Abstract
Sustainable food practices within the food industry are pertinent to allow efficient food supply while not negatively impacting the environment. Alternative proteins have gained the attention of the food industry and consumers. To provide safe novel food products, these protein sources need to be assessed for potential allergen risk to ensure food safety and allow effective labelling to protect the consumer. In this review, the various detection assays applied to target potential allergens in novel and alternative foods are described together with their applications, mechanisms and limitations. Additionally, the use of non-thermal technologies to mitigate the reactivity of food allergens in these new products is explored. Non-thermal techniques including cold plasma, pulsed electric field, ultrasound and gamma irradiation are discussed. This review examines the potential mechanisms by which non-thermal technologies may reduce food allergenicity, primarily through alterations in protein epitopes that could affect antibody recognition. However, it is important to note that the understanding of the precise mechanisms and outcomes in allergen mitigation through these methods remains an area requiring further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Efforts to develop a sustainable food supply are increasing in the market, as a means of mitigating the environmental damage caused by farming and addressing the challenges currently faced in the industry. Animal-based proteins are considered traditional protein sources, but they have been associated with various environmental, ethical, and health issues due to the conventional farming practices used to produce them. These issues include higher levels of greenhouse gas emissions, significant water and land usage, and ethical concerns related to animal welfare. Sustainable food provides us with more options in food selection, including meat substitutes and alternative proteins. Alternative proteins mainly refer to the proteins that can provide an equal protein supply for daily human consumption but are derived from sources such as plants, microorganisms, algae and even insects [55]. In comparison to traditional protein sources, insects and pulses require less water and land for cultivation, while algae are naturally rich in fresh and sea water [5, 12, 145, 155]. Recently, the potential of various alternative proteins, including pulses, algae, plant-based meat alternatives, and insects has been investigated [59, 148]. Insects, for example, are a rich source of protein, omega-3 fatty acids, vitamins and essential minerals like iron and calcium [65]. Insects are also more efficient at converting feed into protein, making them a more sustainable option. Moreover, pulses, such as lentils, chickpeas, and beans are a nutritious source of protein and fibre, as well as B vitamins, iron, and potassium, which make them a healthy and beneficial alternative source of protein [12, 18, 154]. Algae is rich in protein, fibre, vitamins, fatty acids and minerals, which are essential for maintaining a healthy diet [53, 137]. Algae has also a low environmental impact, as it can help absorb carbon dioxide from the atmosphere and use it for microalgae cultivation [28]. An often overlooked alternative protein source is canola meal, a byproduct of the canola oil extraction process. After extracting the oil from rapeseed, canola meal still retains an impressive protein content and is commonly used worldwide as animal feed, second only to soybean meal [34].
However, alongside the exploration of the potential of alternative protein sources, the critical aspect of food safety emerges as a pivotal area of concern. There are several potential concerns of food safety related to the presence of microbes, toxins, or allergens that might have adverse effects on the health of consumers, and therefore, it is precise their risk assessment, detection and control [143]. In particular, this review focuses on the potential presence of allergens in alternative food proteins.
Allergies are triggered by the immune system in response to a foreign substance. This allergic reaction can induce different symptoms that can range from mild (e.g. cough, rhinitis) to severe (e.g. anaphylaxis), depending on the type and severity of the response [40]. Food allergies are defined as adverse, immune-related reactions that occur in particular individuals and can be immunoglobulin E (IgE) and cell-mediated reactions [103]. A growing body of research indicates that certain food allergens continue to provoke allergic reactions despite significant. alterations to their three-dimensional structures following gastrointestinal digestion [158]. The prevalence of food allergies (FAs) appears to have risen in recent decades [121]. Furthermore, Sicherer and Sampson [130] reported that the incidence of recorded food allergies increased by up to 10% over four years. Thus, food allergies are a global health concern. The introduction of novel alternative proteins in the agri-food sector may lead to a food safety risk and therefore, a proper risk evaluation of the presence of food allergens in these products has to be conducted [56]. Considering the potentially adverse impacts that may be caused by the presence of food allergens in these novel protein sources. It is important to minimise the presence of allergens present in final products. In order to minimise the risk of allergic reactions in patients caused by an abnormal immune response to certain food allergens, a number of approaches are adopted such as detection of the allergens, risk assessment and reduction of the allergen employed [29, 30, 123, 160]. Commonly, food allergens are proteins or peptides that present a specific region or specific regions formed by several amino acids, also known as epitopes. Epitopes, which are segments typically comprising 8–26 amino acids, are categorized into linear and conformational types [172]. Linear epitopes consist of sequential amino acids, whereas conformational epitopes are formed by amino acids that are non-sequential but spatially close [158]. Both linear and conformational epitopes can be recognized by specific IgE. The allergens’ three-dimensional configuration can be significantly changed by digestion in the gastrointestinal tract, affecting the availability and types of epitopes for IgE binding [158]. However, linear epitopes are deemed crucial for food allergens as they are more adept at maintaining their structure throughout the digestive process compared to conformational epitopes, positioning them as the primary epitopes with the potential to initiate allergic responses [87, 142, 158, 172].
In response to the growing demand for sustainable food options, the exploration of alternative proteins has gained significant attention. In this study, we have narrowed our scope to allergen detection and control methods within alternative protein sources, such as pea, soybean, seaweed and other protein sources. This intentional emphasis arises from our examination of the current literature, which has highlighted gaps and limitations in the development of alternative protein sources. This review seeks to enrich the existing body of knowledge by examining the evolution and current landscape of alternative proteins in the European context, with a particular focus on their impact on the food industry. A critical aspect of this exploration is the identification of allergens originating from these novel protein sources. The review also meticulously scrutinizes potential food allergens found in alternative proteins and evaluates current detection assays used to identify and mitigate allergen presence. Different types of assays, such as analysis based on proteomics, immunoblotting assays and DNA-based assays, are presented with their specific limitations and advantages, crucial for effective labelling and ensuring consumer safety. Additionally, in this review, the use of non-thermal technologies to mitigate the reactivity of food allergens in these new products is proposed. Cold plasma (CP), High Pressure Processing (HPP), Pulsed Electricity Field (PEF), Ultrasound (US) and gamma irradiation technologies are described with particular emphasis on describing the effects and mechanisms of each technology on allergen mitigation. It is therefore hoped that this review could provide valuable information to highlight the importance of alternative proteins in the sustainable food landscape and the critical need to address allergens associated with these sources, contributing some insights to both researchers and industry professionals for the development of safer and more sustainable food products for consumers.
Potential Food Allergens of Alternative Food Proteins
Table 1 summarises the potential main food allergens found in alternative protein sources. Research in the field of insect allergens is relatively undeveloped. A few studies have successfully identified certain allergens, which are documented in the recognized database (http://www.allergen.org/). The identified allergens include such as Pro c 2 (arginine kinase) in red swamp crayfish, Der f 4 (α-amylase ) and Der p 4 (α-amylase ) in dust mite, and Per a 17 (α-tublin), Bla g 12 (chitinase) in cockroach [25, 36, 117]. In plant-based proteins, peas, a member of the legume group, have been considered as an alternative protein source. Pis s 1 (vicilin), Pis s 2 (convicilin), and Pis s 3 (non-specific lipid transfer protein-nsLTP) belonging to legumin-like globulin proteins were identified as the allergens of pea, reported by and accepted as food allergens by the International Union of Immunological Sciences [138]. In a comprehensive study on pea allergens, two sulfur-rich albumin proteins, PA1 and PA2, found in peas, were shown to trigger an immune response [138].
When discussing food allergens, it is worth mentioning the phenomenon known as cross-reactivity. The mechanism of cross-reactivity among allergens is complex, potentially stemming from the homologous protein structural similarities, identical amino acid sequences, or even shared common epitopes within the proteins [153]. Thus, an individual can experience a similar allergic reaction to another substance that they were not exposed to before [23]. For instance, cross-reactivity between lupines and peanuts or soybeans is a common phenomenon in individuals allergic to peanuts or soybean that were not previously exposed to lupines [3, 153]. Specifically, the study done by Sirtori et al. [131] revealed a sequence homology of 63% between allergen Ara h 1 (vicilin) in peanuts and β-conglutin precursor from lupins when comparing peptides from reactive regions in lupin peptides.
The use of seaweed as a novel alternative protein is well known. Currently, to the best of the authors’ knowledge scarce evidence reported seaweed as a potential source of allergenic proteins. However, a risk associated with seaweed consumption remains in terms of its ability to induce an allergic reaction. Allergic reactions caused by algae species may be caused indirectly (e.g. as feed for organisms such as shrimps, crustaceans, etc.) [169]. Furthermore, shellfish (crustaceans and molluscs) can be found in seaweed and act as parasites in the seaweed’s natural habitat and seaweed cultivation spaces. Therefore, shellfish allergens are one of the major causes of allergic reactions in seaweeds [17]. For instance, a reported case of an allergic reaction to seaweed was when a patient allergic to Crustacean protein (tropomyosin) consumed a seaweed product contaminated with bycatch [60]. Although studies are limited to seaweed, it has been reported that allergenic proteins were found in the green Ulva species [7, 139]. By comparing potentially allergenic proteins extracted from Ulva spp. in the Allergenic Protein Database, Polikovsky et al. [116] found that the protein responsible for the allergenicity of this protein in Ulva spp. was superoxide dismutase. Few studies reporting allergic reactions to certain algae species are described in the literature. As an example, generalized urticaria with facial angioedema was observed in an individual after the consumption of sushi containing red seaweed (Chondrus crispus and Palmaria palmata) [139]. Another alternative protein source, Spirulina, which belongs to the Cyanobacteria family, is shown to induce allergic rhinitis and dermatitis when aerosol particles invade a patient’s respiratory system [89]. The first case of Spirulina-related anaphylaxis was reported in 2010 when the photosynthetic pigment phycocyanin was discovered to be a potentially allergenic protein [111].
Allergen Detection
The most effective and established method of reducing and controlling the risk of allergic reactions is to detect allergens in foods and food substances and label them accordingly [45]. Deoxyribonucleic acid (DNA) and proteins in food are the principal targets for allergen detection [45, 118]. Direct analysis method targets the specific allergenic proteins, while indirect methods detect the DNA (as markers). Some of the classical assays will be introduced in this review, including the mass spectrometry-based assay, SDS-PAGE Western blotting, Enzyme-Linked Immunosorbents assay (ELISAs) and polymerase chain reaction (PCR) techniques. A comparative summary of the various methods mentioned above is shown in Table 2.
Mass Spectrometry-Based Proteomic Methods
Mass spectrometry (MS) is central to the field of proteomics and the utilisation of proteomic techniques to identify food allergens has given rise to what is referred to as ‘allergenomics’ [4]. In the MS method, samples are ionised and broken down into gaseous ions according to mass-to-charge (m/z) ratios in the protein or peptide molecule, which are then identified by their molecular weight and analysed for their isotopic abundance. Following this, the specific spectral information of extracted or digested proteins or peptides is matched to known protein information in protein databases such as the National Centre for Biotechnology Information [93, 144]. Various software and algorithms are utilized in the protein/peptide analysis process, which includes tools such as MASCOT, SEQUEST, and Andromeda, in addition to commonly used software like MaxQuant, PEAKS Studio, and Protein Prospector [14, 88, 93, 122]. MS-based proteomics enables qualitative and quantitative analysis of allergenic proteins. The workflow of this method is illustrated in Fig. 1.
Depending on the level of information required, two alternative types of analytical strategies are used in MS-based assays, namely “bottom-up” analysis and “top-down’’. Specifically, the “top-down” technique is a direct analysis of intact proteins and their fragmentation products at the protein level [99]. On the other hand, when proteins are digested into peptides or polypeptides during digestion, the “bottom-up” technique is typically used to analyse the information provided by these molecules at the peptide level [74, 99]. Shotgun proteomics applies a “bottom-up” strategy [37], which is an undirected approach to protein discovery and identification [35]. Shotgun proteomics most commonly starts with the proteins in the digestion mixture, followed by the separation of the resulting peptides by liquid chromatography [61]. Another commonly used proteomic assay assisting MS-based methods is selected reaction monitoring (SRM) also known as multiple reaction monitoring (MRM) proteomics, which is used to quantify known allergenic proteins [93]. A triple quadrupole mass spectrometer is the standard spectrometer for SRM assay, with ionised proteins or protein fragments separated and filtered via three phases [93]. The stringent selection criteria reduce interference from isobaric precursor ions and improve the performance of SRM in terms of peptide quantification rates and measurement sensitivity [35, 114].
According to Monaci and Visconti [99], the MS-based methods offer greater advantages in the sensitive detection of processed foods that have been heat-treated or chemically preserved, even when food processing causes changes in protein structure [149]. In addition, the MS-based approach enables the quantification of allergens in a wide range of protein matrices [93]. Along with the previously discussed strategies and software that support MS techniques for allergen detection. Data independent acquisition (DIA) has emerged as a bioinformatics-based data processing approach. DIA systematically fragments all precursor ions within specific m/z intervals, enabling the effective detection and analysis of even low-abundance ions. This method ensures a comprehensive peptide scan of potential allergenic proteins, minimizing false negatives and improving the detection and analysis of low-abundance allergens. The capacity of DIA to detect both known and unknown allergens in complex food matrices strengthens allergen detection processes, contributing significantly to food safety applications [14]. Uvackova et al. [146] detected and quantified 15 allergenic protein isoforms and early identification of several peptides in four epitopes of two specific allergenic proteins by using the MS-based techniques and alongside the DIA approach. As a developing method, mass spectrometry also can be performed either in a specific allergen protein or in a matrix of allergen proteins. In addition, it is an effective tool to achieve the goal of quantifying allergens in food not only at the protein level but also at the peptide level [74]. However, MS-based proteomics assays have limitations such as the simultaneous elution of large numbers of different proteins or peptides occurring when a significant number of proteins or proteins elute from LC, and ion suppression occurs [61, 74]. The mass spectrum then shows a superposition of many peaks due to the appearance of many different proteins [74] which lead to less sensitivity. In addition, the limitations of this technology are also reflected in the high cost of the equipment and skilled operators are demanded in the analysis process [93].
Immunoassay
Allergens and antibodies have a strong affinity for one another, and the immunoassay detects allergens by binding antibodies to epitopes of the antigen. Figure 2 illustrates the basic principles of the immunoassay in detecting food allergens. Western blot and enzyme-linked immunosorbent assay (ELISA) are two classic immunoassays for the detection of allergens in food.
Western Blot
The western blot, also known as the immunoblot, is a classic method for identifying proteins. It is typically used following sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) for food allergy testing [120]. Western blot procedure involves three primary steps: protein separation by SDS-PAGE, transfer of separated proteins onto a membrane, and specific detection through antibody probing. Protein separation was completed by using the SDS-PAGE technique, which is based on the interaction between proteins and SDS. During this, proteins are denatured and uniformly negatively charged by binding to SDS [118, 133], ensuring their separation during electrophoresis is based solely on molecular weight, rather than their intrinsic charge. After separation, proteins are transferred to a membrane with a high affinity for proteins (e.g., nitrocellulose or polyvinylidene difluoride (PVDF)) [118]. SDS-PAGE can be one-dimensional (1D) for separating proteins based on their molecular weight [74, 110], or two-dimensional (2D), which includes preceding steps of separation by isoelectric point for more detailed proteome mapping [74].
The detection of target proteins in the western blot assay involves a series of steps culminating in chromatic changes due to enzymatic reaction. Initially, allergenic proteins separated by SDS-PAGE are transferred from the gel into the membrane and immobilized on the membrane. During antibody probing, specific antigens within these proteins are then targeted by primary antibodies, which bind to them with high specificity [16]. The secondary antibody recognizes and binds to the primary antibodies [69, 77]. Secondary antibodies can be labelled with radioisotopes, enzymes, or fluorophores, with the latter two being the preferred methods for signal generation due to common practice [9, 91]. Enzyme-conjugated secondary antibodies are prevalent, with horseradish peroxidase (HRP) and alkaline phosphatase (AP) being commonly employed due to their efficiency in catalyzing chromogenic or fluorogenic reactions with the substrates [9, 91]. Colour development occurs upon substrate addition triggered by the reaction between the enzyme-labelled antibody and the substrate. This signal, which correlates with the presence and quantity of the target antigen, is then detected and analyzed, often through enhanced chemifluorescence (ECF) methods. It has been therein reported that when the substrate is cleaved by the enzyme, a higher fluorescence end product is formed and a more stable signal (absorbance) occurs, along with a greater linear range of protein detection than the traditional one that does not use ECF [91]. Allergen analysis can then be completed by spectrophotometry. In the end, the analysis of allergens, or other proteins of interest, can be quantitatively assessed using spectrophotometry.
There are some limitations of western blot that must be considered. Firstly, this is a time-consuming assay that requires a lot of time for protein migration and sample preparation [74]. Secondly, the western blot is used for allergen detection at a quantitative or semi-quantitative level, so if the allergens need to be identified, other methods such as MS, are required.
ELISA
Another classic immunoassay for the detection of food allergens is the enzyme-linked immunosorbent assay (ELISA), which detects known allergens and involves specific and enzyme-labelled antibodies [45]. Similar to the western blot, the ELISA process involves enzyme-labelled antibodies binding to specific target antigens. The enzymatic activity of these antibodies, upon interaction with a specific substrate, generates a colourimetric or fluorescent signal, indicating the presence of the antigen [74]. The two epitopes in the antigen are specifically recognised and bound by two types of antibodies, where the primary antibody specifically binds to the allergen and the enzyme-labelled antibody binds to the antibody-antigen (Ab-Ag) complex and reacts with the substrate and forms a coloured product.
There are two types of ELISA for the detection of allergens, based on different purposes, namely the “sandwich” ELISA and competitive ELISA. In a “sandwich” ELISA, a capture polyclonal antibody specific to the target allergen is bound to the solid phase (e.g. multi-well plate) and the capture antibody selectively binds to the food antigen present in the sample, forming an Ag-Ab complex. Subsequently, an enzyme-labelled analyte-specific antibody, which recognizes a different epitope on the antigen, binds to the Ag-Ab complex. The enzyme reacts with the substrate showing a colour change, and the absorbance of the coloured product correlates positively with the allergen concentration [45, 161]. Given that the antigen must offer more than one epitope, the “sandwich” ELISA approach is suited for large molecules of antigens (EFSA Panel on Dietetic Products & Allergies [45], Schubert-Ullrich et al [124]. As the other classic ELISA method, competitive ELISA is used for the detection of small molecules of allergens, where the concentration is indirectly detected (EFSA Panel on Dietetic Products & Allergies [45]). In a competitive ELISA, the reference antigen is immobilised on a plate. The reference antigen and the antigen in the food sample compete to bind to the enzyme-labelled analyte-specific antibody and form the Ag-Ab complex. The enzyme-substrate reaction shows a colour change. When the plate is washed, Ag-Ab formed by the food antigen is washed away, and then the remaining Ag-Ab in the plate is the reference antigen-antibody complex. Therefore, the absorbance of the coloured product is negatively correlated with the allergen concentration when performing the analysis (EFSA Panel on Dietetic Products & Allergies [45], Wu et al [161]. However, one limitation of ELISA, as with all other immunoassay tests, is cross-reactivity [74]. The recognition between antigen and antibody is based on the epitopes rather than the entire antigens. Therefore, the uniqueness of the epitope determines the specific recognition of the antibody [74]. When the antibodies do not fully bind to one antigen but bind to other allergens with that epitope, cross-reactivity can occur, resulting in a false positive test result (EFSA Panel on Dietetic Products & Allergies [45]). In addition, the sensitivity is strongly influenced by changes in the conformation of proteins after food processing [32]. Since the epitope plays a critical role in antibody recognition when food processing such as roasting and boiling alters the structure (quaternary) of the protein, the epitope will shift position (fold or hide) and the ELISA test’s sensitivity will be diminished [74].
Other Detection Assays
Other methods that can be used for the detection of allergens include enzyme-allegro sorbent test (EAST), radioallergosorbent test (RAST), lateral flow devices (LFDs), dip sticks, rocket immune electrophoresis (RIE), dot immunoblots, protein microarrays, and protein biosensors. The principle of EAST and RAST is similar to that of ELISA, albeit the RAST test employs a radioisotope-labelled antibody and the colour products are measured using a gamma counter [74]. As a simplified ELISA test, LFDs and dipsticks employ a similar principle where the Ag-Ab complex is coloured during the test. Dot immunoblotting, on the other hand, is similar to western blot, except that it does not require pre-separation of proteins and is much easier to perform (EFSA Panel on Dietetic Products & Allergies [45, 118]. RIE employs the precipitation and migration of Ag-Ab complexes during electrophoresis. In addition, the graphs displayed during electrophoresis often resemble rockets, hence the name RIE [118]. This method is often used for semi-quantitative analysis. Antibodies are fixed as microarrays identifying and qualifying the specific antigens in the protein microarrays method. In addition, a number of assay combinations and innovations are gradually being discovered, such as ELISA inductively coupled plasma MS [22], DNA microarrays [13], Surface Plasmon Resonance [2], Magneto-Chemical Sensor [85], smartphone-based immunoassays [123]. The validity of the immunoassay will be affected by the processing of the food due to changes in protein structure during processing. Specific recognition and binding between antigens and antibodies will be affected by alteration in protein structure, which will adversely affect the effectiveness of the immunoassay.
Deoxyribonucleic Acid-Based Methods
DNA has a stable structure and is resistant to treatment processes such as heating, chemical reactions and hydrolysis. These properties provide an alternative method for quantifying allergens [66]. As a marker, DNA is a powerful tool for detecting food allergens when the concentration level of an allergen is low within a food sample or when the allergens in the processed food may be altered (EFSA Panel on Dietetic Products & Allergies [45]). Polymerase chain reaction (PCR) is a widely DNA-based detection technique and it involves rapidly amplifying the DNA fragment of the allergenic protein of interest, generating many copies and using the genetic code to identify the suspected allergenic protein in the food [74].
There are two types of PCR amplification of DNA, end-point PCR and real-time PCR (EFSA Panel on Dietetic Products & Allergies [45]). End-point PCR is used to identify the presence of the allergenic DNA fragment, which is a qualitative method. While real-time PCR is used for quantitative assay [62]. In addition, some novel methods based on PCR were developed to detect food allergens. The multiplex PCR method allowed the simultaneous identification of several allergenic DNA fragments [78]. Ultrafast PCR system allowed a shorter time thermal cycle in PCR (20 min, 40 cycles) with the microfluidic chips to detect allergens in food [73].
Although PCR is an alternative assay in cases where immunoassays are not applicable, the use of PCR to amplify DNA sequences does not always reveal the presence of allergenic proteins in the food matrix. This technique focuses on specific DNA fragments encoding amino acids within active peptide regions, rather than analyzing the entire protein. Consequently, it only offers gene-level information and does not directly reflect the structural and functional changes of proteins (EFSA Panel on Dietetic Products & Allergies [45], Mulalapele & Xi [102]. The allergenic potential of proteins depends not only on their amino acid sequences but also on factors such as tertiary structures and post-translational modifications (e.g., folding or glycosylation) [1]. However, PCR is unable to directly detect the structural changes in the protein, which are crucial for understanding protein expression levels and modifications. Moreover, certain issues associated with PCR technology such as non-specific sequence amplification and challenges in amplifying GC-rich DNA sequences, can lead to unsatisfactory results [135]. The presence of small amounts of DNA in allergenic foods can sometimes also result in false-negative outcomes (EFSA Panel on Dietetic Products & Allergies [45]). These challenges underscore the need for caution when relying solely on PCR for allergen detection.
Non-Thermal Mitigation of Allergens
Several studies highlighted the use of food processing technologies as a potential allergen mitigation strategy. In terms of reducing food allergenicity, traditional heat treatments are divided into moist heat (e.g., autoclaving, boiling, frying at 120°C) and dry heat (e.g., microwave, baking, roasting at 150°C) [129]. Heat-induced protein denaturation, changes in the conformational structure of the allergen, particularly in secondary and tertiary structures, the formation and breaking of new molecular bonds and intermolecular aggregation, lead to changes in the reactivity of the allergen [100]. Vanga et al. [150] explained the effect of heat treatment on proteins, where the structure of proteins, especially tertiary structures, was lost when temperatures reached 50–60°C. However, consequently, heat can negatively affect the quality of food products. High temperatures could cause chemical reactions of amino acids and certain components of the food (carbohydrates), leading to a change in colour (e.g., browning) or texture of the food [129]. Even protein allergenicity can occur or be maintained after heat treatment. Heat processing may result in the formation of “neo-allergenic” ingredients or epitopes, in other words, heat processing enhances the allergenicity of certain allergenic foods [24, 39, 92]. According to Davis and Williams [39], one patient with a reported allergy had an allergic reaction after consuming fish that had been cooked but did not experience a reaction after consuming raw fish. The heat-treated protein β-lactoglobulin was found to be reactive to the antibody, whereas the native protein was not. It was inferred that the refolding of conformational epitopes remaining in the denatured protein formed new allergenic epitopes or maintained the original allergenicity. The degradation of secondary and tertiary structures and non-covalent interactions that occur in the protein structure caused by thermal treatment, can affect the allergenicity of the protein by either eliminating or exposing conformational epitopes. This can lead to a decrease or increase in allergenicity [29]. Although it is not clear whether a ‘neo-allergic’ ingredient occurs when proteins are treated with nonthermal techniques, there is great interest in developing nonthermal mitigation techniques. To our current understanding, many food processing methods entail a heat effect. However, to counteract the negative impacts on food quality associated with traditional thermal processing, non-thermal technologies strive to process foods with minimal temperature increase. The heating effect becomes significant during prolonged technology application, whereas shorter processing durations typically prevent heat from interfering with the outcomes of non-thermal processing. In addition to this, given that some allergenic proteins, such as TM in shrimp, caseins in milk, and vicilin in peanuts, are heat-resistant [11, 33, 43, 112], pivoting towards non-thermal technologies represents a growing trend in reducing allergens in food. Nonthermal techniques including pulsed light, HPP, CP, gamma irradiation treatment, US, PEF, fermentation treatment and enzyme hydrolysis treatment, ribonucleic acid (RNA) interference techniques, magnetic beads techniques, were discussed thoroughly by Chizoba Ekezie et al. [29], based on the various applications of the techniques and their impact on allergenicity. According to the literature, techniques, such as CP, HPP, PEF, US and gamma irradiation are promising methods that show huge potential in mitigating allergens in traditional food protein. However, there is a lack of research on the effectiveness of these techniques for mitigating allergens in alternative proteins, such as those from algae, peas, lupine and insect, which have not been investigated.
Considering these challenges, the application of nonthermal treatment in the reduction of allergens in food is a promising area of research. Because of the similarities and cross-reactivity between some allergens and traditional allergens, such as tropomyosin in seaweed can trigger allergic reactions similar to those caused by tropomyosin in seafood such as shrimp, lobster and crustacean seafood [17]. Therefore, studies applying non-thermal techniques to treat traditional allergens can yield valuable insights. These similarities provide valuable perspective on the application of similar techniques to address allergens in foods from alternative protein sources. Table 3 summarises several studies that focus on the effect of different nonthermal processing techniques on food samples and allergens.
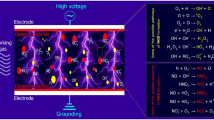
Physical processing methods-including heat, pressure, radiation, and ultrasound-primarily influence the allergenicity of food proteins by modifying their secondary and tertiary structures, which can lead to the destruction, masking, or revelation of conformational epitopes. In contrast, linear epitopes are predominantly altered by biochemical interventions, such as fermentation and enzymatic hydrolysis [150]. These alterations at the epitopes have the potential to either attenuate or amplify allergenicity [119]. Table 4 gives an overview of the mechanism and limits of those techniques in mitigating allergens of alternative proteins. Figure 3 provides a comprehensive depiction of the hypothesized impact of non-thermal processing technologies, including CP, HPP, US, and Gamma Radiation, on the modification of protein allergenic properties.
Cold Plasma
Plasma is the fourth state of matter and is produced by the ionization of gas at ambient or atmospheric temperature. CP has been employed in the food industry as a decontamination method to target microbes, serving as an innovative approach in food preservation technologies [44, 46]. Due to the capability of CP to alter protein structure, it has been recently investigated for its potential to reduce allergenicity in foods. The exact mechanism of CP is not well understood, one of the most prevalent hypotheses is that the reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated by the plasma gas react with antigens and alter their protein structure or binding sites between antigen and antibodies [29, 38, 132, 141, 152]. The effectiveness of CP treatment usually depends on the duration and temperature of the treatment [104]. When combined with time [26], temperature, and glycation [157], as hurdle technologies in food processing, cold plasma (CP) shows enhanced performance in reducing allergenicity. According to the effect of plasma on mitigating the allergens from alternative protein sources, numerous research studies have speculated on diverse mechanisms. Figure 3 outlines the potential pathways through which plasma acts to mitigate the effects of food allergens.
In a study investigating the application of CP to treat soybean allergens, reduced immunoreactivity of allergens after CP treatment was speculated to be due to the oxidation of amino acids in proteins by ROS, leading to protein fragmentation and disruption of antibody binding sites [96]. Similarly, while studying the effect of CP on dried defatted peanut flour and whole peanut, Venkataratnam et al. [152] also observed decreased antigenicity of Ara h 1 in CP-treated peanuts. According to the further detection of protein structure by using the circular dichroism, it was reported that the secondary structure showed alterations and concluded that the alteration of the antigen was due to the ROS and RNS. As previously mentioned, tropomyosin, an allergenic protein present in seaweed proteins, is exposed in post-harvest environments and adheres to seaweed surfaces. Despite the lack of specific research on treating this allergen in seaweed proteins, insights can be gleaned from studies on CP processing of tropomyosin in other seafood, offering theoretical support for potential applications in reducing the allergenicity of seaweed-derived proteins. For instance, Ekezie et al. [47] investigated the effect of cold argon plasma treatment on the binding capacity of King Prawn tropomyosin, observing a decrease in the recognition capability for both immunoglobulin E (17.6% reduction) and immunoglobulin G (IgG) (26.87% reduction) post- treatment. It is concluded from that study, which encompassed the examination of the primary structure of the protein and the scond and tertiary structure, that the reduced antibody binding to tropomyosin was due to oxidative modifications by ROS impacting both the linear and conformational epitopes.
CP offers the advantages of being ecologically sustainable, and no significant loss and changes in colours and texture [51, 109]. However, there are some limitations regarding this technology. Firstly, the investment in a CP system is a significant cost compared to other heat treatments such as boiling and roasting. Secondly, as the mechanisms of CP allergen reduction are not fully understood, the application of this technology should be carefully studied. Further investigations are required to elucidate this. Finally, according to Boehm et al. [19, 52], possible cytotoxic activity associated with CP-treated fluids should be taken into account when conducting relevant studies. Therefore, more investigations on toxicity will be needed in the future to secure this technology.
High-Pressure Processing
HPP, also known as high hydrostatic processing, is a non-thermal processing technology applied in the food industry. High-Pressure Processing (HPP) has been demonstrated to effectively deactivate spoilage-causing microorganisms and enzymes, thus its potential for reducing allergenicity in food proteins is being rigorously evaluated [8, 27]. HPP treatment is effective in reducing the allergenicity of proteins while maintaining the sensory quality of food samples [72]. High pressure affects the secondary structure of proteins, by damaging the non-covalent bonds such as high-pressure breaks the hydrogen bonds [63, 64, 97]. Studies have shown that high pressure induced the formation of multimers, leading to the burial of immune active sites (conformational epitopes), which is a possible mechanism for reducing allergenicity [107]. It was reported that high pressure was effective in extracting or releasing allergens from the membrane, thus facilitating the enzymatic hydrolysis of these molecules [72].
Many studies have been conducted to investigate the relationship between changes in protein structure and the reduction in protein allergenicity caused by high pressure. One of the prominent areas of research involving the application of HPP in the development of safe food products from alternative protein sources focuses on soybean protein isolation (SPI), particularly in infant milk powder formulations. Li et al. [81] conducted a study demonstrating that HPP significantly reduced the allergenicity of SPI by 45.5% at 300 MPa for 15 min, suggesting its potential incorporation into infant milk formulas. Further mass spectrometry analysis revealed that HPP treatment led to changes in the subunits of 7 S globulin and 11 S globulin, resulting in alterations in allergenicity profiles. In another study, the allergenicity of tropomyosin was effectively reduced after shrimps were treated with high pressure, especially when the pressure was increased from 100 to 500 MPa [89]. Zhang et al. [168] studied the structural changes of squid hemocyanin (Hc) after HPP treatment and the reduction in the content of α-helix indicated that the secondary structure of squid Hc changed after HPP treatment. The comparison of control and treated samples, particularly the amount of α-helix and random coils in the samples confirmed that HPP weakened the binding capacity of IgE and IgG. However, there were no significant differences between the HPP-treated samples and the control in terms of the tertiary structure according to the indicators (surface hydrophobicity index and free sulfhydryl content). Pan et al. [107] found that HPP significantly reduced the immunoreactivity of Ara h 1 in treatments at 400 MPa and 600 MPa. Furthermore, an increase in particle size was also found in the 400 MPa treated Ara h1. It was therein confirmed that the secondary structure changed when 400 MPa acted on Ara h1, suggesting that the effect of HPP treatment on allergenicity was associated with changes in secondary structure. Based on the results of fast protein liquid chromatography or molecular weight analysis and particle size distribution for particle size analysis, it was discovered that when allergens were treated beyond 400 MPa, unfolded proteins aggregated and became multimers, resulting in a reduction in allergenicity. It was elaborated that such alteration in the protein results in the concealment of the immunological active site leading to the allergens not being identified. However, in some studies, the effect of HPP processing does not have an effect on reducing allergenicity. Lavilla et al. [79] reported the effect of high pressure on the peach allergen protein caused it to unfold, exposing the epitope and making it more readily recognisable thus increasing the potential of the major allergen in peach to elicit an allergic reaction. Given the varying performances in reducing the allergenicity of the protein, Zhou et al. [173] hypothesised that the two opposite effects on antigens caused by HPP treatment were related to the linear epitopes of antigens. It has been noted that linear epitope-dominated antigens did not perform well in HPP processing. Moreover, the variability in the effects of HPP in reducing allergenicity may be caused by the detection assays used in the research [80]. Therefore, futher studies are required to investigate the effects and mechanisms of HPP work on allergens to better apply the technique.
As mentioned previously, HPP may not may not consistently reduce the allergenicity of protein antigens, particularly those with linear epitopes. Studies have shown that the allergenicity of specific allergens was reduced through the synergistic application of HPP with other factors, such as temperature and proteolysis [46, 75, 79, 80, 89]. This suggests that the hurdle approach, which combines high pressure with additional methods could potentially offer a more robust solution for reducing allergenicity across a wider spectrum of food allergens.
Pulsed Electric Field (PEF)
PEF applies high voltage pulses (10–80 kV cm− 1) over a very short period of time (millisecond or microsecond) to food for various processing applications, resulting in the disruption of cellular structures [54, 126]. In the food industry, PEF is widely applied in the food industry and has been proven to significantly enhance meat quality, such as improving tenderness and extending the shelf-life of foods [42, 54]. Recent research has discovered that PEF shows great potential to reduce the immune activity of allergens [140, 163]. Although the exact mechanism of PEF on allergens has not been clearly elucidated, there is evidence to suggest that PEF processing may induce changes in protein structure, including alterations in hydrogen bonds and hydrophobic interactions within the secondary and tertiary structures, potentially affecting the concealment or exposure of conformational epitopes [83, 165]. Wei et al. [159] reported that the linear unfolding of the secondary structure of the ovalbumin protein molecule increased with increasing pulse intensity (ranging from 27 to 53 kV cm− 1) and treatment time (ranging from 720 to 4320 µs).
Yang et al. [163] showed a loss of immunity to Ovalbumin antigens (OVA) when OVA was isolated and purified from fresh eggs and treated with PEF at 35 kV cm− 1 and 180 µs. The conformational structure of OVA was altered after treatment with PEF, which was confirmed when analysing the OVA samples using circular dichroism, UV absorption and fluorescence spectroscopy. Moreover, they implied that the binding of IgG to OVA or IgE to OVA was influenced by these structural changes. A similar result was obtained by Li et al. [84] when treating one of the main types of allergens (ovomucin) in egg whites with PEF. PEF was reported to reduce the ability of IgG and IgE to ovomucin at 30 kV cm− 1. Structural changes in the treated proteins were also confirmed with the help of scanning electron microscopy, Fourier transform infrared (FTIR) and CD spectroscopy, SDS-PAGE methods, and analysis of changes in surface hydrophobicity and total sulfhydryl content. Thus a correlation between the spatial conformation and the ability of IgE and IgG to bind to ovalmucin was indicated therein.
Same to HPP, PEF can modify the proteins in food products while offering the advantage of being non-destructive. However, as few studies have been conducted on the treatment of foods with PEF, its mechanisms have not been clearly described. In this way, there is limited scientific guidance on the use of PEF to mitigate allergens in foods for operational purposes, limiting its application in food allergen mitigation. In addition, PEF technology can be effective independently for various food processing applications, such as microbial inactivation and improving textural properties. The efficacy of the PEF in certain areas, like reducing allergenicity or enhancing protein digestibility, can be significantly enhanced when combined with other methods like proteolysis [82]. This synergistic approach, known as hurdle technology, allows for wider applications in food processing but also adds complexity to the implementation process. Therefore, more efforts should be made to carry out research on PEF in food allergen mitigation, especially focusing on understanding the underlying mechanisms.
Ultrasound
Ultrasound applies power ultrasonic waves with 20–100 kHz to a sample, this energy generates a gradient of pressure and temperature within a matrix, inducing physical changes and stimulating chemical reactions [126]. It has been widely used as a non-thermal approach to keep fruits and vegetables fresh by sterilizing the microbial inside [68], extraction [76], oxidation [86] and homogenization [136]. The exploration of US technology in food preservation has prompted a closer examination of its potential for addressing other concerns within the food industry. US has been observed to induce modifications in the structure of proteins, including allergens, while simultaneously extending the shelf life of food products. As shown in Fig. 3, the possible mechanism of US is the cavitation effect that US treatment has on the food sample, altering the structure of the allergen protein [128]. The energy released during the cavitation process propagates through the ultrasonic medium, generating active free radicals, particularly hydroxyl radicals (OH); these factors cause damage to the peptide bonds, leading to changes in protein structure that may reduce allergenicity [29, 31, 126, 170]. Yang et al. [162] investigated the effect of ultrasound on soybean sprouts in which the samples were subjected to different ultrasonic conditions at 100, 200 and 300 W with a holding time of 30 min. According to the study, the IgE binding capacity of the US- treated sample considerably decreased. It was claimed that the degradation of the allergen to a number of peptides and amino acids after US treatment allowed the epitopes of the allergen to be destroyed. Wang et al. [156] studied casein (CN), an allergenic protein in cow’s milk. Treatment of fresh milk and purified CN in the US revealed a decrease in the binding capacity of IgE to CN, indicating that the US mitigated the IgE binding capacity of CN in milk. Despite CN being characterized by its linear peptide chains, which typically indicate the presence of linear epitopes, the study observed only a slight reduction in the IgE-binding capacity of CN. This suggests that while CN may predominantly contain linear epitopes, the ultrasound treatment might also impact the secondary or tertiary structures of protein to some extent, thereby affecting conformational epitopes. The result of SDS-PAGE and transmission Electron Microscopy (TEM) analyses confirmed that the reported reduction in allergenicity was significantly related to the alteration of conformational epitopes in CN. Another study conducted by X. Dong et al. [43] on the effect of US treatment on allergenic proteins in shrimp, showed that ultrasound treatment is a potentially effective method to mitigate allergens in shrimp. In this study, analysis of changes in tropomyosin (TM) content obtained by ELISA and changes in the banding intensity obtained by SDS-PAGE showed that shrimp allergenicity decreased with increasing duration of US treatment. FTIR analysis revealed alterations in secondary structures post-treatment, with a rise in β-sheets and α-helices alongside a declines in turns and unordered structures. Multiple studies have observed a correlative phenomenon where ultrasound treatment is linked to changes in protein secondary and tertiary structures, potentially reducing allergenicity by modifying conformational epitopes.
Compared to conventional treatment of allergens, the US is environmentally friendly, energy-efficient and inexpensive [29]. However, studies by Gallo et al. [50, 166] concluded that there were some negative effects on food quality, particularly on colour, flavour and even nutritional value, when food was treated using US at high power levels for a long time. Therefore, future studies should aim to optimize US parameters, such as intensity, duration, and frequency, to mitigate allergens effectively while preserving the sensory and nutritional attributes of food products.
Gamma Irradiation
Food irradiation has been recognized for its role in pasteurizing microbial (Pi, Yang, et al [113]), and gamma irradiation was found to be an effective non-thermal technology used to decrease the allergenicity of food products [108]. Gamma irradiation is a form of ionising radiation and the process of food irradiation treatment involves the irradiation of food or raw materials by highly energetic and penetrating rays [108, 115, 127]. Gamma irradiation has a dual mechanism in mitigating allergens. The first one involved the direct impact of gamma photons on proteins, with the absorbed energy inducing structure changes by breaking covalent bonds, leading to changes in linear and conformational epitopes [108, 151, 171]. Another mechanism is that after radiation, free radicals produced from water molecules react with amino acids, causing decarboxylation, reduction and oxidation of sulfhydryl groups, further modifying the protein structure [29, 108, 151]. The mechanism of gamma radiation’s effect on allergens is depicted in Fig. 3, including the modification in either linear or conformational epitopes. Van der Spiegel et al. [147] established that the changes in the protein molecular were irreversible in the gamma-irradiated allergens.
The application and mechanism of Gamma irradiation application on food allergens have been investigated for a long time. Zhenxing et al. [171] confirmed that gamma irradiation was potentially beneficial in reducing the allergenicity of shrimps, especially in processing shrimp extract with optimal functionality. It was found that the allergenicity of shrimp extract decreased with increasing irradiation dose, while the allergenicity of shrimp muscle decreased with increasing irradiation up to more than 10 kGy of radiation treatment. The results have been therein interpreted that the different effects of irradiation on the samples might be related to the radiolysis of water. There are other components of shrimp muscle, such as lipids, that protect proteins from being catalysed by free radicals formed by small doses of irradiation. The interpretation was supported by a study conducted by Muanghorn et al. [101], which focused on the effects of gamma irradiation on the allergenicity of freshwater prawns. An increase in protein band density was shown on immunoblot images at lower irradiation levels (5–7 kDa), suggesting that lipids might protect proteins from ROS attack, potentially leading to increased protein allergenicity. Moreover, the study demonstrated the gamma irradiation only at irradiation levels of 15 kGy showed the best effect on the food samples, with a notable decrease in tropomyosin band density. Another study conducted by Meinlschmidt et al. [96] who applied gamma irradiation to soy protein isolates found that gamma irradiation was effective with a complete reduction in Gly m6 when the applied dose exceeded 100 kGy.
Gamma Irradiation offers an option for reducing allergens in food without causing sensory damage to the food. However, some limitations also accompany the advantages of gamma radiation. Firstly, the most effective gamma radiation for handling food allergens is 25 kGy or more, however, the FDA set a safe radiation level for food use of only 10 kGy in 2004 [49]. Secondly, there is a list of food and food ingredients that can be treated with ionising radiation according to the regulation of the European Union (EU, [48]). However, most of the food or food ingredients in the list are traditional foods, like chicken meat, fish, milk, eggs, onions, garlic, dried fruit, and rice. As alternative protein foods including seaweed and insects are novel foods there may be some legislative issues to overcome. Finally, key consumers are sceptical of residues in irradiated food, leading to lower customer acceptance [127]. In addition, there were currently insufficient substantial studies to assess the relationship between irradiation dose and allergenic effects [108, 119]. Therefore, in order to ensure the safe and effective development and dissemination of irradiation treatment in food allergens, and to optimise gamma treatments for food allergenicity reduction, more detailed and targeted radiation studies (e.g., the range of effective radiation doses required for specific food allergens) should be investigated in depth in the future.
Discussion on the Impact of Protein Physicochemical Properties on Allergen Modification
The allergenic potential of a protein, or the ability to elicit allergic reactions, is modulated by multiple attributes such as its solubility, stability to digestion, molecular size, and conformational structure [1, 57, 94]. Research above has shown that food processing can significantly reduce the risk of food allergies by altering these protein attributes, thereby modifying their allergenic properties. Structural changes in protein are critical for both conformational and linear epitopes, which play a crucial role in the exposure or concealment of immune-reactive sites [119]. In addition, the physicochemical properties of proteins, including solubility and digestibility, can affect their interactions with the immune reaction due to modification of proteins during processing [126]. For food allergens, the solubility and stability of the protein to be digested are critical, as these factors can impact the allergenicity of the protein and sometimes the assessment of allergens in food [119]. This section focuses on discussing how changes in solubility and digestibility lead to varying outcomes in protein allergenicity.
Solubility
Numerous studies indicate a potential link between solubility and protein allergenicity [15, 20, 21]. A common hypothesis is that the aggregation and denaturation of protein post-treatment, due to changes such as the masking or unveiling of target epitopes, affect allergenicity. However, the solubility-allergenicity relationship is complicated by the unpredictable alterations in epitopes within the protein structure. Generally, processing leads to protein molecule aggregation, concealing certain conformational epitopes and thus, potentially decreasing allergenicity in tests [95]. For instance, heating processing induces the formation of insoluble aggregates of the allergens Ara h 1 and Ara h 6 in peanuts, which impedes antibody recognition by concealing native epitopes, thus resulting in a diminished allergenicity assessment [15, 90]. Recent research has further confirmed the relationship between protein solubility and its allergenic potential. Nugraha et al. [105] investigated the effects of extraction buffers on the solubility and immunoreactivity of Pacific oyster allergens, finding that buffers with high salt and pH levels enhanced the protein solubility, which subsequently resulted in increased IgE reactivity. In the study by Kasera et al. [71], the effects of thermal processing and gamma irradiation on the allergenicity and solubility of proteins from three leguminous plants (peanut, black gram and kidney bean) were investigated. It was found that thermal processing led to a decrease in the solubility of proteins from all three legumes, accompanied by a reduction in their IgE binding capacity. An interesting result was observed by Kasera et al. [71] when they verified whether the decrease in allergenicity was due to the aggregation of allergenic proteins into insoluble fractions during thermal processing. Immunological analysis was conducted on the insoluble protein fractions of the three legumes. The findings revealed that only in the case of kidney beans, there was no significant decrease in IgE binding capacity. Thermal treatment reduced the solubility of kidney bean proteins, causing them to aggregate and form less detectable insoluble aggregates in immunological assays. However, different result obtained from similar tests on peanuts and black gram which is the IgE binding capacity reduced significantly in the insoluable fraction of the samples.The discrioency between two results indicating that there is no comfirmed correlation between the protein aggregation, solubility and allergenicity. Therefore, a decrease in solubility does not consistently result in lower allergenicity, suggesting that the impact varies among different allergens. There is another research which applied the ultrasound and pressure homogenizer to treat the CN in milk, found that the solubility increased while the allergenicity reduced after the treatment [58]. Further review on aggregation and sensitization within protein families suggests that protein aggregation can enhance the allergenic potential of 2 S albumins. In contrast, for legume proteins and cereal prolamins, similar aggregative processes may lead to a reduction in allergenicity [33]. The observed correlation between solubility and allergenicity post-treatment indicates that solubility might act as a preliminary indicator for allergenic alterations. Nevertheless, a comprehensive understanding emerges only when solubility assessments are integrated with examinations of protein structure and epitope mappings, thereby elucidating the processing-induced modifications in allergenic properties.
Digestibility
In the context of food allergen research, the impact of food processing on protein digestibility and allergenic potential is complex and multifaceted. Digestibility encompasses not only the ability of the protein to be cleaved into smaller molecules, such as small peptides and single amino acids, but also the extent to which these breakdown products are absorbed by the human body. Digestibility is a crucial factor influencing the allergenic potential of proteins. However, when proteins are digested, their epitopes may be exposed, hidden, or even degraded [174]. This change in epitope unpredictability directly affects the protein’s ability to bind to antibodies, a key factor in triggering allergic reactions. The correlation between digestibility and the allergenicity is complex.
Highly digestible proteins generally have their epitopes disintegrated more easily, reducing their potential to cause allergies [134]. Conversely, proteins that resist digestion can maintain their structure, including epitopes, and may cross the intestinal barrier to elicit an immune response, heightening allergy risks [106, 119]. For instance, in the study conducted by Jin et al. [70], high-pressure processing high-pressure processing led to the unfolding of the protein structure of the squid allergen Tod p1. This structural change not only disrupted the conformational epitopes, thereby reducing its capacity to bind IgE antibodies and consequently its allergenicity but also exposed new cleavage sites for digestive enzymes, enhancing its digestibility. However, there are some cases of increased allergy due to increased digestibility. During the digestion of autoclaved peanuts, some proteins are efficiently processed, improving overall digestibility. However, particular allergenic fragments, especially those near 42 kDa, persist or are more evident. This suggests that despite the general increase in digestibility, the allergenic potential of some components persists [6, 10, 41].
The digestibility and allergenic potential of food proteins can undergo simultaneous changes due to modifications in the protein structure. However, the unpredictable digestion patterns of epitopes in allergenic proteins make it challenging to determine the allergenicity level of a processed protein solely based on its digestibility. Future research should focus on elucidating these complex interactions, potentially through in vivo studies, to better predict the allergenicity of processed food proteins [33, 70].
Conclusion
This review highlights advancements in the study of alternative proteins and their allergenic properties, focusing on innovative allergen detection methods such as MS-based proteomics and ELISA, alongside the emerging role of non-thermal processing techniques like CP, US, HPP, PEF and gamma irradiation in allergen mitigation. These innovations hold the potential for diminishing allergenicity, though challenges remain, especially in comprehensively understanding the impact on allergenic epitopes. Current research primarily on allergen processing mechanisms predominantly addresses changes in conformational epitopes. However, linear epitopes, as the most prevalence epitopes in food protein, require more focused research. on allergen mitigation. Integrating hurdle technology could address these challenges, enhancing both allergen modification and microbial safety. Furthermore, the translation of reductions in allergenicity from in vitro studies to real-world outcomes remains uncertain, largely due to variability in protein digestibility. These challenges underscore the necessity for more in-depth research on processing effects on epitope modifications and their implications for allergenicity.
As the field moves forward, the need for in-depth research becomes evident, particularly studies that bridge the gap between in vitro allergenicity assessments and actual clinical outcomes in humans. Future explorations should leverage hurdle technology to devise safer, hypoallergenic food solutions, taking into account both conformational and linear epitope modifications to achieve effective allergen management. This comprehensive strategy is crucial for advancing our understanding and capability to mitigate allergenicity in alternative protein sources.
Data Availability
Data are available within the article or its supplementary materials.
References
Aalberse RC (2000) Structural biology of allergens. J Allergy Clin Immunol 106(2):228–238
Abdulhalim I, Zourob M, Lakhtakia A (2008) Surface plasmon resonance for biosensing: a mini-review. Electromagnetics 28(3):214–242
Aguilera-Insunza R, Iturriaga C, Mariñanco A, Venegas L, Aravena G, Perez-Mateluna G, Baptista-Dias N, Borzutzky A, Wandersleben T (2023) High prevalence of lupin allergy among patients with peanut allergy: identification of γ-conglutin as major allergen. Ann Allergy Asthma Immunol 130(2):225–232
Akagawa M, Handoyo T, Ishii T, Kumazawa S, Morita N, Suyama K (2007) Proteomic Analysis of Wheat Flour Allergens. J Agric Food Chem 55(17):6863–6870. https://doi.org/10.1021/jf070843a
Anusha Siddiqui S, Bahmid NA, Mahmud CM, Boukid F, Lamri M, Gagaoua M (2022) Consumer acceptability of plant-, seaweed-, and insect-based foods as alternatives to meat: a critical compilation of a decade of research. Crit Rev Food Sci Nutr, 1–22
Apostolovic D, Stanic-Vucinic D, De Jongh HH, De Jong GA, Mihailovic J, Radosavljevic J, Radibratovic M, Nordlee JA, Baumert JL, Milcic M (2016) Conformational stability of digestion-resistant peptides of peanut conglutins reveals the molecular basis of their allergenicity. Sci Rep 6(1):29249
Banach J, Hoek-van den Hil E, van der Fels‐Klerx H (2020) Food safety hazards in the European seaweed chain. Compr Rev Food Sci Food Saf 19(2):332–364
Barba FJ, Terefe NS, Buckow R, Knorr D, Orlien V (2015) New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Res Int 77:725–742
Bass JJ, Wilkinson DJ, Rankin D, Phillips BE, Szewczyk NJ, Smith K, Atherton PJ (2017) An overview of technical considerations for Western blotting applications to physiological research. Scand J Med Sci Sports 27(1):4–25
Bavaro SL, Di Stasio L, Mamone G, De Angelis E, Nocerino R, Canani RB, Logrieco AF, Montemurro N, Monaci L (2018) Effect of thermal/pressure processing and simulated human digestion on the immunoreactivity of extractable peanut allergens. Food Res Int 109:126–137. https://doi.org/10.1016/j.foodres.2018.04.021
Besler M, Steinhart H, Paschke A (2001) Stability of food allergens and allergenicity of processed foods. J Chromatogr B Biomed Sci Appl 756(1–2):207–228
Bessada SMF, Barreira JCM, Oliveira MBPP (2019) Pulses and food security: dietary protein, digestibility, bioactive and functional properties. Trends Food Sci Technol 93:53–68. https://doi.org/10.1016/j.tifs.2019.08.022
Bettazzi F, Lucarelli F, Palchetti I, Berti F, Marrazza G, Mascini M (2008) Disposable electrochemical DNA-array for PCR amplified detection of hazelnut allergens in foodstuffs. Anal Chim Acta 614(1):93–102. https://doi.org/10.1016/j.aca.2008.03.027
Bianco M, Ventura G, Calvano CD, Losito I, Cataldi TR (2023) Food allergen detection by mass spectrometry: from common to novel protein ingredients. Proteomics 23(23–24):2200427
Blanc F, Vissers YM, Adel-Patient K, Rigby NM, Mackie AR, Gunning AP, Wellner NK, Skov PS, Przybylski‐Nicaise L, Ballmer‐Weber B (2011) Boiling peanut Ara h 1 results in the formation of aggregates with reduced allergenicity. Mol Nutr Food Res 55(12):1887–1894
Blancher C, Jones A (2001) SDS-PAGE and western blotting techniques. Metastasis research protocols. Springer, pp 145–162
Blikra MJ, Altintzoglou T, Løvdal T, Rognså G, Skipnes D, Skåra T, Sivertsvik M, Noriega Fernández E (2021) Seaweed products for the future: using current tools to develop a sustainable food industry. Trends Food Sci Technol 118:765–776. https://doi.org/10.1016/j.tifs.2021.11.002
Boeck T, Sahin AW, Zannini E, Arendt EK (2021) Nutritional properties and health aspects of pulses and their use in plant-based yogurt alternatives. Compr Rev Food Sci Food Saf 20(4):3858–3880. https://doi.org/10.1111/1541-4337.12778
Boehm D, Heslin C, Cullen PJ, Bourke P (2016) Cytotoxic and mutagenic potential of solutions exposed to cold atmospheric plasma. Sci Rep 6(1):21464–21464. https://doi.org/10.1038/srep21464
Cabanillas B, Cuadrado C, Rodriguez J, Hart J, Burbano C, Crespo JF, Novak N (2015) Potential changes in the allergenicity of three forms of peanut after thermal processing. Food Chem 183:18–25
Cabanillas B, Maleki SJ, Rodríguez J, Cheng H, Teuber SS, Wallowitz ML, Muzquiz M, Pedrosa MM, Linacero R, Burbano C (2014) Allergenic properties and differential response of walnut subjected to processing treatments. Food Chem 157:141–147
Careri M, Elviri L, Mangia A, Mucchino C (2007) ICP-MS as a novel detection system for quantitative element-tagged immunoassay of hidden peanut allergens in foods. Anal Bioanal Chem 387(5):1851–1854
Chadwick SJ (2008) Chapter 2 - Principles of Allergy Management. In J. H. Krouse, M. J. Derebery, & S. J. Chadwick (Eds.), Managing the Allergic Patient (pp. 19–72). W.B. Saunders. https://doi.org/10.1016/B978-141603677-7.50006-6
Chang X, Zhou X, Tang Y, Zhang Y, Yuan J, Li X, Yang A, Tong P, Wu Z, Chen H (2022) Effect of Processing on the structure and allergenicity of Peanut Allergen Ara h 2 Roasted in a Matrix. J Agric Food Chem 70(2):626–633. https://doi.org/10.1021/acs.jafc.1c06828
Chen H-L, Mao H-Y, Cao M-J, Cai Q-F, Su W-J, Zhang Y-X, Liu G-M (2013) Purification, physicochemical and immunological characterization of arginine kinase, an allergen of crayfish (Procambarus clarkii). Food Chem Toxicol 62:475–484
Cheng J-H, Wang H, Sun D-W (2023) Insight into the IgE-binding sites of allergenic peptides of tropomyosin in shrimp (Penaeus chinensis) induced by cold plasma active particles. Int J Biol Macromol 234:123690
Cheng JH, Wang H, Sun DW (2022) An overview of tropomyosin as an important seafood allergen: Structure, cross-reactivity, epitopes, allergenicity, and processing modifications. Compr Rev Food Sci Food Saf 21(1):127–147
Chew KW, Yap JY, Show PL, Suan NH, Juan JC, Ling TC, Lee D-J, Chang J-S (2017) Microalgae biorefinery: high value products perspectives. Bioresour Technol 229:53–62. https://doi.org/10.1016/j.biortech.2017.01.006
Chizoba Ekezie F-G, Cheng J-H, Sun D-W (2018) Effects of nonthermal food processing technologies on food allergens: a review of recent research advances. Trends Food Sci Technol 74:12–25. https://doi.org/10.1016/j.tifs.2018.01.007
Cho CY, Oles C, Nowatzke W, Oliver K, Garber EAE (2017) Cross-reactivity profiles of legumes and tree nuts using the xMAP® multiplex food allergen detection assay. Anal Bioanal Chem 409(25):5999–6014. https://doi.org/10.1007/s00216-017-0528-y
Corzo-Martínez M, Villamiel M, Moreno FJ (2017) Impact of High-intensity Ultrasound on Protein Structure and Functionality during Food Processing. In Ultrasound in Food Processing (pp. 417–436). https://doi.org/10.1002/9781118964156.ch16
Costa J, Ansari P, Mafra I, Oliveira MBPP, Baumgartner S (2014) Assessing hazelnut allergens by protein- and DNA-based approaches: LC-MS/MS, ELISA and real-time PCR. Anal Bioanal Chem 406(11):2581–2590. https://doi.org/10.1007/s00216-014-7679-x
Costa J, Villa C, Verhoeckx K, Cirkovic-Velickovic T, Schrama D, Roncada P, Rodrigues PM, Piras C, Martín-Pedraza L, Monaci L (2022) Are physicochemical properties shaping the allergenic potency of animal allergens? Clin Rev Allergy Immunol 62(1):1–36
Croat JR, Berhow M, Karki B, Muthukumarappan K, Gibbons WR (2016) Conversion of Canola meal into a high-protein feed additive via solid-state fungal incubation process. J Am Oil Chem Soc 93(4):499–507. https://doi.org/10.1007/s11746-016-2796-7
Croote D, Quake SR (2016) Food allergen detection by mass spectrometry: the role of systems biology. NPJ Syst Biology Appl 2(1):1–10
Cui Y-b, Yu L-l, Teng F-x, Wang N, Zhou Y, Yang L, Zhang C-b (2016) Dust mite allergen Der f 4: expression, characterization, and IgE binding in pediatric asthma. Pediatr Allergy Immunol 27(4):391–397. https://doi.org/10.1111/pai.12544
Dani FR, Pieraccini G (2020) Chapter Four - Proteomics of arthropod soluble olfactory proteins. In P. Pelosi & W. Knoll (Eds.), Methods in Enzymology (Vol. 642, pp. 81–102). Academic Press. https://doi.org/10.1016/bs.mie.2020.04.069
Dasan BG, Boyaci IH (2018) Effect of Cold Atmospheric plasma on inactivation of Escherichia coli and Physicochemical properties of Apple, Orange, Tomato juices, and Sour Cherry Nectar. Food Bioprocess Technol 11(2):334–343. https://doi.org/10.1007/s11947-017-2014-0
Davis PJ, Williams SC (1998) Protein modification by thermal processing. Allergy (Copenhagen) 53(46 Suppl):102–105. https://doi.org/10.1111/j.1398-9995.1998.tb04975.x
de Gier S, Verhoeckx K (2018) Insect (food) allergy and allergens. Mol Immunol 100:82–106. https://doi.org/10.1016/j.molimm.2018.03.015
Di Stasio L, Picariello G, Mongiello M, Nocerino R, Canani RB, Bavaro S, Monaci L, Ferranti P, Mamone G (2017) Peanut digestome: identification of digestion resistant IgE binding peptides. Food Chem Toxicol 107:88–98
Dong M, Xu Y, Zhang Y, Han M, Wang P, Xu X, Zhou G (2020) Physicochemical and structural properties of myofibrillar proteins isolated from pale, soft, exudative (PSE)-like chicken breast meat: effects of pulsed electric field (PEF). Innovative food Sci Emerg Technol 59:102277
Dong X, Wang J, Raghavan V (2020) Effects of high-intensity ultrasound processing on the physiochemical and allergenic properties of shrimp. Innovative food Sci Emerg Technol 65:102441. https://doi.org/10.1016/j.ifset.2020.102441
Dong X, Wang J, Raghavan V (2021) Critical reviews and recent advances of novel non-thermal processing techniques on the modification of food allergens. Crit Rev Food Sci Nutr 61(2):196–210
EFSA Panel on Dietetic Products, N., &, Allergies (2014) Scientific opinion on the evaluation of allergenic foods and food ingredients for labelling purposes. EFSA J 12(11):3894
Ekezie F-GC, Cheng J-H, Sun D-W (2018) Effects of nonthermal food processing technologies on food allergens: a review of recent research advances. Trends Food Sci Technol 74:12–25
Ekezie F-GC, Sun D-W, Cheng J-H (2019) Altering the IgE binding capacity of king prawn (Litopenaeus Vannamei) tropomyosin through conformational changes induced by cold argon-plasma jet. Food Chem 300:125143. https://doi.org/10.1016/j.foodchem.2019.125143
EU. (2009) 2009/C 283/02. List of Member States’ authorisations of food and food ingredients which may be treated with ionising radiation. https://doi.org/https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.C_.2009.283.01.0005.01.ENGhttps://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.C_.2009.283.01.0005.01.ENG
Food, Administration D (2004) Food allergen and labeling and consumer protection (FALCP) act of 2004 (Public Law 108–282, Title II). US FDA
Gallo M, Ferrara L, Naviglio D (2018) Application of ultrasound in food science and technology: a perspective. Foods 7(10):164. https://doi.org/10.3390/foods7100164
Gavahian M, Chu Y-H, Khaneghah M, Barba A, F. J., Misra NN (2018) A critical analysis of the cold plasma induced lipid oxidation in foods. Trends Food Sci Technol 77:32–41. https://doi.org/10.1016/j.tifs.2018.04.009
Gavahian M, Khaneghah AM (2020) Cold plasma as a tool for the elimination of food contaminants: recent advances and future trends. Crit Rev Food Sci Nutr 60(9):1581–1592
Geada P, Moreira C, Silva M, Nunes R, Madureira L, Rocha CM, Pereira RN, Vicente AA, Teixeira JA (2021) Algal proteins: production strategies and nutritional and functional properties. Bioresour Technol 332:125125
Gómez B, Munekata PE, Gavahian M, Barba FJ, Martí-Quijal FJ, Bolumar T, Campagnol PCB, Tomasevic I, Lorenzo JM (2019) Application of pulsed electric fields in meat and fish processing industries: an overview. Food Res Int 123:95–105
Grossmann L, Weiss J (2021) Alternative protein sources as Technofunctional Food Ingredients. Annual Rev food Sci Technol 12(1):93–117. https://doi.org/10.1146/annurev-food-062520-093642
Hadi J, Brightwell G (2021) Safety of alternative proteins: Technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein. Foods 10(6):1226. https://doi.org/10.3390/foods10061226
Haidar E, Lakkis J, Karam M, Koubaa M, Louka N, Debs E (2023) Peanut allergenicity: an insight into its Mitigation using Thermomechanical Processing. Foods 12(6):1253
Han T, Wang M, Wang Y, Tang L (2020) Effects of high-pressure homogenization and ultrasonic treatment on the structure and characteristics of casein. Lwt 130:109560. https://doi.org/10.1016/j.lwt.2020.109560
Hartmann C, Siegrist M (2017) Consumer perception and behaviour regarding sustainable protein consumption: a systematic review. Trends Food Sci Technol 61:11–25. https://doi.org/10.1016/j.tifs.2016.12.006
Hashimoto H, Hongo T, Hayashi C, Nakamura K, Nakanishi K, Ikeda M, Adachi R, Akiyama H, Teshima R, Yano T (2015) A method for the detection of shrimp/prawn and crab DNAs to identify allergens in dried seaweed products. Japanese J Food Chem Saf 22(1):1–10. https://doi.org/10.18891/jjfcs.22.1_1
He Z (2015) 5 - Protein inference in shotgun proteomics. In Z. He (Ed.), Data Mining for Bioinformatics Applications (pp. 39–49). Woodhead Publishing. https://doi.org/10.1016/B978-0-08-100100-4.00005-3
Hirao T, Hiramoto M, Imai S, Kato H (2006) A novel PCR method for quantification of buckwheat by using a unique internal standard material. J Food Prot 69(10):2478–2486
Huang H-W, Yang BB, Wang C-Y (2014) Effects of high pressure processing on immunoreactivity and microbiological safety of crushed peanuts. Food Control 42:290–295. https://doi.org/10.1016/j.foodcont.2014.02.030
Huang HW, Hsu CP, Yang BB, Wang CY (2014) Potential utility of high-pressure processing to address the risk of food allergen concerns. Compr Rev Food Sci Food Saf 13(1):78–90
Imathiu S (2020) Benefits and food safety concerns associated with consumption of edible insects. NFS J 18:1–11
Iniesto E, Jiménez A, Prieto N, Cabanillas B, Burbano C, Pedrosa MM, Rodríguez J, Muzquiz M, Crespo JF, Cuadrado C (2013) Real time PCR to detect hazelnut allergen coding sequences in processed foods. Food Chem 138(2–3):1976–1981
Jappe U, Karstedt A, Warneke D, Hellmig S, Böttger M, Riffelmann FW, Treudler R, Lange L, Abraham S, Dölle-Bierke S, Worm M, Wagner N, Ruëff F, Reese G, Knulst AC, Becker WM (2021) Identification and purification of novel low-molecular-weight lupine allergens as components for personalized diagnostics. Nutrients 13(2):1–21. https://doi.org/10.3390/nu13020409
Jiang Q, Zhang M, Xu B (2020) Application of ultrasonic technology in postharvested fruits and vegetables storage: a review. Ultrason Sonochem 69:105261. https://doi.org/10.1016/j.ultsonch.2020.105261
Jin S, Kennedy RT (2015) New developments in Western blot technology. Chin Chem Lett 26(4):416–418. https://doi.org/10.1016/j.cclet.2015.01.021
Jin Y, Deng Y, Qian B, Zhang Y, Liu Z, Zhao Y (2015) Allergenic response to squid (Todarodes pacificus) tropomyosin Tod p1 structure modifications induced by high hydrostatic pressure. Food Chem Toxicol 76:86–93. https://doi.org/10.1016/j.fct.2014.12.002
Kasera R, Singh AB, Kumar R, Lavasa S, Prasad KN, Arora N (2012) Effect of thermal processing and γ-irradiation on allergenicity of legume proteins. Food Chem Toxicol 50(10):3456–3461. https://doi.org/10.1016/j.fct.2012.07.031
Khan MU, Ahmed I, Lin H, Li Z, Costa J, Mafra I, Chen Y, Wu Y-N (2019) Potential efficacy of processing technologies for mitigating crustacean allergenicity. Crit Rev Food Sci Nutr 59(17):2807–2830
Kim M-J, Kim H-I, Kim J-H, Suh S-M, Kim H-Y (2018) Rapid on-site detection of shrimp allergen tropomyosin using a novel ultrafast PCR system. Food Sci Biotechnol 28(2):591–597. https://doi.org/10.1007/s10068-018-0479-x
Kirsch S, Fourdrilis S, Dobson R, Scippo M-L, Maghuin-Rogister G, De Pauw E (2009) Quantitative methods for food allergens: a review. Anal Bioanal Chem 395(1):57–67. https://doi.org/10.1007/s00216-009-2869-7
Kleber N, Maier S, Hinrichs J (2007) Antigenic response of bovine β-lactoglobulin influenced by ultra-high pressure treatment and temperature. Innovative food Sci Emerg Technol 8(1):39–45
Kumar K, Srivastav S, Sharanagat VS (2021) Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason Sonochem 70:105325. https://doi.org/10.1016/j.ultsonch.2020.105325
Kurien BT, Scofield RH (2006) Western blotting. Methods 38(4):283–293. https://doi.org/10.1016/j.ymeth.2005.11.007
Kutateladze T, Bitskinashvili K, Sapojnikova N, Kartvelishvili T, Asatiani N, Vishnepolsky B, Datukishvili N (2021) Development of multiplex PCR coupled DNA chip technology for assessment of endogenous and exogenous allergens in GM soybean. Biosens (Basel) 11(12):481. https://doi.org/10.3390/bios11120481
Lavilla M, Orcajo J, Díaz-Perales A, Gamboa P (2016) Examining the effect of high pressure Processing on the allergenic potential of the major allergen in peach (Pru p 3). Innovative Food Sci Emerg Technol 38:334–341. https://doi.org/10.1016/j.ifset.2016.06.021
Lavilla M, Puértolas E, Orcajo J (2020) HPP impact to reduce allergenicity of foods. Present and future of high pressure processing. Elsevier, pp 113–138
Li H, Jia Y, Peng W, Zhu K, Zhou H, Guo X (2018) High hydrostatic pressure reducing allergenicity of soy protein isolate for infant formula evaluated by ELISA and proteomics via Chinese soy-allergic children’s sera. Food Chem 269:311–317. https://doi.org/10.1016/j.foodchem.2018.07.001
Li Y, Ding J, Dong L, Sun N, Lin S (2023) Mechanism of targeted regulation of ovalbumin epitopes by pulsed electric field-assisted alcalase treatment. J Agric Food Chem 71(27):10417–10426
Li Y, Zhang S, Ding J, Zhong L, Sun N, Lin S (2022) Evaluation of the structure-activity relationship between allergenicity and spatial conformation of ovalbumin treated by pulsed electric field. Food Chem 388:133018
Li Y, Zhang S, Jiang P, Zhong L, Lin S, Sun N (2021) Exploration of structure-activity relationship between IgG1 and IgE binding ability and spatial conformation in ovomucoid with pulsed electric field treatment. Food Sci Technol 141:110891. https://doi.org/10.1016/j.lwt.2021.110891
Lin H-Y, Huang C-H, Park J, Pathania D, Castro CM, Fasano A, Weissleder R, Lee H (2017) Integrated magneto-chemical sensor for on-site food allergen detection. ACS Nano 11(10):10062–10069
Lin Y, Feng L, Li X, Chen Y, Yin G, Zhou W (2020) Study on ultrasound-assisted oxidative desulfurization for crude oil. Ultrason Sonochem 63:104946. https://doi.org/10.1016/j.ultsonch.2019.104946
Liu C, Sathe SK (2018) Food allergen epitope mapping. J Agric Food Chem 66(28):7238–7248
Liu M, Huan F, Li M, Han T, Xia F, Yang Y, Liu Q, Chen G, Cao M, Liu G (2021) Mapping and IgE-binding capacity analysis of heat/digested stable epitopes of mud crab allergens. Food Chem 344:128735
Long F, Yang X, Wang R, Hu X, Chen F (2015) Effects of combined high pressure and thermal treatments on the allergenic potential of shrimp (Litopenaeus vannamei) tropomyosin in a mouse model of allergy. Innovative food Sci Emerg Technol 29:119–124. https://doi.org/10.1016/j.ifset.2015.03.002
Luo C, Hu C, Gao J, Li X, Wu Z, Yang A, Chen H (2013) A potential practical approach to reduce Ara h 6 allergenicity by gamma irradiation. Food Chem 136(3–4):1141–1147
MacPhee DJ (2010) Methodological considerations for improving Western blot analysis. J Pharmacol Toxicol Methods 61(2):171–177
Maleki SJ, Viquez O, Jacks T, Dodo H, Champagne ET, Chung S-Y, Landry SJ (2003) The major peanut allergen, Ara h 2, functions as a trypsin inhibitor, and roasting enhances this function. J Allergy Clin Immunol 112(1):190–195
Marzano V, Tilocca B, Fiocchi AG, Vernocchi P, Mortera SL, Urbani A, Roncada P, Putignani L (2020) Perusal of food allergens analysis by mass spectrometry-based proteomics. J Proteom 215:103636
Masilamani M, Commins S, Shreffler W (2012) Determinants of food allergy. Immunol Allergy Clin 32(1):11–33
Mattison CP, Bren-Mattison Y, Vant-Hull B, Vargas AM, Wasserman RL, Grimm CC (2016) Heat-induced alterations in cashew allergen solubility and IgE binding. Toxicol Rep 3:244–251. https://doi.org/10.1016/j.toxrep.2015.12.009
Meinlschmidt P, Ueberham E, Lehmann J, Reineke K, Schlüter O, Schweiggert-Weisz U, Eisner P (2016) The effects of pulsed ultraviolet light, cold atmospheric pressure plasma, and gamma-irradiation on the immunoreactivity of soy protein isolate. Innovative food Sci Emerg Technol 38:374–383. https://doi.org/10.1016/j.ifset.2016.06.007
Meng X, Bai Y, Gao J, Li X, Chen H (2017) Effects of high hydrostatic pressure on the structure and potential allergenicity of the major allergen bovine β-lactoglobulin. Food Chem 219:290–296. https://doi.org/10.1016/j.foodchem.2016.09.153
Mildenberger J, Stangeland JK, Rebours C (2022) Antioxidative activities, phenolic compounds and marine food allergens in the macroalgae Saccharina Latissima produced in integrated multi-trophic aquaculture systems. Aquaculture 546:737386. https://doi.org/10.1016/j.aquaculture.2021.737386
Monaci L, Visconti A (2009) Mass spectrometry-based proteomics methods for analysis of food allergens. TRAC Trends Anal Chem 28(5):581–591
Mondoulet L, Paty E, Drumare MF, Ah-Leung S, Scheinmann P, Willemot RM, Wal JM, Bernard H (2005) Influence of Thermal Processing on the allergenicity of peanut proteins. J Agric Food Chem 53(11):4547–4553. https://doi.org/10.1021/jf050091p
Muanghorn W, Konsue N, Sham H, Othman Z, Mohamed F, Mohd Noor N, Othman N, Akmal MN, Ahmad Fauzi NSS, Dewaprigam Solomen NP, M. M., Abdull Razis AF (2018) Effects of gamma irradiation on tropomyosin allergen, proximate composition and mineral elements in giant freshwater prawn (Macrobrachium rosenbergii). J Food Sci Technol 55(5):1960–1965. https://doi.org/10.1007/s13197-018-3104-3
Mulalapele LT, Xi J (2021) Detection and inactivation of allergens in soybeans: a brief review of recent research advances. Grain Oil Sci Technol 4(4):191–200
Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, Cardona V, Dubois A, duToit G, Eigenmann P, Fernandez Rivas M, Halken S, Hickstein L, Høst A, Knol E, Lack G, Marchisotto MJ, Niggemann B, Nwaru BI, Papadopoulos NG, Poulsen LK, Santos AF, Skypala I, Schoepfer A, van Ree R, Venter C, Worm M, Vlieg-Boerstra B, Panesar S, de Silva D, Soares-Weiser K, Sheikh A, Ballmer-Weber BK, Nilsson C, de Jong NW, Akdis CA, Anaphylaxis EFA, Allergy EF, Guidelines A (2014) EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy (Copenhagen) 69(8):1008–1025, the, E. F. A., & Anaphylaxis Guidelines. https://doi.org/10.1111/all.12429
Ng SW, Lu P, Rulikowska A, Boehm D, O’Neill G, Bourke P (2021) The effect of atmospheric cold plasma treatment on the antigenic properties of bovine milk casein and whey proteins. Food Chem 342:128283–128283. https://doi.org/10.1016/j.foodchem.2020.128283
Nugraha R, Ruethers T, Johnston EB, Rolland JM, O’Hehir RE, Kamath SD, Lopata AL (2021) Effects of extraction buffer on the solubility and immunoreactivity of the Pacific Oyster allergens. Foods 10(2):409
Pali-Schöll I, Untersmayr E, Klems M, Jensen-Jarolim E (2018) The effect of digestion and digestibility on allergenicity of food. Nutrients 10(9):1129
Pan D, Tang B, Liu H, Li Z, Ma R, Peng Y, Wu X, Che L, He N, Ling X, Wang Y (2020) Effect of high hydrostatic pressure (HHP) Processing on immunoreactivity and spatial structure of Peanut Major Allergen Ara h 1. Food Bioprocess Technol 13(1):132–144. https://doi.org/10.1007/s11947-019-02382-z
Pan M, Yang J, Liu K, Xie X, Hong L, Wang S, Wang S (2021) Irradiation technology: an effective and promising strategy for eliminating food allergens. Food Res Int 148:110578–110578. https://doi.org/10.1016/j.foodres.2021.110578
Pankaj SK, Wan Z, Keener KM (2018) Effects of cold plasma on food quality: a review. Foods 7(1):4. https://doi.org/10.3390/foods7010004
Pastorello EA, Trambaioli C (2001) Isolation of food allergens. J Chromatogr B Biomed Sci Appl 756(1–2):71–84
Petrus M, Culerrier R, Campistron M, Barre A, Rougé P (2010) First case report of anaphylaxis to spirulin: identification of phycocyanin as responsible allergen. Allergy 65(7):924–925. https://doi.org/10.1111/j.1398-9995.2009.02257.x
Pi X, Sun Y, Fu G, Wu Z, Cheng J (2021) Effect of processing on soybean allergens and their allergenicity. Trends Food Sci Technol 118:316–327
Pi X, Yang Y, Sun Y, Wang X, Wan Y, Fu G, Li X, Cheng J (2021) Food irradiation: a promising technology to produce hypoallergenic food with high quality. Crit Rev Food Sci Nutr 62(24):6698–6713
Picotti P, Aebersold R (2012) Selected reaction monitoring–based proteomics: workflows, potential, pitfalls and future directions. Nat Methods 9(6):555–566. https://doi.org/10.1038/nmeth.2015
Pillai SD, Shayanfar S (2016) Electron Beam Technology and other Irradiation Technology Applications in the Food Industry. Top Curr Chem (2016) 375(1):1–20. https://doi.org/10.1007/s41061-016-0093-4
Polikovsky M, Fernand F, Sack M, Frey W, Müller G, Golberg A (2019) In silico food allergenic risk evaluation of proteins extracted from macroalgae Ulva sp. with pulsed electric fields. Food Chem 276:735–744. https://doi.org/10.1016/j.foodchem.2018.09.134
Pomés A, Schulten V, Glesner J, da Silva Antunes R, Sutherland A, Bacharier LB, Beigelman A, Busse P, Frazier A, Sette A (2021) IgE and T cell reactivity to a Comprehensive Panel of Cockroach Allergens in Relation to Disease. Front Immunol 11:621700–621700. https://doi.org/10.3389/fimmu.2020.621700
Poms RE, Klein CL, Anklam E (2004) Methods for allergen analysis in food: a review. Food Addit Contam 21(1):1–31. https://doi.org/10.1080/02652030310001620423
Rahaman T, Vasiljevic T, Ramchandran L (2016) Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci Technol 49:24–34
Ree Rv, Akkerdaas J, Zuidmeer L (2006) 5 - immunoblotting in allergen detection. Elsevier Ltd, pp 98–108. https://doi.org/10.1533/9781845690557.2.98
Ring J, Akdis C, Lauener R, Schäppi G, Traidl-Hoffmann C, Akdis M, Ammann W, Behrendt H, Bieber T, Biedermann T, Bienenstock J, Blaser K, Braun-Fahrländer C, Brockow K, Buters J, Crameri R, Darsow U, Denburg JA, Eyerich K, Frei R, Galli SJ, Gutermuth J, Holt P, Koren H, Leung D, Müller U, Muraro A, Ollert M, O’Mahony L, Pawankar R, Platts-Mills T, Rhyner C, Rosenwasser LJ, Schmid-Grendelmeier P, Schmidt-Weber CB, Schmutz W, Simon D, Simon HU, Sofiev M, van Hage M, van Ree R (2014) Global Allergy Forum and Second Davos Declaration 2013 Allergy: barriers to cure–challenges and actions to be taken. Allergy 69(8):978–982. https://doi.org/10.1111/all.12406
Rodríguez Mallón A, Javier González L, Guzmán E, Bechara PE, Sanches GH, Pousa GS, Cabrera S, Cabrales G, Garay A, H., Mejías R (2020) Functional and mass spectrometric evaluation of an anti-tick antigen based on the P0 peptide conjugated to Bm86 protein. Pathogens 9(6):513
Ross GMS, Bremer MGEG, Nielen MWF (2018) Consumer-friendly food allergen detection: moving towards smartphone-based immunoassays. Anal Bioanal Chem 410(22):5353–5371. https://doi.org/10.1007/s00216-018-0989-7
Schubert-Ullrich P, Rudolf J, Ansari P, Galler B, Führer M, Molinelli A, Baumgartner S (2009) Commercialized rapid immunoanalytical tests for determination of allergenic food proteins: an overview. Anal Bioanal Chem 395(1):69–81. https://doi.org/10.1007/s00216-009-2715-y
Sengar AS, Thirunavookarasu N, Choudhary P, Naik M, Surekha A, Sunil CK, Rawson A (2022) Application of power ultrasound for plant protein extraction, modification and allergen reduction – A review. Appl Food Res 2(2):100219. https://doi.org/10.1016/j.afres.2022.100219
Shah F, Shi A, Ashley J, Kronfel C, Wang Q, Maleki SJ, Adhikari B, Zhang J (2019) Peanut allergy: characteristics and approaches for mitigation. Compr Rev Food Sci Food Saf 18(5):1361–1387
Shahbaz HM, Akram K, Ahn J-J, Kwon J-H (2016) Worldwide Status of Fresh fruits irradiation and concerns about Quality, Safety, and Consumer Acceptance. Crit Rev Food Sci Nutr 56(11):1790–1807. https://doi.org/10.1080/10408398.2013.787384
Shriver S, Yang W, Chung S-Y, Percival S (2011) Pulsed ultraviolet light reduces immunoglobulin E binding to Atlantic white shrimp (Litopenaeus setiferus) extract. Int J Environ Res Public Health 8(7):2569–2583
Shriver SK, Yang WW (2011) Thermal and Nonthermal Methods for Food Allergen Control. Food Eng Rev 3(1):26–43. https://doi.org/10.1007/s12393-011-9033-9
Sicherer SH, Sampson HA (2018) Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 141(1):41–58. https://doi.org/10.1016/j.jaci.2017.11.003
Sirtori E, Resta D, Arnoldi A, Savelkoul HFJ, Wichers HJ (2011) Cross-reactivity between peanut and lupin proteins. Food Chem 126(3):902–910. https://doi.org/10.1016/j.foodchem.2010.11.073
Sruthi NU, Josna K, Pandiselvam R, Kothakota A, Gavahian M, Mousavi Khaneghah A (2022) Impacts of cold plasma treatment on physicochemical, functional, bioactive, textural, and sensory attributes of food: a comprehensive review. Food Chem 368:130809. https://doi.org/10.1016/j.foodchem.2021.130809
Sule R, Rivera G, Gomes AV (2023) Western blotting (immunoblotting): history, theory, uses, protocol and problems. Biotechniques 75(3):99–114
Sun N, Liu Y, Liu K, Wang S, Liu Q, Lin S (2022) Gastrointestinal fate of food allergens and its relationship with allergenicity. Compr Rev Food Sci Food Saf 21(4):3376–3404
Tabatabaei MS, Islam R, Ahmed M (2021) Applications of gold nanoparticles in ELISA, PCR, and immuno-PCR assays: a review. Anal Chim Acta 1143:250–266. https://doi.org/10.1016/j.aca.2020.08.030
Taha A, Ahmed E, Ismaiel A, Ashokkumar M, Xu X, Pan S, Hu H (2020) Ultrasonic emulsification: an overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci Technol 105:363–377. https://doi.org/10.1016/j.tifs.2020.09.024
Tanna B, Mishra A (2019) Nutraceutical potential of seaweed polysaccharides: structure, bioactivity, safety, and toxicity. Compr Rev Food Sci Food Saf 18(3):817–831
Taylor SL, Marsh JT, Koppelman SJ, Kabourek JL, Johnson PE, Baumert JL (2021) A perspective on pea allergy and pea allergens. Trends Food Sci Technol 116:186–198. https://doi.org/10.1016/j.tifs.2021.07.017
Thomas I, Siew LQ, Watts TJ, Haque R (2019) Seaweed allergy. J Allergy Clin Immunol Pract 7(2):714–715
Tobajas AP, Agulló-García A, Cubero JL, Colás C, Segura-Gil I, Sánchez L, Calvo M, Pérez MD (2020) Effect of high pressure and pulsed electric field on denaturation and allergenicity of Pru p 3 protein from peach. Food Chem 321:126745. https://doi.org/10.1016/j.foodchem.2020.126745
Tolouie H, Mohammadifar MA, Ghomi H, Hashemi M (2018) Cold atmospheric plasma manipulation of proteins in food systems. Crit Rev Food Sci Nutr 58(15):2583–2597
Tordesillas L, Berin MC, Sampson HA (2017) Immunology of food allergy. Immunity 47(1):32–50
Tsagkaris AS, Nelis JL, Ross G, Jafari S, Guercetti J, Kopper K, Zhao Y, Rafferty K, Salvador JP, Migliorelli D (2019) Critical assessment of recent trends related to screening and confirmatory analytical methods for selected food contaminants and allergens. TRAC Trends Anal Chem 121:115688
Tu C, Sheng Q, Li J, Ma D, Shen X, Wang X, Shyr Y, Yi Z, Qu J (2015) Optimization of search engines and postprocessing approaches to maximize peptide and protein identification for high-resolution Mass Data. J Proteome Res 14(11):4662–4673. https://doi.org/10.1021/acs.jproteome.5b00536
Ullmann J, Grimm D (2021) Algae and their potential for a future bioeconomy, landless food production, and the socio-economic impact of an algae industry. Org Agric 11(2):261–267
Uvackova L, Skultety L, Bekesova S, McClain S, Hajduch M (2013) MSE Based Multiplex Protein Analysis Quantified Important Allergenic Proteins and detected relevant peptides carrying known epitopes in wheat grain extracts. J Proteome Res 12(11):4862–4869. https://doi.org/10.1021/pr400336f
Van der Spiegel M, Noordam M, Van der Fels-Klerx H (2013) Safety of novel protein sources (insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr Rev Food Sci Food Saf 12(6):662–678
van der Weele C, Feindt P, van der Jan A, van Mierlo B, van Boekel M (2019) Meat alternatives: an integrative comparison. Trends Food Sci Technol 88:505–512. https://doi.org/10.1016/j.tifs.2019.04.018
van Hengel AJ (2007) Food allergen detection methods and the challenge to protect food-allergic consumers. Anal Bioanal Chem 389(1):111–118
Vanga SK, Singh A, Raghavan V (2017) Review of conventional and novel food processing methods on food allergens. Crit Rev Food Sci Nutr 57(10):2077–2094. https://doi.org/10.1080/10408398.2015.1045965
Vaz AF, Souza MP, Carneiro-da-Cunha MG, Medeiros PL, Melo AM, Aguiar JS, Silva TG, Silva-Lucca RA, Oliva ML, Correia MT (2012) Molecular fragmentation of wheat-germ agglutinin induced by food irradiation reduces its allergenicity in sensitised mice. Food Chem 132(2):1033–1039
Venkataratnam H, Sarangapani C, Cahill O, Ryan CB (2019) Effect of cold plasma treatment on the antigenicity of peanut allergen Ara h 1. Innovative food Sci Emerg Technol 52:368–375. https://doi.org/10.1016/j.ifset.2019.02.001
Villa C, Costa J, Mafra I (2020) Lupine allergens: clinical relevance, molecular characterization, cross-reactivity, and detection strategies. Compr Rev Food Sci Food Saf 19(6):3886–3915. https://doi.org/10.1111/1541-4337.12646
Vogelsang-O’Dwyer M, Zannini E, Arendt EK (2021) Production of pulse protein ingredients and their application in plant-based milk alternatives. Trends Food Sci Technol 110:364–374. https://doi.org/10.1016/j.tifs.2021.01.090
Wade M, Hoelle J (2020) A review of edible insect industrialization: scales of production and implications for sustainability. Environ Res Lett 15(12):123013. https://doi.org/10.1088/1748-9326/aba1c1
Wang C, Xie Q, Wang Y, Fu L (2020) Effect of Ultrasound Treatment on Allergenicity reduction of milk casein via colloid formation. J Agric Food Chem 68(16):4678–4686. https://doi.org/10.1021/acs.jafc.9b08245
Wang F-Q, Cheng J-H, Keener KM (2023) Changing the IgE binding capacity of tropomyosin in shrimp through structural modification induced by cold plasma and glycation treatment. Foods 12(1):206
Wang S, Lin S, Liu K, Liu Y, Liu Q, Sun N (2023) Digestion-Resistant Linear Epitopes as Dominant contributors to strong allergenicity of Tropomyosin in Antarctic Krill (Euphausia superba). J Agric Food Chem 71(44):16739–16751
Wei J-N, Zeng X-A, Tang T, Jiang Z, Liu Y-Y (2018) Unfolding and nanotube formation of ovalbumin induced by pulsed electric field. Innovative food Sci Emerg Technol 45:249–254. https://doi.org/10.1016/j.ifset.2017.10.011
Weng X, Gaur G, Neethirajan S (2016) Rapid detection of food allergens by microfluidics ELISA-based optical sensor. Biosens (Basel) 6(2):24–24. https://doi.org/10.3390/bios6020024
Wu L, Li G, Xu X, Zhu L, Huang R, Chen X (2019) Application of nano-ELISA in food analysis: recent advances and challenges. TRAC Trends Anal Chem 113:140–156. https://doi.org/10.1016/j.trac.2019.02.002
Yang H, Gao J, Yang A, Chen H (2015) The ultrasound-treated soybean seeds improve edibility and nutritional quality of soybean sprouts. Food Res Int 77:704–710. https://doi.org/10.1016/j.foodres.2015.01.011
Yang W, Tu Z, Wang H, Zhang L, Gao Y, Li X, Tian M (2017) Immunogenic and structural properties of ovalbumin treated by pulsed electric fields. Int J Food Prop 20(sup3):S3164–S3176. https://doi.org/10.1080/10942912.2017.1396479
Yang W, Tu Z, Wang H, Zhang L, Gao Y, Li X, Tian M (2018) Immunogenic and structural properties of ovalbumin treated by pulsed electric fields. Int J Food Prop 20(sup3):S3164–S3176. https://doi.org/10.1080/10942912.2017.1396479
Yang W, Tu Z, Wang H, Zhang L, Kaltashov IA, Zhao Y, Niu C, Yao H, Ye W (2018) The mechanism of reduced IgG/IgE-binding of β-lactoglobulin by pulsed electric field pretreatment combined with glycation revealed by ECD/FTICR-MS. Food Funct 9(1):417–425
Yuan S, Li C, Zhang Y, Yu H, Xie Y, Guo Y, Yao W (2021) Ultrasound as an emerging technology for the elimination of chemical contaminants in food: a review. Trends Food Sci Technol 109:374–385. https://doi.org/10.1016/j.tifs.2021.01.048
Zhang B, Tan C, Zou F, Sun Y, Shang N, Wu W (2022) Impacts of cold plasma technology on sensory, nutritional and safety quality of food: a review. Foods 11(18):2818
Zhang Y, Deng Y, Zhao Y (2017) Structure-based modelling of hemocyanin allergenicity in squid and its response to high hydrostatic pressure. Sci Rep 7(1):1–10
Zhang Y, Ren Y, Bi Y, Wang Q, Cheng K-W, Chen F (2019) Seafood allergy and potential application of high hydrostatic pressure to reduce seafood allergenicity. Int J Food Eng, 15(8)
Zhang Z, Zhang X, Chen W, Zhou P (2018) Conformation stability, in vitro digestibility and allergenicity of tropomyosin from shrimp (Exopalaemon modestus) as affected by high intensity ultrasound. Food Chem 245:997–1009. https://doi.org/10.1016/j.foodchem.2017.11.072
Zhenxing L, Hong L, Limin C, Jamil K (2007) The influence of gamma irradiation on the allergenicity of shrimp (Penaeus vannamei). J Food Eng 79(3):945–949
Zhou F, He S, Sun H, Wang Y, Zhang Y (2021) Advances in epitope mapping technologies for food protein allergens: a review. Trends Food Sci Technol 107:226–239
Zhou H, Wang C, Ye J, Chen H, Tao R, Cao F (2016) Effects of high hydrostatic pressure treatment on structural, allergenicity, and functional properties of proteins from ginkgo seeds. Innovative Food Sci Emerg Technol 34:187–195. https://doi.org/10.1016/j.ifset.2016.02.001
Zhou H, Wu Z, Chang X, Tang Y, Yuan J, Li X, Yang A, Tong P, Chen H (2021) The effect of roasting on peanut allergens’ digestibility, allergenicity, and structure. Food Bioscience 44:101454
Acknowledgements
The authors would like to acknowledge the U-Protein project funded by the Irish Department of Agriculture, Food and Marine (DAFM) under the Food Institutional Research Measure (Grant No. 2019PROG702). Gaoya Dong and Zhipeng Hu would also like to acknowledge the financial support from University College Dublin (UCD) and China Scholarship Council (CSC, China) for their PhD studies under the UCD-CSC Scholarship scheme.
Funding
Open Access funding provided by the IReL Consortium
Author information
Authors and Affiliations
Contributions
First author: Writing - original draft, Formal analysis, Investigation.Others: Investigation.2nd Last author: Supervision, Funding acquisition, Resources, Writing - review & editing.Last author: Supervision, Funding acquisition, Resources, Writing - review & editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dong, G., Hinds, L.M., Soro, A.B. et al. Non-Thermal Processing Technologies for Allergen Control in Alternative Protein Sources for Food Industry Applications. Food Eng Rev (2024). https://doi.org/10.1007/s12393-024-09378-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12393-024-09378-2