Chen BX, et al. Myocardial activity at 18F-FAPI PET/CT and risk for sudden cardiac death in hypertrophic cardiomyopathy. Radiology. 2023;306(2):e221052. https://doi.org/10.1148/radiol.221052.
Lin K, Chen X, Xue Q, Yao S, Miao W. Diffuse uptake of [68Ga]Ga-FAPI in the left heart in a patient with hypertensive heart disease by PET/CT. J Nucl Cardiol. 2022;29(6):3596–8. https://doi.org/10.1007/s12350-021-02646-2.
Siebermair J, Kessler L, Kupusovic J, Rassaf T, Rischpler C. Cardiac fibroblast activation detected by 68Gallium-FAPI-46 positron emission tomography-magnetic resonance imaging as a sign of chronic activity in cardiac sarcoidosis. Eur Heart J Case Rep. 2022;6(1):ytac005. https://doi.org/10.1093/ehjcr/ytac005.
Guo W, Chen H. 68Ga FAPI PET/MRI in cardiac amyloidosis. Radiology. 2022;303(1):51. https://doi.org/10.1148/radiol.211951.
Wang X, Guo Y, Gao Y, Ren C, Huang Z, Liu B, et al. Feasibility of 68Ga-labeled fibroblast activation protein inhibitor PET/CT in light-chain cardiac amyloidosis. JACC Cardiovasc Imaging. 2022;15(11):1960–70. https://doi.org/10.1016/j.jcmg.2022.06.004.
Treutlein C, Distler JHW, Tascilar K, Fakhouri SC, Györfi AH, Atzinger A, et al. Assessment of myocardial fibrosis in patients with systemic sclerosis using [68Ga]Ga-FAPI-04-PET-CT. Eur J Nucl Med Mol Imaging. 2023;50(6):1629–35. https://doi.org/10.1007/s00259-022-06081-4.
Si J, Zhang X, Chen N, Sun F, Du P, Li Z, et al. Case report: Multimodal imaging guides the management of an eosinophilic leukemia patient with eosinophilic myocarditis and intracardiac thrombus. Front Cardiovasc Med. 2022;9:903323. https://doi.org/10.3389/fcvm.2022.903323.
Finke D, Heckmann MB, Herpel E, Katus HA, Haberkorn U, Leuschner F, et al. Early detection of checkpoint inhibitor-associated myocarditis using 68Ga-FAPI PET/CT. Front Cardiovasc Med. 2021;8:614997. https://doi.org/10.3389/fcvm.2021.614997.
Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, et al. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol. 2015;87:194–203. https://doi.org/10.1016/j.yjmcc.2015.08.016.
Zhang M, Quan W, Zhu T, Feng S, Huang X, Meng H, et al. [68Ga]Ga-DOTA-FAPI-04 PET/MR in patients with acute myocardial infarction: potential role of predicting left ventricular remodeling. Eur J Nucl Med Mol Imaging. 2023;50(3):839–48. https://doi.org/10.1007/s00259-022-06015-0. A potential predictive role for FAPI imaging is here depicted, in regard to the prediction of ventricular remodelling.
Shi X, Lin X, Huo L, Li X. Cardiac fibroblast activation in dilated cardiomyopathy detected by positron emission tomography. J Nucl Cardiol. 2022;29(2):881–4. https://doi.org/10.1007/s12350-020-02315-w.
Zhang X, Song W, Qin C, Lan X. Different displays of 13N-NH3 myocardial perfusion and cardiac 68Ga-FAPI PET in immune checkpoint inhibitor-associated myocarditis-induced heart failure. Eur J Nucl Med Mol Imaging. 2023;50(3):964–5. https://doi.org/10.1007/s00259-022-06018-x.
Weber BN, Stevens E, Perez-Chada LM, Brown JM, Divakaran S, Bay C, et al. Impaired coronary vasodilator reserve and adverse prognosis in patients with systemic inflammatory disorders. JACC Cardiovasc Imaging. 2021;14(11):2212–20. https://doi.org/10.1016/j.jcmg.2020.12.031.
Thackeray JT, Bengel FM. Molecular imaging of myocardial inflammation with positron emission tomography post-ischemia: a determinant of subsequent remodeling or recovery. JACC Cardiovasc Imaging. 2018;11(9):1340–55. https://doi.org/10.1016/j.jcmg.2018.05.026.
Caobelli F, Taqueti VR. Molecular imaging of myocardial inflammation: more evidence toward a causative role in cardiovascular disease. Curr Radiopharm. 2021;14(3):171–2. https://doi.org/10.2174/187447101403210715152013.
Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38(3):187–97. https://doi.org/10.1093/eurheartj/ehw002.
Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, Kansal P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94(6):730–6. https://doi.org/10.1136/hrt.2007.122622.
Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128(5):2127–43. https://doi.org/10.1172/JCI98215.
Funding
Open access funding provided by University of Bern
Author information
Authors and Affiliations
Contributions
CP, PF, and IR contributed to the draft of the manuscript and to retrieve relevant papers. IB and AR gave critical suggestions. FC contributed to the draft of the paper and supervised the entire work.
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
Not applicable for review papers.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Popescu, C.E., Ferro, P., Gotuzzo, I. et al. 68Ga-FAPi: Pathways and Diagnosis in Cardiac Imaging. Curr Cardiovasc Imaging Rep 16, 93–101 (2023). https://doi.org/10.1007/s12410-023-09583-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12410-023-09583-3